Abstract
Presently several evidences support an association between acute myocardial infarction and influenza infection. The pathophysiology rationale rests on the release of inflammation cytokines, rupture of atherosclerotic plaque, and triggering of prothrombotic events leading to coronary artery occlusion. Several observational evidences support a potential role of influenza vaccine in cardiovascular prevention. It is estimated that the efficacy of influenza vaccine in preventing myocardial infarction could range between 15% and 45%. Notwithstanding the clear recommendation of numerous guidelines concerning patients with cardiovascular diseases, vaccination rates are still low in the high-risk groups. Influenza vaccine as preventive measure of cardiovascular disease still awaits support from randomized clinical trials. Nonetheless, considering the favourable cost-efficacy and safety profile of influenza vaccination, its use should be encouraged in everyday clinical practice.
Keywords: Myocardial infarction, Influenza infection, Plaque rupture, Influenza vaccine, Acute coronary syndrome
Introduction
Although several studies have analysed the association between various types of infection and acute cardio and cerebrovascular events, convincing evidence of a stronger association has emerged for the flu syndrome. The epidemiological relationship between acute myocardial infarction (AMI) and flu syndrome was first observed in 1930 with an increased mortality due to cardiovascular causes in conjunction with the epidemic influenza peak.1 To date, during influenza epidemics, there is an increased rate of hospitalization and death from cardio- and cerebrovascular diseases, and especially for AMI. In this review, we will, therefore, examine the possible causal link between influenza infection and AMI. In general, the search for a causal link is a stimulating but often problematic process. The nine guiding principles that must be respected to provide the foundation for a valid argumentation of this process are the evidence of strength of association, a temporal relation, consistency, coherence, analogy, and biological plausibility; biological gradient, experimental evidence, and specificity.2 On the basis of these principles, we can now hypothesize the existence of a true causal relationship between flu syndrome and acute coronary syndrome (ACS). Arguing this causality is, moreover, not an intellectual exercise for its own sake, since if demonstrated, it would entail important repercussions in the clinical setting as a means to provide further cardiovascular prevention through the systematic use of vaccination.
Epidemiological evidence
Several studies have shown the existence of an association between respiratory infections and the development of AMI. However, in many of them, the clinical diagnosis of infectious disease was neither sensitive nor specific for the confirmation of influenza virus infection. In this regard, Smeeth et al.,3 Who had included 20 486 subjects with a first myocardial infarction and 19 063 subjects with a first acute cerebrovascular event had shown that the risk for both events was higher within the first 3 days from an acute respiratory infection; the incidence ratio for myocardial infarction was 4.95 [95% confidence interval (CI): 4.43–5.53] and the stroke rate of 3.19 (95% CI: 2.81–3.62). More recently, Warren-Gash et al.,4 in a case–control study, analysed 11 208 subjects hospitalized for ACS, of which 3927 with recent acute respiratory infection. They showed a significantly higher risk of AMI during the first 3 days of acute respiratory infection, with an incidence ratio of 4.19 (95% CI: 3.18–5.53) and a progressive reduction in risk over time. Among other things, it emerged that infections occurring during the influenza epidemic and those coded as ‘likely to be influenza’ were associated with a consistent higher risk of incidence of AMI.
Several studies have, therefore, tried to demonstrate the association between influenza and SCA through laboratory confirmation of influenza infection; the results, however, did not always seem convincing. However, these are evidences deriving from case–control studies which, as such, are limited by defects (bias) of selection, confounding factors, and reduced sample size. In 1980, Pönkä et al.5 conducted a case–control study including a total of 49 patients with myocardial infarction (MI) subjected to the determination of the antibody titter for 22 viruses including that for Influenza A. From the results of the study, there were no significant differences in the antibody titter between patients with AMI and controls. Therefore, this limited series of cases did not confirm the hypothesis that a viral infection, including that with Influenza A virus, was associated with AMI. Macintyre et al.6 then designed a case–control study in which the cases were represented by subjects with AMI, while the controls were ambulatory subjects without AMI. The primary outcome measure was laboratory evidence of influenza infection. Of 559 participants, 34/275 cases and 19/284 controls were positive for laboratory virus determination for influenza virus [odds ratio (OR) 1.97; 95% CI: 1.09–3.54]; for statistical analysis; however, the flu infection was not a significant predictor for AMI. A case–control study conducted in China in 2012 showed instead that patients with AMI had, compared to controls, an OR for the determination of antibodies for Influenza A and B, respectively of 5.5 (95% CI: 1.3–23.0) and 20.3 (95% CI: 5.6–40.8). Although this study demonstrates the existence of a strong association between influenza infection and AMI, it is limited by the fact that the results derived from serological tests are less robust than those derived from the influenza virus laboratory research.7 In this regard, a very recent study, published in the New England Journal of Medicine in January 2018, evaluated the association between laboratory-confirmed influenza infection with highly specific methods and hospitalization for IMA. In it, Kwong et al. identified 364 hospitalizations for AMI from a year before to a year after the laboratory test positive result for influenza. Winds of these hospitalizations occurred during the defined ‘risk’ interval, that is in the first 7 days from the date of detection of the flu; the remaining 344 fell in the period defined as the ‘control interval’, that is, from the year prior to the following year the risk interval. The researchers pointed out that the incidence of admission due to AMI was six times higher in the first 7 days after laboratory confirmation of the influenza infection compared to the control interval, and no increase in incidence was observed after the seventh day (the admission incidence ratio for IMA during the risk interval was 6.05; 95% CI: 3.86–9.5). Furthermore, a subgroup analysis showed that the incidence ratio was higher for more adult subjects, for Type B influenza and for patients affected by a first myocardial infarction; however, these subgroup analyses did not have sufficient statistical power to demonstrate the existence of significant differences.
The incidence of AMI was still high even after infection with non-influenza respiratory virus, although minor compared to the secondary forms of influenza infection. Specifically, an incidence ratio for AMI emerged for Type B influenza (95% CI: 4.37–23.38), 5.17 for Type A influenza (95% CI: 3.02–8.84), 3.51 for respiratory syncytial virus (95% CI: 1.11–11.12), and 2.77 for other viruses (95% CI: 1.23–6.24).8 According to these most recent results, therefore acute respiratory infections, and in particular—but not only—the flu, would seem to act as triggers for AMI.
Pathophysiological rational assumptions
The crucial role of inflammation in the development of acute coronary syndrome (ACS) is now well known; in this sense, it would not be surprising how an acute infection, with all its inflammatory potential, can contribute to the development of myocardial infarction.9 The physiopathological characteristics of AMI are heterogeneous; while Type 1 myocardial infarction is secondary to thrombosis due to rupture and/or plaque ulceration, Type 2 myocardial infarction is defined as a myocardial necrosis secondary to a discrepancy between oxygen supply and demand.10 Influenza can be clearly related to Type 2 myocardial infarction due to tachycardia, fever, hypoxia, and changes in vessel tone; and in this case, it is the septic state, rather than coronary thrombosis, the underlying cause of the flu-related myocardial necrosis. However, it has been hypothesized that the influenza through multiple mechanisms can induce or facilitate occlusive phenomena on pre-existing subcritical atherosclerotic plaques. Following the release of inflammatory cytokines, the flu syndrome can favour the triggering of a prothrombotic state, facilitating platelet activation and endothelial dysfunction.11,12 Simultaneously, the sympathetic activation and the increased concentration of endogenous catecholamines determine a hyperdynamic cardiovascular response and changes in systemic and coronary vasal tone, with consequent vasoconstriction. Coronary vasoconstriction causes narrowing of the vasal lumen and, due to increased frictional stress (shear stress), further platelet activation.13 Added to this are changes in the volume status, such as hypovolaemia or hypervolaemia. All these factors at the same time are responsible for an increased biomechanical stress on pre-existing atherosclerotic coronary plaques which facilitate their rupture.14
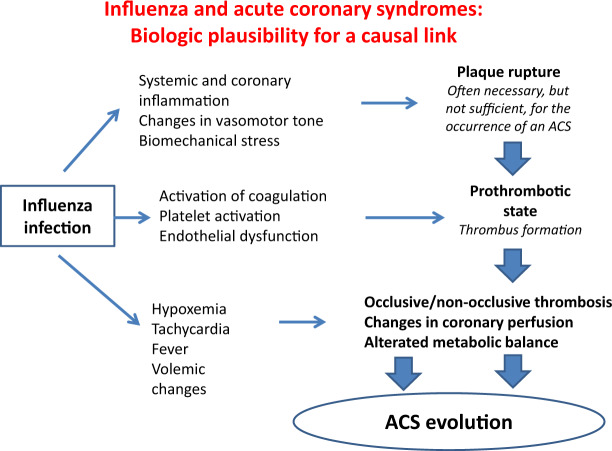
Influenza infection, in addition to inducing a systemic inflammatory response, would appear to have a direct inflammatory effect on atherosclerotic plaque and coronary arteries, as evidenced experimentally in murine models. Specifically, it was observed that in apo-lipoprotein E-knockout mice, an animal model used for the study of atherosclerosis, infection with influenza virus induces acute inflammation, proliferation of smooth muscle cells, and fibrin deposition in pre-existing atherosclerotic plaques, showing an ‘histopathological evolution similar to that of culprit lesions of an ACS15 (Figure 1).
Figure 1.
The multiple pathways that can lead from a flu syndrome to an acute coronary syndrome. It has been hypothesized that the flu infection leads to acute myocardial infarction through the development of thrombosis on pre-existing atherosclerotic plaques. The increased systemic and coronary inflammatory state and the increased biomechanical stress facilitate the rupture of pre-existing atherosclerotic plaques, with exposure of the underlying thrombogenic material. Add to this, the increased platelet activation, phenomena of endothelial dysfunction and the imbalance of the blood coagulation system in favour of procoagulant factors with consequent formation of occlusive or subocclusive thrombus. Furthermore, the acute infection causes tachycardia, hypoxia, fever, and changes in the volume status. This contributes to the evolution of acute coronary syndrome as it increases metabolic demand and accentuates the discrepancy between oxygen demand and supply. ACS, acute coronary syndrome.
Influenza vaccination for secondary prevention of coronary heart disease
‘Indirect’ evidence of a possible association between influenza and AMI derives from studies showing that influenza vaccination is effective in the prevention of ischaemic heart disease. This, in addition to strengthening the causality between the two factors, would highlight the important clinical repercussion from the association. The results of some observational studies show that the protective efficacy of influenza vaccination from new coronary events in secondary prevention is between 19% and 45%.6 This is a range of effectiveness substantially similar to that obtained with other cardiovascular prevention measures widely accepted in clinical practice, including the cessation of smoking (range of efficacy estimated at 32–43%), anti-hypertensive therapy (range efficacy from 17% to 25%) and the use of statins (efficacy range from 19% to 30%).16 In a meta-analysis of case–control studies published in 2015, Barnes et al.17 estimated the association between influenza vaccination and occurrence of AMI. A total of eight studies were identified in which the cases were subjects with first MI or recurrent MI. The results showed a protective efficacy of influenza vaccination against AMI equal to 29% (95% CI: 9–44%). However, only two small randomized clinical trials have evaluated the protection provided by the influenza vaccine against cardiac events in subjects with pre-existing cardiovascular disease, the FLU vaccination in ACSs and planned percutaneous coronary interventions (FLUVACS)18 and the influence vaccination study in secondary prevention from coronary ischaemic events in coronary artery disease (FLUCAD).19
The FLUVACS, published in 2003, was a randomized, prospective, parallel-group, placebo-controlled trial conducted in hospitalized patients whose goal was to test the potential beneficial effect of influenza vaccination in secondary prevention. Two hundred patients hospitalized for myocardial infarction were enrolled within 72 h of the event and 101 patients to be subjected to elective coronary angioplasty. Subjects were randomized to receive influenza or placebo vaccination. In the intention to treat analysis, the primary outcome measure, i.e. the incidence of cardiovascular death at 1 year, was significantly lower among patients receiving vaccination than in the control group (6% and 17%, respectively; 95% CI: 0.17–0.71; P = 0.002).18 However, the results of the subsequent FLUCAD trial, a double-blind placebo-controlled trial in which 658 patients were already in optimal medical therapy for cardiovascular disease were contrasting. In it, 325 subjects received influenza vaccine and 333 received placebo. The average follow-up was 298 days. With regard to the primary outcome measure of cardiovascular death, the results showed no statistically significant differences between the two treatment groups (the cumulative incidence of events was 0.63% in the vaccine group and 0.76% in the control group; 95% CI: 0.15–7.56, P = 0.95). However, the authors showed a tendency to the lower incidence of the composite outcome measure of cardiovascular death, myocardial infarction, and coronary revascularization (major adverse cardiac event, MACE) although not statistically significant. However, influenza vaccination statistically significantly reduced the composite outcome measure of MACE occurrence or hospitalizations due to ischaemia compared to the placebo group.19 In a meta-analysis of these randomized trials, Warren-Gash et al.4,17 showed that influenza vaccination was protective against IMA, although a statistical analysis of grouping on a random-effects model did not show statistically significant differences (relative risk 0.85, 95% CI: 0.44–1.64).
Despite the plausibility of a beneficial effect of influenza vaccination in the prevention of cardiovascular disease, few studies have been conducted to explain the possible molecular mechanism of this phenomenon. It is likely that the flu vaccination may have its protective effect by preventing the activation of the previously described inflammatory pathways. On the other hand, in a study published in 2014, Veljkovic et al.,20 using a virtual spectroscopy method for the analysis of protein–protein interactions, showed a cross-reactivity between the antibody induced by the influenza vaccination and the human bradykinin receptor (bradykinin B2 receptor, BKB2R). The results of the study show that the interaction of the antibody with the BKB2R stimulates the production of nitroxide (NO) and induces cardio-protection by increase in myocardial perfusion both through NO-mediated vasodilation and through phenomena of neo-angiogenesis.
Arguing causality
Based on the available evidence, the causal link cannot be defined as certain but at least highly probable. There is a strength of association, as some data, although observational, show that the flu syndrome is associated with an increased risk of ACS; this increase is transient and higher in the first days after infection; therefore, a temporal relationship is highlighted. There is a principle of consistency since many studies, although different because they are conducted on different populations using different designs and different methods of statistical analysis, are globally consistent in results. A principle of coherence is respected: the possibility that the influenza triggers an ACS agrees with the current scientific data according to which both conditions have the same seasonality (winter peak), occur in people with similar characteristics (subjects at higher risk), and both show a similar pathophysiology in the immune, coagulation, and vascular systems. There is a principle of analogy, according to which acute infections can trigger other vascular events, such as stroke similar to ACS and, analogously, ACS can be triggered by other situations or stressful events. There is a strong biological plausibility. However, although an ideal causal link should consist of a univocal relationship between a specific factor and a given outcome (principle of specificity), this criterion is rarely met also in other already widely accepted causal relationships, such as tobacco consumption and development of lung tumour. Therefore, in light of the other characteristics of association, its absence by itself would not be sufficient to disqualify our argument.9 Furthermore, although there are more generic evidences that show the existence of a biological gradient between type and severity of an infection and ACS risk, specific data to report to the flu syndrome are lacking. For example, the risk of myocardial infarction would be greater after lower respiratory tract infections rather than after urinary tract infection. Furthermore, in pneumococcal infections, the more severe the infection would be, the higher the risk of ACS would result.3 Finally, there is a lack of solid experimental evidence, since only two randomized clinical trials have been conducted, the FLUVACS and the FLUCAD, with conflicting results.
Conclusions
The large number of published papers suggests that various viral and bacterial infections, acute or chronic, may be associated with an increased risk of AMI. Why does the influenza remain a potential focus of particular importance? On the one hand, influenza is one of the most frequent respiratory infections, on the other, it is the only respiratory virus for which it is possible to achieve effective prophylaxis with vaccination.4 Based on this analysis, we can, therefore, deduce that it is highly probable that influenza has a causal role in triggering ACS. However, in the age of evidence-based medicine fundamental elements for its effective demonstration are lacking, i.e. strong data derived from randomized prospective clinical trials.20 However, these syndromes are multifactorial, and no factor can be considered an absolutely necessary and sufficient causal element. Therefore, acute infection must be seen as a component of a set of causes in which complex interactions with other factors determine the development of a coronary event. Although there is evidence to support the role of influenza vaccination for the prevention of ischaemic heart disease, vaccination is not considered a priority preventive measure in the clinical setting. In fact, despite being recommended by many guidelines in different categories of patients at risk, vaccination rates remain low. In this context, while we await strong results, we are still encouraged to use vaccination as a preventive measure against ischaemic heart disease, considering its favourable cost-effectiveness and safety profile. In the clinical setting, this requires awareness so that there is a change in the paradigm that often sees influenza vaccination as a simple preventive measure against the infectious situation only, rather than an additional cardiovascular preventive strategy.
Conflict of interest: none declared.
References
- 1. Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998-2009. J Infect Dis 2012;206:1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 3. Smeeth L, Thomas SL, Hall AJ, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004;351:2611–2618. [DOI] [PubMed] [Google Scholar]
- 4. Warren-Gash C, Smeeth L, Hayward AC.. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 2009;9:601–610. [DOI] [PubMed] [Google Scholar]
- 5. Pönkä A, Jalanko H, Pönkä T, Stenvik M.. Viral and mycoplasmal antibodies in patients with myocardial infarction. Ann Clin Res 1981;13:429–432. [PubMed] [Google Scholar]
- 6. Macintyre CR, Heywood AE, Kovoor P, et al. Ischaemic heart disease, influenza and influenza vaccination: a prospective case control study. Heart 2013;99:1843–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan X, Yang W, Sun X, et al. Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm Res 2012;61:591–598. [DOI] [PubMed] [Google Scholar]
- 8. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018;378:345–353. [DOI] [PubMed] [Google Scholar]
- 9. Corrales-Medina VF, Madjid M, Musher DM.. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010;10:83–92. [DOI] [PubMed] [Google Scholar]
- 10. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2018. [DOI] [PubMed] [Google Scholar]
- 11. Vallance P, Collier J, Bhagat K.. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet 1997;349:1391–1392. [DOI] [PubMed] [Google Scholar]
- 12. Levi M, Keller TT, van Gorp E, et al. Infection and inflammation and the coagulation system. 2003;60:26–39. [DOI] [PubMed] [Google Scholar]
- 13. Ardlie NG, McGuiness JA, Garrett JJ.. Effect on human platelets of catecholamines at levels achieved in the circulation. Cardiovasc Res 1985;58:251–259. [DOI] [PubMed] [Google Scholar]
- 14. Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 15. Bermudez-Fajardo A, Oviedo-Orta E.. Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis 2011;217:97–105. [DOI] [PubMed] [Google Scholar]
- 16. MacIntyre CR, Mahimbo A, Moa AM, et al. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart 2016;102:1953–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes M, Heywood AE, Mahimbo A, et al. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart 2015;101:1738–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurfinkel EP, Leon de, la FR, Mendiz O, et al. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J 2004;25:25–31. [DOI] [PubMed] [Google Scholar]
- 19. Ciszewski A, Bilinska ZT, Brydak LB, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J 2008;29:1350–1358. [DOI] [PubMed] [Google Scholar]
- 20. Veljkovic V, Glisic S, Veljkovic N, et al. Influenza vaccine as prevention for cardiovascular diseases: possible molecular mechanism. Vaccine 2014;32:6569–6575. [DOI] [PubMed] [Google Scholar]