Abstract
Myocardial infarction in the absence of obstructive coronary stenosis (MINOCA) is a syndrome with several causes, characterized by clinical evidence of myocardial infarction and coronary angiographically normal or almost normal (stenosis ≤50%). MINOCAs represent about 10% of acute coronary syndromes. The causes of MINOCA are manifold and can be classified on the basis of the mechanism in epicardial (unstable plaque not manifested by angiography, epicardial spasm and coronary dissection) or microvascular. The latter in turn can be divided into intrinsic (microvascular spasm, Takotsubo syndrome and coronary embolization) and extrinsic (myocarditis). In the former, the dysfunctional microcirculation causes myocardial necrosis due to reduction of the lumen due to vasoconstriction and / or obstruction, while in the latter, the compression of the lumen occurs ab extrinsic due to myocardial edema. Note that the prognosis of MINOCA is extremely variable and depends on the underlying cause with high risk clinical subsets. A correct diagnostic procedure includes first level tests (clinical / anamnestic examination, ECG, myocardial necrosis enzyme dosage, trans-thoracic echocardiogram, coronary angiography, ventriculogram) and second level tests (intracoronary imaging, coronary vasomotor test, cardiac nuclear magnetic resonance and trans-esophageal or contrast ultrasound). Through this process, it is possible to identify the cause of MINOCA, fundamental for targeting therapy on the disease mechanism, thus constituting a typical example of precision medicine.
Keywords: Myocardial infarction, MINOCA, Personalized medicine
Introduction
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a condition with different causes, characterized by clinical evidence of myocardial infarction (MI) and angiographically normal or minimally obstructive (≤50% stenosis) coronary arteries. MINOCA represent ∼10% of acute coronary syndromes. The causes of MINOCA are several and can be classified according to the pathologic mechanism in epicardic (unstable plaque not detectable at angiography, epicardial spasm, and coronary dissection) or microvascular. The latter can then be divided into intrinsic causes (microvascular spasm, Takotsubo syndrome, and coronary embolism), and extrinsic (myocarditis). For the former microcirculatory dysfunction determines myocardial necrosis due to vasoconstriction and/or obstruction of the vessels lumen, on the other hand, in myocarditis, the vessel lumen is compressed ab extrinseco by myocardial oedema. Prognosis of MINOCA, interestingly, is very variable and related to the underlying cause, with some high-risk clinical subsets.
An appropriate diagnostic work-up includes first-level tests (accurate history and physical examination, electrocardiogram, blood work for MI, transthoracic echocardiography, coronary angiogram, and ventriculogram), and second-level tests (intravascular coronary imaging, coronary vasomotor testing, cardiac magnetic resonance, and transoesophageal echocardiography). Such diagnostic work-up would allow the definition of the cause for MINOCA, which is instrumental in focusing the treatment on the specific disease mechanism, thus embodying the essence of precision medicine.
Definition and epidemiology of MINOCA
Myocardial infarction in the absence of obstructive coronary stenosis (MINOCA) is a syndrome with various causes, characterized by clinical evidence of myocardial infarction (AMI) (according to the third universal definition of infarction) and angiographically normal or almost normal coronary.1 The cut-off generally accepted in the literature to define a stenosis as non-obstructive is related to lesions that angiography occupy the lumen of the vessel ≤50%.1 However, it is useful to underline that this cut-off value defines the haemodynamic meaning of a stenosis and its impact on the coronary reserve, while it does not provide information on the composition of the atherosclerotic lesion and its stability. Finally, there should be no other clinically evident causes that justify the acute presentation.
On the basis of data obtained from registers related to AMI, a prevalence of MINOCA was extrapolated between 5% and 25%,1 but a contemporary study based on a current cohort of patients suggests a prevalence of 8.8%, which more appropriately reflects estimates of daily clinical activity.2
Pathogenesis of MINOCA and diagnostic procedure
The mechanisms underlying the MINOCA are manifold. A recently proposed classification distinguishes epicardial from microvascular causes (Table 1).1
Table 1.
Diagnostic test and treatment according to aetiology of myocardial infarction with non-obstructive coronary arteries
| Mechanism | Diagnosis | Treatment |
|---|---|---|
| Epicardial causes Vasospasm Eccentric plaque |
Acetylcolin test and intracoronary ergonovine IVUS and OCT |
Calcium antagonist, nitrates, inhibitors of Rho-kinases Statinee, ACE inhibitors/sartans, beta-blockers |
| Microvascular Intrinsic causes Takotsubo syndrome Microvascular Spasm |
Ventriculogram, Cardiac RMN with contrast, echocardiogram with contrast, and adenosine Intracoronary acetylcholine |
Treatment of heart failure Calcium antagonists, inhibitors of Rho-kinases |
| Coronary embolism Extrinsic Parvovirus B19 myocarditis |
Coronary angiogram Identification of embolic cause Cardiac RMN with contrast endomyocardial biopsy |
According to the embolic causeTreatment of heart failure |
IVUS, intravascular echography; OCT, optical coherence tomography; RMN, nuclear magnetic resonance.
Epicardial mechanisms
Unstable plaques and coronary dissection
Rupture or erosion of an atherosclerotic plaque falls under the IA classification of Type I.3 Plaque rupture and erosion can occur in areas where the vessel appears normal at coronary angiography or presents only alterations of its profile. In this case, myocardial necrosis is mediated by occlusive or sub-occlusive thrombosis, which in the event of plaque rupture is consequent to the release of the pro-thrombogenic content of the underlying necrotic core, while in the case of erosion it is consequent to the apoptosis of endothelial cells with subsequent exposure of pro-thrombogenic material and, in particular, hyaluronic acid.4
Thrombosis can be associated with thromboembolism, due to detachment of part of the thrombus, and vasospasm, which in both cases contribute to myocardial necrosis, following microvascular obstruction and protracted ischaemia.
The theory of thrombolysis and autolysis of a coronary thrombosis has been proposed; spontaneous thrombolysis is believed to be an endogenous protective mechanism against the formation of a thrombus, even in the presence of a plaque rupture,5 explaining why the coronary artery appears normal to angiography, despite having caused myocardial necrosis.5
Spontaneous coronary dissection, more frequent in young women, typically causes AMI, producing vascular lumen obstruction, although this may not be recognized by coronary angiography and leads to the diagnosis of MINOCA.6 The fibro-muscular dysplasia of the media and intima appears to be at the basis of the phenomenon and can be recognized in other vascular areas. Hormonal changes, pregnancy, and childbirth may favour its appearance.6
Epicardial coronary spasm
The spasm of an epicardial coronary artery reflects a hyper-reactivity of the coronary smooth muscle to endogenous or exogenous stimuli (such as spasms induced by cocaine or methamphetamine).7 Spasm more frequently produces transient ischaemia, but can sometimes last, leading to persistent ischaemia at the base of the AMI. MINOCA may be the first presentation of vasospastic angina or an interim manifestation of the already known chronic disorder.7
The prevalence of variant angina in patients with MINOCA varies in the literature depending on the definition of coronary spasm, the race of the patient or the stimuli used to ‘trigger’ the spasm. In a recent study, the incidence of epicardial spasm after administration of acetylcholine was 46% in patients with MINOCA.8
Microvascular mechanisms
Microvascular causes can lead to myocardial necrosis with ‘intrinsic’ or ‘extrinsic’ mechanisms. In the first case, the reduction or interruption of the myocardial flow is linked to phenomena concerning the vassal lumen, which is obliterated either by epicardial vasospasm or by microvascular obstruction linked to thromboembolism. In the second case, the impairment of the myocardial flow is linked to phenomena that ab extrinseco obliterate the vassal lumen by compression, as in the case of inflammatory phenomena. In this case, the interstitial oedema is responsible for the compression of the coronary microcirculation with consequent myocardial necrosis linked to protracted ischaemia.
Intrinsic microvascular causes
Microvascular coronary spasm
About 25% of MINOCA cases are caused by microvascular spasm.9 In this case, it is the microcirculation that responds in an exaggerated manner to vasoconstrictor stimuli. The diagnosis can be obtained when the intracoronary administration of acetylcholine reproduces the symptomatology and the ECG-graphical modifications (such as the depression ST or the elevation of at least 0.1 mV or the inversion of T waves in at least two contiguous derivations) similar to that reported by the patient in spontaneous episodes, without evidence of spasm of an epicardic artery (i.e. a reduction of the lumen ≥90%).9
Takotsubo cardiomyopathy
Takotsubo cardiomyopathy (TTS) frequently appears as an acute coronary syndrome with ST-segment changes generally accompanied by the release of markers of myocardial necrosis. The ST-segment elevation is the most frequent electrocardiographic alteration. The clinical presentation may, in some cases, be more severe, with acute heart failure up to shock.1–10
The extent of myocardial dysfunction is variable and in the classical forms it concerns the left ventricular apex; however, it saves the basal segments. The transient nature of myocardial dysfunction suggests reversible disease mechanisms. In particular, transient sympathetic overactivation has been called into question, with massive release of catecholamines.10 Myocardial necrosis in TTS would be the consequence of the toxic effect of catecholamines on the myocardium and of vascular mechanisms, such as the microvascular spasm responsible for myocardial ischaemia. From the first descriptions, coronary vasospasm has been considered as a plausible causal factor.10,11 Note that Angelini et al., in a prospective study, confirmed the development of coronary spasm in patients with TTS who were tested for acetylcholine. Sub-occlusive spasm was associated with echocardiographic evidence of transient left ventricular dysfunction, similar to that observed during TTS.10 In agreement, the role of microvascular dysfunction has been documented with other indices. Some authors have described a reduced thrombolysis in MI Frame Count during an emergency coronary study, with improvement of the coronary reserve at 1-month follow-up, although this is not a constant finding. It is significant to point out that using contrast echocardiogram shows a form of reversible microvascular dysfunction in patients with TTS.10 The clear perfusion defect in the segments of the left ventricle affected by the dysfunction, unlike the patients with ST-elevation myocardial infarction (STEMI), improves transiently following the intracoronary infusion of Adenosine and regresses definitively to 1 month of follow-up, strengthening the pathogenetic role of dysfunction microvascular ischaemia followed by postischaemic stunning.10
Coronary thromboembolism
Coronary embolism starting from the left heart (atrial fibrillation, valvular diseases, endoventricular thrombus, and cardiac tumours) or paradox in the presence of dx-sn shunt (PFO and DIA),11 can cause MINOCA from microcirculation involvement. A hereditary thrombophilic disorder, such as factor V Leiden deficiency, C/S protein deficiency frequently occurs. Thrombophilic screening performed on patients with MINOCA showed a prevalence of ∼14% of these disorders.11
Extrinsic microvascular causes
Myocarditis
It is debated whether to include myocarditis in MINOCA, at least in some cases. We believe that only some forms, with more microvascular involvement than myocardial, can be included. However, as already discussed in myocarditis myocardial oedema can compress the microcirculation ab extrinseco, contributing to the release of cardiac enzymes for reduction of myocardial flow and for direct damage of cardiomyocites mediated by the pathogen.
Especially, Parvovirus B19 can cause a myocardial and microvascular involvement that mimics the MINOCA. Endothelial cells are the specific target of Parvovirus B19, thanks to the Blood group P antigen.12 Chest pain and changes in the ST segment to the ECG, in the absence of coronary obstruction, in patients with viral myocarditis, could be caused by intense coronary microvascular constriction, as a result of coronary vasculitis, and microcirculatory dysfunction. In agreement, Yilmaz et al.12 showed that, after the administration of acetylcholine, patients with myocarditis mimicking MINOCA, they showed coronary spasm at the level of the distal segments of the epicardial vessels, probably extending to the microvascular level.
Myocardial infarction Type II and MINOCA of unclear origin
In case of profound mismatch between oxygen demand and perfusion, a Type 2 AMI may occur, even in the absence of obstructive coronary artery disease.3
On the other hand, it is possible that cardiac magnetic resonance imaging (MRI) is completely normal, despite an increase in troponins. This is probably due to the fact that myocardial oedema, with an ab extrinseco mechanism, can lead to impairment of the myocardial flow, with consequent release of cardiac enzymes, without however causing more serious myocardial damage and subsequent replacement fibrosis. In some cases, the release of markers of myocardial necrosis is not accompanied by evidence of late enhancement to MRI.10
Note that in a cohort of patients who underwent both MRI and intravascular ultrasound (IVUS), 25% had plaque rupture and a normal MRI. Therefore, microembolization phenomena can also explain this discrepancy.13 Coronary spasm, on the other hand, can induce minimal troponin elevation, with nuclear magnetic resonance in the limits.14 These apparently contradictory phenomena can be explained taking into account that the resolution of the MRI, although very high, is however limited. Therefore, foci of patchy myocardial necrosis that can cause troponin increase may be too small to be visible as late enhancement to MRI.10
Diagnostic procedure
Figure 1 suggests a possible diagnostic course in MINOCA.
Figure 1.
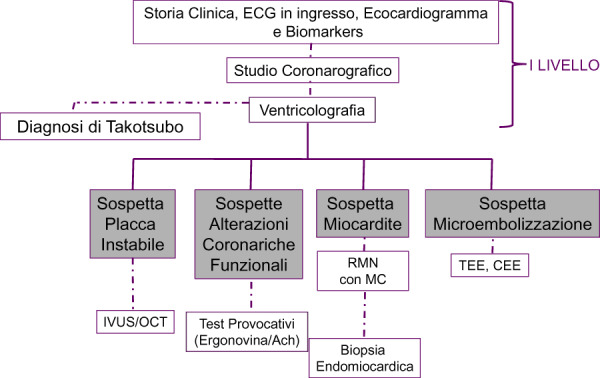
Diagnostic course in MINOCA.
Clinical history, ECG, cardiac enzymes, echocardiography, coronary angiography, and ventriculogram are the first level investigations to identify the causes of MINOCA.1 The clinical presentation, in fact, may point to the suspicion of myocarditis (fever and recent infections), the echocardiogram may lead to suspect embolic causes or Takotsubo’s disease, which will be confirmed by ventriculogram.10 Ventriculogram can also direct towards an ‘epicardial pattern’, in the presence of regional kinetic anomalies, limited to the territory of a single epicardial coronary vessel, or to a ‘microvascular pattern’ in the case in which the functional alteration involves a more extended territory covered by a single vessel.
However, in many cases, second-level investigations are required to arrive at the definition of the MINOCA mechanism. Intravascular ultrasound and more recently optical coherence tomography (OCT) have shown plaque rupture or erosion in 20-30% of MINOCA.4 Such imaging tests may also be useful in suspected coronary dissection.6
Coronary vasomotor tests (intracoronary injection of acetylcholine or ergonovine) have been shown to be safe and able to diagnose epicardial or microvascular spasm even in the acute phase.14
If myocarditis is suspected, cardiac MRI is essential both in the acute phase to confirm the diagnosis and for risk stratification. It may be useful to repeat MRI during follow-up to more accurately assess the progress of ventricular function and response to therapy.1 MRI is the only method able to highlight myocardial oedema which, as already mentioned, can contribute to myocardial ischaemia and consequent necrosis.
The transthoracic, the transoesophageal echocardiogram, and the use of the contrast medium are the cornerstones of the diagnosis of cardiac embolism11 Finally, it should be noted that subclinical MRI evidence of MI was documented in 10.8% of patients with oval foramen.15
MINOCA prognosis
Prognostic data on MINOCA are conflicting. Beltrame’s group shows that all-cause mortality at 12 months is lower in MINOCA compared with that in patients with MI and obstructive coronary artery disease (4.7% vs. 6.7%).11 According to these data, in the wide ACTION register, the MINOCA is associated with a lower in-hospital mortality than infarcts with obstructive coronary artery disease.16 However, a recent retrospective analysis of ACUITY patients showed that, comparing patients with non-STEMI (NSTEMI) and CAD with MINOCA, the latter presented a greater risk of mortality at 1 year (4.7% vs. 3.6%); however, linked to an increase of non-cardiac deaths (2.1% vs. 1.2%), against a higher rate at 1 year of recurrent MI and repeated revascularizations in NSTEMI.2 In the VIRGO study, which focused on youthful presentations (<55 years), MINOCA had a similar 12-month prognosis compared to infarcts with obstructive coronary artery disease and was prevalent in women.17 Finally, a 14% mortality at 4.5 years of follow-up was reported in the Swedish register, with a prevalence of non-cardiovascular deaths.18
This contradiction in the data published in the literature may depend on the populations enrolled in the studies, on the underlying mechanism and on the fact that even in MINOCA, there are high-risk subsets (Table 2).
Table 2.
Prognosis of myocardial infarction with non-obstructive coronary arteries
| Study | Population | Rate of MINOCA | Follow-up | Prognosis | Mortality |
|---|---|---|---|---|---|
| SWEDEHEART18 | 199 163 patients with first MI | 4.6% | 4.5 years | Rate of MACE (all-cause mortality; re-AMI, stroke, heart failure) 24% | Total mortality in MINOCA 14% (58% non-cardiovascular) |
| VIRGO17 | 2690 patients with coronary angiogram between 18 and 55 years for MI female/male ratio: 2:1 | 11.1% | 1 year | Quality of life according to SAQ similar MINOCA vs. MI-CAD | Total mortality in MINOCA 0.6% |
| ACTION16 | 322 523 patients with MI | 5.9% | In-hospital | Rate of MACE (all-cause mortality; re-AMI, heart failure): MINOCA vs. MI-CAD 4.9% vs. 9.9% | Total mortality in MINOCA vs. MI-CAD (1.1% vs. 2.1%) |
| ACUITY2 | 13 800 patients with ACS at moderate-high risk, undergoing coronary angiogram within 72 h | 8.8% | 1 anno | Rate of MACE (death or myocardial infarction) MINOCA vs. MI-CAD 6.8% vs. 14.1% | Total mortality in MINOCA vs. MI-CA (4.7% vs. 3.6%) (non-cardiac 44% of MINOCA vs. 33% of MI-CAD) |
SWEDEHEART: Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy. VIRGO: Results from the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients study. ACTION Registry: Acute Coronary Treatment and Intervention Outcomes Network Registry. ACUITY Trial: Acute Catheterization and Urgent Intervention Triage Strategy Trial.
ACS, acute coronary syndrome; MACE, major cardiac events; MI, myocardial infarction; MI-CAD, myocardial infarction with obstructive coronary disease; SAQ: Seattle angina questionnaire.
MINOCA high-risk subset with epicardial mechanism
In cases of unstable plaque that was not manifest on angiography but documented with IVUS/OCT, recent data suggest that plaque ruptures are associated with a worse prognosis than the presence of an intact fibrous cap.19 It should be noted that the severity of atherosclerosis is a prognostic predictor in MINOCA and that the C Reactive Protein at admission is a marker of worse prognosis.18 The mechanisms underlying IMA have recently been classified by Crea and Libby4 in inflammatory plaque ruptures (with abundant macrophage infiltrate and inflammatory cells), non-inflammatory plaque ruptures (in which a mechanical trigger is at the base of the ruptures), plaque erosions, and hyper-reactive smooth plaques to vasoconstrictor stimuli. Among these, recent data not yet published (Crea et al.), suggest that inflammatory ruptures are at higher risk of recurrent events. Therefore, when at the basis of MINOCA there is a plaque rupture with evidence of local or systemic inflammation, the follow-up must be particularly close due to the high risk of atherothrombotic recurrences.
For the vasospastic forms, the abnormal response to the test with ergonovine or acetylcholine is associated with a worse prognosis, both in relation to major events (such as death from all cause, death of cardiac origin, re-hospitalization for acute coronary syndrome) and in relation to the quality of life. The negative prognostic value related to the positivity of the provocative tests seems largely associated with the induction of epicardial coronary spasm. In agreement, the reduction or suspension of the calcium antagonist dose was also correlated with mortality, supporting the crucial role of epicardial spasm in the onset of fatal events.8
For spontaneous dissections, intra-hospital and long-term survival are excellent, although the risk of recurrence of acute events reported is high (27%/5 years).6
MINOCA high-risk subset with microvascular mechanism
As for microvascular spasm, although the prognosis is good in the short term, there are two aspects to consider. The first concerns the angina symptom that can occur, compromising the quality of life of the patient (up to 36%, despite the use of calcium antagonists).1 The second aspect concerns the long-term prognosis. Microvascular spasm is the expression of a microcirculatory dysfunction that numerous studies with invasive and non-invasive methods (positron emission tomography) have associated with a long-term benign prognosis.20 Microcirculatory dysfunction indeed predicts both hard cardiovascular endpoints (death, infarction), and non-cardiac mortality, as an expression of systemic risk.8
In TTS, the mortality reported during the acute phase of hospitalization is ∼4–5%, a percentage comparable to that of STEMI, at the time of primary angioplasty.10 Significant is the fact that, despite the subsequent normalization of ventricular function and the absence, in most cases, of significant coronary disease, mortality following discharge is greater than in the healthy population of the same age. On a sample of 1750 cases, Templin et al.10 report a 30-day mortality rate of 5.9% and a mortality rate of 5.6% per patient per year.
As for myocarditis, an infarct-like pattern has been associated with a better prognosis than the non-infarct presentation, and the evidence of late gadolinium enhancement as a predictor of adverse prognosis.21
Prognostic data relating to patients with paradoxical embolism and MINOCA are extracted essentially from case reports and are mainly influenced by the underlying causes,15 such as the MINOCA prognosis with Type 2 infarction.
Conflict of interest: none declared.
References
- 1. Niccoli G, Scalone G, Crea F.. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 2015;36:475–481. [DOI] [PubMed] [Google Scholar]
- 2. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, Stone GW.. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy Trial. Circ Cardiovasc Interv 2014;7:285–293. [DOI] [PubMed] [Google Scholar]
- 3. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Eur Heart J 2018;doi:10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 4. Crea F, Libby P.. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 2017;136:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iqbal SN, Feit F, Mancini GBJ, Wood D, Patel R, Pena-Sing I, Attubato M, Yatskar L, Slater JN, Hochman JS, Reynolds HR.. Characteristics of plaque disruption by intravascular ultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am Heart J 2014;167:715–722. [DOI] [PubMed] [Google Scholar]
- 6. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GBJ.. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–655. [DOI] [PubMed] [Google Scholar]
- 7. Kaski JC, Crea F, Meran D, Rodriguez L, Araujo L, Chierchia S, Davies G, Maseri A.. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation 1986;74:1255–1265. [DOI] [PubMed] [Google Scholar]
- 8. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F.. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J 2018;39:91–98. [DOI] [PubMed] [Google Scholar]
- 9. Mohri M, Koyanagi M, Egashira K, Tagawa H, Ichiki T, Shimokawa H, Takeshita A.. Angina pectoris caused by coronary microvascular spasm. Lancet 1998;351:1165–1169. [DOI] [PubMed] [Google Scholar]
- 10. Pelliccia F, Kaski JC, Crea F, Camici PG.. Pathophysiology of takotsubo syndrome. Circulation 2017;135:2426–2441. [DOI] [PubMed] [Google Scholar]
- 11. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF.. Systematic review of patients presenting with suspected myocardial infarction and non-obstructive coronary arteries (MINOCA). Circulation 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 12. Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert E-M, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U.. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 2008;94:1456–1463. [DOI] [PubMed] [Google Scholar]
- 13. Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GBJ, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS.. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 2011;124:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U.. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients with Acute Coronary Syndrome) Study. J Am Coll Cardiol 2008;52:523–527. [DOI] [PubMed] [Google Scholar]
- 15. Wohrle J, Kochs M, Hombach V.. Prevalence of myocardial scar in patients with cryptogenic cerebral ischemic events and patent foramen ovale. JACC Cardiovasc Imaging 2010;3:8339.. [DOI] [PubMed] [Google Scholar]
- 16. Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get with the Guidelines). Circulation Cardiovasc Qual Outcomes 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 17. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D'Onofrio G.. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 2018;7:pii:e009174. doi:10.1161/JAHA.118.009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nordenskjöld AM, Baron T, Eggers KM, Jernberg T, Lindahl B.. Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. Int J Cardiol 2018;261:18–23. [DOI] [PubMed] [Google Scholar]
- 19. Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F.. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377–1384. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, Harrington M, Hainer J, Rimoldi O, Dorbala S, Bhatt DL, Blankstein R, Camici PG, Di Carli MF.. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ammirati, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, Mantovani R, Varrenti M, Pedrotti P, Conca C, Mafrici A, Grosu A, Briguglia D, Guglielmetto S, Perego GB, Colombo S, Caico SI, Giannattasio C, Maestroni A, Carubelli V, Metra M, Lombardi C, Campodonico J, Agostoni P, Peretto G, Scelsi L, Turco A, Di Tano G, Campana C, Belloni A, Morandi F, Mortara A, Cirò A, Senni M, Gavazzi A, Frigerio M, Oliva F, Camici PG. E; Registro Lombardo delle Miocarditi. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: the Multicenter Lombardy Registry. Circulation 2018;138:1088–1099. [DOI] [PubMed] [Google Scholar]


