Abstract
Arterial blood pressure (BP) is a continuous variable, with a physiology characterized by significant variability stemming from the complex interaction among haemodynamic factors, neuronal reflexes, as well as hormonal, behavioural, and environmental stimuli. The homoeostatic response accounts for the physiologic variability in BP in normotensive individuals, which is more evident in hypertensive patients. Blood pressure variability is a complex phenomenon, which could be classified in various types: very short term (beat to beat), short term (during 24 h), mid-term (day by day), long term (<5 years), and very long term (>5 years). Accurate measurement of BP variability represents a complex and often controversial endeavour, despite several methodological approaches are available. Albeit a prognostic significance has been demonstrated for some indicators of BP variability, the clinical significance of this measurement is still uncertain. In fact, none of the indicators presently available for BP variability, including early morning BP rise, substantially affects, and redefines, the cardiovascular risk of the hypertensive patient, over and beyond the mere BP values. Accordingly, in defining the cardiovascular risk, the focus should be on the absolute BP values, which remain the most relevant risk factor, and the one more susceptible to modification with both non-pharmacologic and pharmacologic treatment.
Keywords: Blood pressure variability, Arterial hypertension, ABPM, Standard deviation, Microcirculation
Characteristics of blood pressure variability
Arterial pressure is a continuous variable characterized physiologically by marked oscillations deriving from the complex interaction among haemodynamic, reflex neuronal, humoral, behavioural, and environmental factors.1,2 Being a homoeostatic response, these oscillations occur physiologically in normotensive subjects and tend to be more pronounced in hypertensive subjects. Blood pressure (BP) variability is a complex phenomenon, classifiable in different types (Table 1).2,3 The very short-term variability is identifiable in the beat-beat variations, and is due to the interaction between baroceptive reflexes, the sympathetic nervous system, the renin–angiotensin–aldosterone system, nitric oxide release, and behavioural alterations. The short-term variability, intended as pressure variations over 24 h, is mainly characterized by circadian modulations. Among the most relevant there is the physiological nocturnal reduction of systolic and diastolic BP, which in normal subjects, defined as ‘dippers’, is about 10–20%. In some subjects, hypertensive phenotype species, defined as ‘non-dippers’, this reduction is <10%, while in the ‘reverse dippers’ there is even a pressure increase during the night.1,2,4 A second source of circadian variability is represented by a sometimes sharp increase in arterial pressure during the first hours of the morning, called ‘morning surge’ or ‘morning rise’5; however, its precise definition, especially in terms of numerical threshold, is still very controversial.
Table 1.
Different types of blood pressure variability and their determinants
| Types of blood pressure variability | Determinants |
|---|---|
| Very short-term variability (beat-to-beat) |
|
| Short-term variability (in 24 h) |
|
| Medium-term variability (day-day) |
|
| Long-term variability (visit-visit < 5 years) and very long-term variability (visit-visit > 5 years) |
|
Medium-term variability, i.e. day-day and long-term variability, represented by visit-visit and season-season variability, are influenced by behavioural, environmental (temperature, altitude, etc.) factors but also by adherence of the patient to antihypertensive therapy.1,2,4
Evaluation of pressure variability
The precise measurement of BP variability is also complex and sometimes controversial.6
The beat-to-beat variability has been measured for years through the invasive Oxford intra-arterial method, up until the advent of the ‘Penaz method’, based on finger sensors that exploit the photoplethysmography technique.1,2 Short-term variability was also extensively assessed through non-invasive 24-h ambulatory measurement (ambulatory blood pressure monitoring, ABPM), with measurements every 15–30 min.4 From 24-h pressure records, it is possible to estimate 24-h variability by calculating the standard deviation of mean systolic, diastolic and mean arterial pressure values, and the coefficient of variation, which provides more reliable information by partially correcting the direct proportionality observed between values average and BP variability.4–6 However, the standard deviation and the coefficient of variation of the pressure values are affected by the influence of stressors and day-night differences. For this reason, other indices have been proposed to evaluate the variability in the short term, such as, for example, the real average variability (‘average real variability’, ARV), the average of the absolute differences between consecutive measurements7 and the residual variability (‘residual BPV’), obtained by eliminating, through the spectral analysis, the slowest components of the 24-h BP profile.7 The use of the weighted standard 24-h BP deviation was also suggested (weighted 24-h BP SD), which selectively removes the contribution of night-time dipping and weighs the average of day and night pressure for the day and day night.2,4,8
This last methodological adjustment is motivated by the fact that, as detailed below, an increased BP variability represents a negative prognostic index, in the same way, a reduced nocturnal pressure reduction (which in itself reduces the 24-h pressure variability) is associated with worst prognosis.2,4,8 Consequently, an index that allows the exclusion of night-time dipping or non-dipping from the quantification of BP variability over 24 h could provide more reliable information from the clinical point of view.
The evaluation of medium and long-term variability, on the other hand, is affected by various problems since it is often difficult to obtain such a number of measurements to allow an adequate estimate; furthermore, the measurement in the doctor’s office (‘office BP’) does not always reflect the arterial pressure values observed during the patient’s usual activities and is affected by the ‘white coat’ effect.1 The ABPM can be considered a certainly more reliable method, but its extensive application has organizational limits. The methodological approach most easily applicable to large populations would seem to be home measurement (home blood pressure monitoring) because it allows obtaining repeated measurements day after day under standardized conditions.1
Blood pressure variability, microcirculation, and macrocirculation
The carotid-femoral pulse wave velocity (carotid-femoral pulse wave velocity, cfPWV) is the gold standard for the non-invasive measurement of stiffness or stiffness of large arteries and is recognized as an important predictor of cardiovascular morbidity and mortality in the general population and in the population with high cardiovascular risk.7–9 In elderly patients or in hypertensive patients, PWV is increased due to the greater rigidity of the proximal vessels, whereby the reflected wave travels faster, arriving during the meso-systole, thus increasing the central systolic pressure and the differential pressure.9 Schillaci et al.7 have shown through a multivariate analysis a significant relationship between the rigidity of large arteries and pressure variability in the 24 h. They also showed that the extent of this association depends on the definition of pressure variability: closer correlations were observed between cfPWV and ARV (‘average real variability’), followed by the ‘weighted’ 24-h BP SD. However, the relationship between stiffness and pressure variability would not be a causal relationship, but a vicious circle in which the increase in aortic stiffness would contribute to increasing the variability of arterial pressure in the short term and in turn the increased variability in the 24 h would determine an increase in cfPWV. The increase in aortic stiffness would favour the increased variability of arterial pressure also through reduced sensitivity of baroreceptors, a typical characteristic of altered autonomic modulation in hypertensive subjects.7
Not only the cfPWV but also the indices of estimation of haemodynamics at the central level and of the reflection of the pulse wave, such as central arterial pressure and the augmentation index (AIx) are independent predictors of cardiovascular events and mortality from all causes.10 Omboni et al.10 have documented a statistically significant relationship, even if moderate, not only between the variability indices of 24-h systolic BP and the arterial stiffness indexes but also between pressure variability and pressure wave reflection indices, such as central systolic BP and AIx. Also, in this case,10 as in Schillaci’s study,7 the correlation was closer considering the weighted SD, followed by the ARV.
Also, the alterations of the microcirculation can be associated and an increased pressure variability.
Already in 1992, it was observed that the standard deviation of 24-h arterial pressure evaluated with ABPM correlated with an index of microvascular alteration, the minimum vascular resistance of the forearm.11
It has been hypothesized that there is a reciprocal relationship between microvascular and macrovascular alterations, which can also influence pressure variability.9,12
In essential hypertension a remodelling of the microcirculation is observed, characterized by an increase in the media/vessel lumen.13 These alterations determine an increase in the flow resistance and, therefore, of the arterial pressure values, being able in addition to amplify the effect of each hypertensive stimulus,14 because for the same shortening of the vascular smooth muscle cells the increase of the resistances is much more marked compared to normotensives.15 This effect of ‘vascular amplification’ of the stimuli can, therefore, determine an increase in the variability of the arterial pressure.9,13,15
The alterations of the microcirculation can influence the reflection of the pulsatory wave and, therefore, the central arterial pressure. In fact, the microvascular structure is not only the main site of generation of flow resistance but also, probably, the origin of most wave reflections that generate an increase in central systolic pressure especially in the elderly.9
The media/lumen ratio of small subcutaneous arteries and retinal arterioles is significantly correlated with 24-h systolic and differential pressure and systolic and central differential pressure.16 Therefore, the central differential pressure, indicative of mechanical alterations in large-caliber arteries, is an independent determinant of vascular remodelling in the microcirculation.12,14 This relationship would indicate an association between microvascular and macrovascular structural changes associated with arterial hypertension.12,14 In fact, the increase in the media/lumen ratio and the rarefaction of small arteries are among the main factors involved in inducing an increase in mean arterial pressure, which in turn can directly determine greater rigidity of the great arteries.
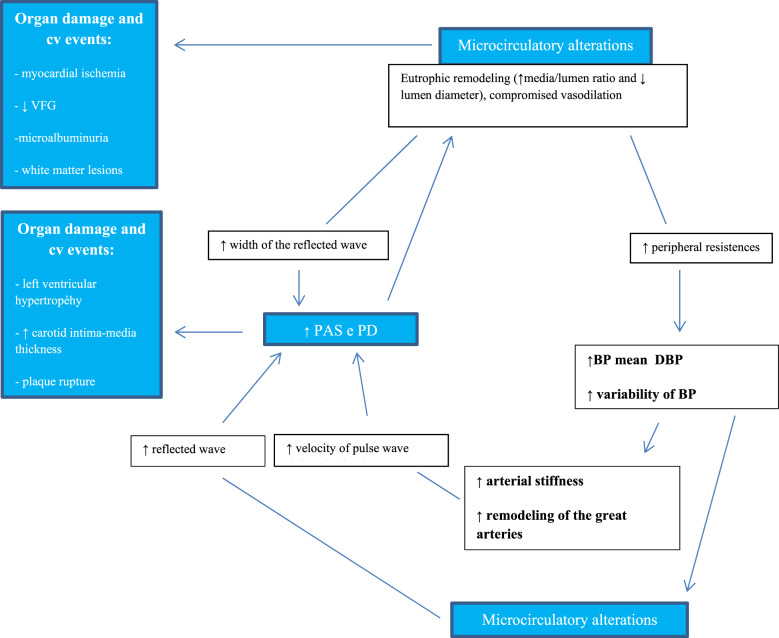
Furthermore, the increased arterial stiffness can lead to an increase in the differential pressure, which in turn can damage the small arteries in the target organs (heart, brain, retina, and kidney) favouring the progression of organ damage.12,14 Therefore, the existence of reciprocal relationships can be postulated, and of a real vicious circle that links the microvascular and macrovascular alterations with the pressure variability9,12,14 (Figure 1).
Figure 1.
Interrelations between microcirculation and macrocirculation; possible effects on blood pressure variability in patients with hypertension / accelerated aging. ↑, increased or increased; ↓, decreased or decreased. PA, blood pressure; PAS, systolic blood pressure; PAD, diastolic blood pressure; PD, differential pressure; VFG, glomerular filtration rate; CV, cardiovascular. Redesigned from references 9 and 12.
It has also been observed that the morphological alterations in the microcirculation could also be involved in the sharp increase in arterial pressure during the first hours of the morning,13 in turn associated with an increase in the incidence of cardiovascular events5 and arterial rigidity.17 Furthermore, the deleterious effect of the sudden rise in BP in the morning could also be mediated by the greater degree of organ damage related to arterial hypertension.17 In the aforementioned study,13 a statistically significant correlation was observed not only between the media/lumen ratio of small subcutaneous resistance arteries and the morning rise in BP but also with the standard deviation of mean arterial pressure measured with l ‘ABPM’, confirming what was previously observed investigating the microcirculation of the forearm.11 However, it is still not clear whether the relationship between microcirculation alterations and morning elevation of BP values is cause-effect type: an increase in the media/lumen ratio of small arteries could cause an accentuated morning peak amplifying hypertensive stimuli, but, at the same time, morning pressure increases could directly damage the wall of small vessels.
Prognostic and clinical significance of blood pressure variability
As previously mentioned, not only the high mean pressure values are considered determinants of cardiovascular adverse events but also the BP variability in the 24 h was identified in various works as a predictor of cardiovascular complications and organ damage marker,2,3 as it reflects sympathetic activation and impairment of baroceptive reflexes3,18 (Table 2).
Table 2.
Different types of blood pressure variability and prognostic relevance
| Types of blood pressure variability | Prognostic relevance |
|---|---|
| Very short-term variability (beat-to-beat) |
|
| Short-term variability (in 24 h) |
|
| Medium-term variability (day-day) |
|
| Long-term variability (visit-visit < 5 years) and very long-term variability (visit-visit > 5 years) |
|
‘Non-dipper’ subjects and subjects with an increased morning pressure rise showed a greater cardiovascular risk.2 However, the prognostic significance of the morning pressure rise is still a matter of debate considering the confounding role of nocturnal pressure reduction: in fact, a lack of pressure reduction during the night could reduce the morning pressure rise, but the two pressure behaviours have an opposite prognostic meaning.2
As mentioned, the increased BP variability in the 24 h was associated, in numerous studies, with organ damage and an increased cardiovascular risk, even independently of mean pressure values2,3; in other studies, however, pressure variability was associated with only a moderate increase in cardiovascular risk, confirming a prevalent role of mean BP.2 Compared with the short-term variability, the visit-visit pressure variability proved to be a good predictor of cardiovascular risk,1,2 of organ damage,2,19 and of all-cause mortality, especially in hypertensive patients in therapy. This figure is not surprising considering that the visit-visit variability may reflect the long-term effectiveness of BP control and treatment adherence.1,2 In the Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm (ASCOT-BPLA) study, increased visit-visit variability and maximum systolic BP were strong predictors of cerebrovascular events independent of mean systolic BP.2
In contrast, in the ELSA study (European Study on Atherosclerosis), visit-visit variability did not contribute to cardiovascular risk prediction in patients with mild/moderate hypertension.2
These conflicting results indicate that the association between visit-visit variability and cardiovascular risk is significantly influenced by the underlying cardiovascular risk level: in low-risk subjects, visit-visit variability does not entail any additional risk with respect to mean arterial BP values, while in high-risk subjects it could be a strong predictor of cardiovascular events and mortality.2 Very recently it has been suggested that the relationship between visit-visit variability and cardiovascular events is very complex and perhaps non-linear, as the risk could increase both for higher and lower BP variability.20
Olesen et al.21 have recently investigated the possible modulatory role of age in the relationship between BP variability, macrovascular alterations and left ventricular hypertrophy. According to these authors, age would play a very modest role.21
A clinically very relevant point is represented by the possibility that the pressure variability is considered or not a therapeutic target: it is still not clear whether it is only a risk factor that accompanies hypertension or whether it is itself an independent risk factor that can be modulated by antihypertensive therapy. Drugs with a long duration of action and a homogeneous effect over 24 h are most likely to reduce BP variations. In clinical practice, the global effect of antihypertensive drugs on BP variability can be assessed by two indices: the trough/peak ratio2 and the smoothness index2,22; both provide information about the duration and distribution of the antihypertensive effect during the dose interval, but the second uses a greater number of information being calculated from the arterial pressure detected with the ABPM in relation to the standard deviation of the change in BP values before and after treatment.
High values of smoothness index seem to be associated with a minor amount of organ damage or a more marked regression of the same during treatment.2
However, the prognostic significance of pressure variability in general still remains very uncertain, especially with regard to the ability to provide additional information with respect to average values.
One of the main limitations is inherent in the lack of standardized and widely shared methods that allow defining and measuring pressure variability and which can be widely applied.2 There is no uniformity of opinions about, for example, the best method to assess the variability of visit-visit or to reduce the role of confounding factors, including adherence or not to therapy.2 Also, the modalities of definition and measurement of the variability in the 24 h are not yet well established: as mentioned the pressure variability increases in proportion to the average values, besides being an important component of our physiological adaptation to the surrounding environment.
Conclusion
In conclusion, further studies are needed to identify standardized methods applicable to the study of pressure variability. This could allow to obtain a better understanding of its actual relationship with cardiovascular risk and, consequently of the potential therapeutic implications, even considering that, at the moment, we do not have drugs with exclusive action on BP variability.2,19 In a recent critical review of the role of BP variability in cardiovascular risk stratification, Asayama et al.23 concluded that none of the currently available pressure variability indices, including the morning pressure rise, could substantially redefine the cardiovascular risk profile of the hypertensive patient, in addition to what is already indicated by BP. Therefore, in the cardiovascular risk stratification, one should concentrate on the absolute levels of arterial pressure, the most relevant and modifiable risk factor both from non-pharmacological measures and, above all, from drug treatment.23 The Guidelines of the European Society of Cardiology and of the European Society of Hypertension regarding the clinical management of arterial hypertension confirm this orientation, as the evaluation of BP variability is considered useful especially in a research field, although it can provide useful complementary information in some circumstances.24
Conflict of interest: none declared.
References
- 1. Parati G, Ochoa JE, Lombardi C, Salvi P, Bilo G.. Assessment and interpretation of blood pressure variability in a clinical setting. Blood Press 2013;22:345–354. [DOI] [PubMed] [Google Scholar]
- 2. Parati G, Ochoa JE, Lombardi C, Bilo G.. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep 2015;17:537.. [DOI] [PubMed] [Google Scholar]
- 3. Parati G, Liu X, Ochoa JE, Bilo G.. Prognostic relevance of blood pressure variability: role of long-term and very long-term blood pressure changes. Hypertension 2013;62:682–684. [DOI] [PubMed] [Google Scholar]
- 4. Chadachan VM, Ye MT, Tay JC, Subramaniam K, Setia S.. Understanding short-term blood-pressure-variability phenotypes: from concept to clinical practice. Int J Gen Med 2018;11:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bilo G, Grillo A, Guida V, Parati G.. Morning blood pressure surge: pathophysiology, clinical relevance and therapeutic aspects. Integr Blood Press Control 2018;11:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stergiou GS, Parati G, Vlachopoulos C, Achimastos A, Andreadis E, Asmar R, Avolio A, Benetos A, Bilo G, Boubouchairopoulou N, Boutouyrie P, Castiglioni P, de la Sierra A, Dolan E, Head G, Imai Y, Kario K, Kollias A, Kotsis V, Manios E, McManus R, Mengden T, Mihailidou A, Myers M, Niiranen T, Ochoa JE, Ohkubo T, Omboni S, Padfield P, Palatini P, Papaioannou T, Protogerou A, Redon J, Verdecchia P, Wang J, Zanchetti A, Mancia G, O’Brien E.. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: current status and future directions. Position statement of the European Society of Hypertension Working Group on blood pressure monitoring and cardiovascular variability. J Hypertens 2016;34:1665–1677. [DOI] [PubMed] [Google Scholar]
- 7. Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G.. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 2012;60:369–377. [DOI] [PubMed] [Google Scholar]
- 8. Bilo G, Giglio A, Styczkiewicz K, et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens 2007;25:2058–2066. [DOI] [PubMed] [Google Scholar]
- 9. Rizzoni D, Agabiti-Rosei C.. Relationships between blood pressure variability and indices of large artery stiffness: does the microvasculature play a role? Hypertens Res 2017;40:348–350. [DOI] [PubMed] [Google Scholar]
- 10. Omboni S, Posokhov IN, Rogoza AN.. Relationships between 24-h blood pressure variability and 24-h central arterial pressure, pulse wave velocity and augmentation index in hypertensive patients. Hypertens Res 2017;40:385–391. [DOI] [PubMed] [Google Scholar]
- 11. Rizzoni D, Muiesan ML, Montani G, Zulli R, Calebich S, Agabiti-Rosei E.. Relationship between initial cardiovascular structural changes and daytime and nighttime blood pressure monitoring. Am J Hypertens 1992;5:180–186. [DOI] [PubMed] [Google Scholar]
- 12. Laurent S, Agabiti-Rosei E. The cross-talk between the macro- and the microcirculation. In Nilsson P, Olsen MH, Laurent S, eds. Early Vascular Aging (EVA): New Directions in Cardiovascular Protection. 2015, pp. 105–118.
- 13. Rizzoni D, Porteri E, Platto C, et al. Morning rise of blood pressure and subcutaneous small resistance artery structure. J Hypertens 2007;25:1698–1703. [DOI] [PubMed] [Google Scholar]
- 14. Rizzoni D, Agabiti-Rosei C, Agabiti-Rosei E.. Hemodynamic consequences of changes in microvascular structure. Am J Hypertens 2017;30:939–946. [DOI] [PubMed] [Google Scholar]
- 15. Folkow B. Physiological aspects of primary hypertension. Physiol Rev 1982;62:347–504. [DOI] [PubMed] [Google Scholar]
- 16. Salvetti M, Agabiti Rosei C, Paini A, Aggiusti C, Cancarini A, Duse S, Semeraro F, Rizzoni D, Agabiti Rosei E, Muiesan ML.. Relationship of wall-to-lumen ratio of retinal arterioles with clinic and 24-hour blood pressure. Hypertens 2014;63:1110–1115. [DOI] [PubMed] [Google Scholar]
- 17. Pucci G, Battista F, Anastasio F, Schillaci G.. Morning pressor surge, blood pressure variability, and arterial stiffness in essential hypertension. J Hypertens 2017;35:272–278. [DOI] [PubMed] [Google Scholar]
- 18. Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell’oro R, Mancia G.. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep 2012;14:333–338. [DOI] [PubMed] [Google Scholar]
- 19. Parati G, Liu X, Ochoa JE.. Clinical relevance of visit-to-visit blood pressure variability: impact on renal outcomes. J Hum Hypertens 2014;28:403–409. [DOI] [PubMed] [Google Scholar]
- 20. Ferreira JP, Duarte K, Pitt B, Dickstein K, McMurray JJV, Zannad F, Rossignol P.. Visit-to-visit blood pressure variation is associated with outcomes in a U-shaped fashion in patients with myocardial infarction complicated with systolic dysfunction and/or heart failure: findings from the EPHESUS and OPTIMAAL trials. J Hypertens 2018;36:1736–1742. [DOI] [PubMed] [Google Scholar]
- 21. Olesen TB, Pareek M, Stidsen JV.. Impact of age on the association between 24-h ambulatory blood pressure measurements and target organ damage. J Hypertens 2018;36:1895–1901. [DOI] [PubMed] [Google Scholar]
- 22. Rizzoni D, Castellano M, Muiesan ML, Porteri E, Agabiti Rosei E.. Beyond trough: peak ratio: a new index of the smoothness of the antihypertensive effect of a drug. High Blood Press 1997;6:110–115. [Google Scholar]
- 23. Asayama K, Schutte R, Li Y, Hansen TW, Staessen JA.. Ambulatory and home blood pressure measurement in the management of hypertension. Blood pressure variability in risk stratification: what does it add? Clin Experim Pharmacol Physiol 2014;41:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Williams B, Mancia G, Spiering W.. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]