Abstract
Brugada syndrome (BrS) has been often described as a purely electrical disease. However, current dogma surrounding this concept has shifted to accept that BrS is associated with structural abnormalities. Brugada syndrome is now associated with epicardial surface and interstitial fibrosis, reduced gap junction expression, increased collagen, and reduced contractility. The ventricular arrhythmias observed in BrS have been linked to an arrhythmogenic substrate (AS) located rather consistently in the right ventricular outflow tract, sparking much debate as to the significance of this anatomical position. The size of the AS is dynamic and can be altered due to a number of factors. A larger AS is associated with reduced contractility, and this impaired mechanical function may be responsible for syncopal episodes in BrS patients in the absence of arrhythmic events. While BrS is generally regarded as a channelopathy, recent studies have now identified also mutations in genes encoding for sarcomeric proteins to be associated with BrS. Future studies should evaluate electromechanical coupling in BrS, including calcium handling and sarcomeric alterations, and evaluate whether BrS should be classified as a cardiomyopathy.
Keywords: Brugada syndrome, Sudden cardiac death, Channelopathy, Cardiomyopathy, Contractility, SCN5A
Introduction
Brugada syndrome (BrS) is characterized by a specific electrocardiographic pattern, classically formed by a right bundle branch block and persistent ST-segment elevation in the right precordial leads. Since its description in 1992, this condition has been associated with ventricular fibrillation and sudden cardiac death (SCD), but considered to be without concomitant structural heart anomalies. More recently, many studies have been published supporting the hypothesis of a real structural heart impairment in Brugada patients.1 Consistent with this hypothesis, interstitial fibrosis and reduced gap junction expression are both emerging features of some BrS patients.1 This suggests that structural abnormalities in BrS patients result from the same cause as the electrical abnormalities, rather than these electrical and structural clinical manifestations resulting from two separate pathologies that ‘overlap’. In our experience, BrS patients show an arrhythmogenic substrate (AS), which is responsible for the electrocardiogram (ECG) abnormalities2 and is frequently found in the right ventricular outflow tract (RVOT). We use a specific software to establish the location, borders, and extension of the AS in all patients who undergo catheter ablation.2 Radiofrequency catheter ablation of the AS has recently been described as a possible treatment aimed to normalize the ECG pattern and prevent ventricular arrhythmia (VA) recurrences.2 In spite of this evidence, BrS is still regarded as a channelopathy by the vast majority of clinicians.
About 25% of molecularly confirmed cases are caused by a heterozygous mutation in the SCN5A gene. This gene encodes the voltage-gated sodium channel subunit (Nav1.5) that plays a pivotal role for heart’s action potential generation. It is still difficult to discern whether BrS might be a single gene disease or an oligo genic trait that shares some genetic causes with other cardiac abnormalities.1 Along these lines, we have previously reported that some genes mutated in BrS are also mutated in cardiomyopathies.3 It is remarkable that none of these patients harbour heterozygous mutations in the 24 genes that are surely associated with BrS (ABCC9, AKAP9, CACNA1C, CACNA2D1, CACNB2, DSG2, GPD1L, HCN4, KCND2, KCND3, KCNE3, KCNE5, KCNH2, KCNJ8, MOG1, PKP2, RANGRF, SCN5A, SCN10A, SCN1B, SCN2B, SCN3B, SEMA3A, TRPM4). Therefore, in order to fully understand the genetics of BrS, additional genes must be screened, and it could be advisable to begin by expanding the search to genes known to be related to muscular diseases.
Electromechanical coupling in Brugada syndrome
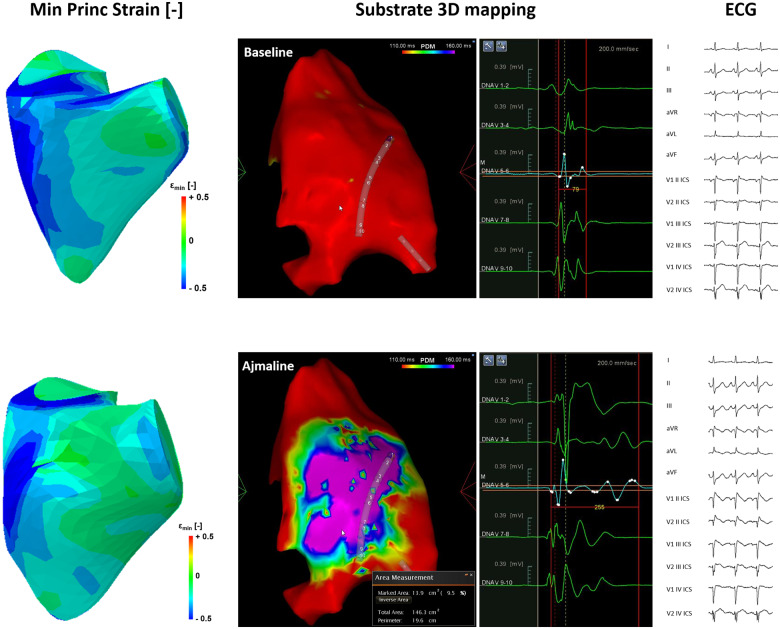
Brugada patients can experience syncopal episodes. It is still thought that the pathogenesis of these episodes is strictly dependent on arrhythmias.4 However, several recent studies have demonstrated reduced contractility in BrS, suggesting that some the syncopal episodes experienced by BrS patients result more likely from reductions in cardiac output due to mechanical dysfunction, rather than from VAs. Ejection fraction (EF) was reduced in several studies, including a reduction in right ventricular ejection fraction,5–7 and even a trend towards a reduced left ventricular ejection fraction.6 In our experience, increases in the size of the dynamic AS during Ajmaline challenge are associated with reduced right ventricular (RV) stroke volume and EF. Furthermore, the minimum principal strain, as measured by 3D transthoracic echocardiography, decreased in the RVOT and RV anterior wall, without significant differences in the inferior RV wall or septum (Figure 1). Catalano et al.5 reported the RVOT diameter, area, and volume were similar between control and BrS patients, but the EF was nevertheless decreased in BrS patients due to an increase in the end-systolic volume. Yet another study, on the other hand, reported a lower end-systolic volume in patients with a spontaneous Type 1 BrS ECG.7 However, patients with the SCN5A sodium channel mutation have been reported to have significantly larger end-diastolic volume and end-systolic volume in the right ventricle and left ventricle, compared to BrS mutated in different genes or volunteers.8 This can be an important clue for the mutual influence between NaV1.5 and muscular proteins.9
Figure 1.
Minimum principal strain (Left), as measured by 3D transthoracic echocardiography, decreased in the right ventricular outflow tract and right ventricular anterior wall, without significant differences in the inferior right ventricular wall or septum, after Ajmaline administration. (Centre) Ajmaline revealed the arrhythmogenic substrate in a patient with a MYBPC3 mutation. Potential duration maps are shown at baseline and after Ajmaline administration, demonstrating the possibility to visualize the arrhythmogenic substrate with Ajmaline in a patient with an MYBPC3 mutation. This patient is negative for mutations normally associated with Brugada syndrome, as described in the full text. (Right) The Type 1 electrocardiogram pattern is visualized after Ajmaline administration.
Moreover, many patients with muscular phenotypes exhibit a high incidence of arrhythmias and syncope. Approximately 1.7% of dilated cardiomyopathy patients harbour a heterozygous pathogenic mutation in the SCN5A gene.10 Brugada syndrome patients negative for variants in the genes normally associated with BrS may exhibit mutations in some genes that today are known as molecular causes of cardiomyopathies. A specific mutation in the sarcomeric gene MYL4 has been purposed as a molecular cause for familial atrial fibrillation.11 The sodium channel scn5Lab is important for the proliferation of embryonic cardiomyocytes in zebrafish.12 A patient with a skeletal muscle sodium channelopathy due to a heterozygous mutation in the SCN4A gene was flecainide-inducible for BrS.13
Finally, a report detailing the shortening of RV ejection time as the Type 1 BrS ECG pattern develops during flecainide challenge attributed this observation to a reduction in the influx of calcium.14 In terms of pathophysiology, contractility can be altered in two main ways. The first is through increases in cytosolic calcium, which results in more calcium available to bind to the myofilaments. This can be achieved by increasing the amount of calcium that enters the cell through, for example, L-type calcium channels or the sodium-calcium exchanger or increasing the release from the sarcoplasmic reticulum. These processes are coupled, since increases in the amount of extracellular calcium entering the cell creates more calcium available to activate ryanodine receptors on the sarcoplasmic reticulum.
The second method by which contractility can be altered is through changes in myofilament calcium sensitivity. Variants in the genes of sarcomeric proteins or post-translational modifications can alter the affinity of troponin C for calcium or alter the rate at which myosin binds to and detaches from actin. Alterations in the affinity for calcium can affect when the calcium is released from the myofilaments, and consequently when it is available to be extruded from the cell or resequestered into the sarcoplasmic reticulum. Thus, alterations in myofilament calcium sensitivity can affect the time required for the cell to ‘reset’ the calcium ions, altering the length of diastole and even resulting in arrhythmias. The effect of myofilament calcium sensitivity on arrhythmia susceptibility has been previously extensively reviewed elsewhere.1 These reports, taken together, detail a clear importance of the electromechanical coupling in BrS and the need to better understand the role of calcium signalling and myofilament involvement.
In addition, some patients affected by BrS show a wide spectrum of mutations in genes coding for calcium channels, such as CACNA1C and CACNB2, as already published recently by our research group.1
The relevance of the arrhythmogenic substrate in Brugada syndrome
A Type 1 BrS pattern is the only ECG marker (spontaneous or provoked) confirming the diagnosis of BrS. In patients suspected of BrS, in which a spontaneous Type 1 pattern is not present, a sodium channel blocking agent, such as Ajmaline, can be used to provoke the Type 1 pattern to diagnose BrS according to International Criteria. Patients with a spontaneously occurring Type 1 pattern are considered at high risk of SCD. However, the presence of a spontaneously occurring Type 2 or Type 3 BrS pattern, or even a normal ECG pattern, does not imply that a patient is not at risk of SCD. In fact, the AS is quite dynamic in BrS, and when the area of the AS is smaller, a Type 1 BrS pattern may not be observed. However, when the patient experiences a triggering event, the AS will grow in size and the Type 1 pattern will be visible.
Several factors contribute to the size of the AS. This can be understood better by studying the many factors that are known to place BrS patients at higher risk of having an arrhythmic event. These factors include fever or an increase in vagal tone, which can occur because of resting, sleeping, digesting a large meal, or vomiting,1 whereas isoproterenol or general anaesthetic can reverse the Type 1 pattern.
The dynamic nature of the AS means that patients can potentially go for years without events and without suspecting a cardiac problem. In fact, sometimes a cardiac arrest in an otherwise seemingly healthy adult may be the first clinical manifestation because there had been no prior event that increased the size of the AS to give prior warning. For example, patients who have not suffered from many high fevers, or who have not been hospitalized and received cardiac monitoring during those fevers, may live many years as a seemingly healthy individual, then seemingly suddenly have an arrhythmic event during a high fever.
The normal contractility of the human heart is influenced by a number of factors, including age, sex, body mass index, drugs, and concomitant pathologies. In our experience, BrS patients exhibit reduced RV stroke volume and EF, and it is likely that BrS patients can be affected by a reduction of contractility, regardless of the differences attributable to age, sex, body mass index, drugs, and concomitant pathologies. This reduction in contractility, including in the absence of arrhythmias, could be the underlying cause of lipothymic, syncopal, and dizziness episodes in this subset of patients.
The genetics of Brugada syndrome—could it be a cardiomyopathy?
Several studies have now identified mutations in genes encoding for sarcomeric proteins to be associated with BrS, including TPM1, TTN, and MYH7.1 We have previously reported a variant in the MYBPC3 gene in two siblings, a brother and sister, with BrS.3 These siblings tested negative for variants in genes in the usual BrS panel, including ABCC9, AKAP9, CACNA1C, CACNA2D1, CACNB2, DSG2, GPD1L, HCN4, KCND2, KCND3, KCNE3, KCNE5, KCNH2, KCNJ8, MOG1, PKP2, RANGRF, SCN5A, SCN10A, SCN1B, SCN2B, SCN3B, SEMA3A, and TRPM4. Their father also carries the same variant and has been diagnosed with hypertrophic cardiomyopathy. The siblings both also tested negative for variants in an additional eight genes, namely MYH7, TNNT2, TNNI3, TPM1, MYL2, ACTC, MYL3, and TNN, which are known to be associated with hypertrophic cardiomyopathy. In the figure, the ECG and potential duration maps can be seen for the female sibling, demonstrating that, after Ajmaline administration, the Type 1 BrS ECG pattern and AS become visible. Alterations in sarcomeric proteins can result in mechanical dysfunction, as well as altered calcium signalling. Future studies should investigate how alterations in these genes could ultimately alter the ECG.
The most commonly mutated gene found in BrS patients is SCN5A. These mutations result in a loss-of-function of the sodium channel, NaV1.5. Phenotypes such as DCM, ARVC, LVNC, early repolarization syndrome, atrial standstill Type 1, atrial fibrillation, long QT syndrome, sick sinus syndrome Type 2, idiopathic ventricular fibrillation, and heart block Type 1A have all also been associated with SCN5A mutations.15 We have previously reported a family with an SCN5A mutation in which the father was diagnosed with LVNC and the daughter with BrS,3 indicating that SCN5A may be important in cases of overlap between BrS and LVNC. Studies have suggested that SCN5A mutations may result in cardiac remodelling, particularly in ARVC, LVNC, and BrS.15 Homozygous knockout of SCN5A (SCN5A-/SCN5A-) in mice is embryonically lethal due to heart defects.15 Therefore, SCN5A mutations are associated with both channelopathies and cardiomyopathies, and perhaps result in cardiac remodelling secondary to altered excitation-contraction coupling.
Conclusion
In conclusion, BrS is not only associated with RV epicardial electrical abnormalities, but also with reduced contractility, which may lead to syncopal episodes even in the absence of clear arrhythmic events. Considering all the data, regardless of the genetic cause, reductions in contractility in BrS and cardiomyopathy patients appear to be the common denominator that may contribute to syncopal episodes in both these groups of patients. This mechanical dysfunction is associated with an AS, the area of which is dynamic in nature. When the area of the AS is large, it becomes visible on the ECG as a Type 1 BrS pattern. The genetics of this are not well understood, but appear to be somehow linked to pathways that ultimately merge into the excitation-contraction coupling, thus affecting not only the electrophysiology, but also the mechanical function of the heart. Future studies should evaluate electromechanical coupling in BrS, including calcium handling and sarcomeric alterations, and evaluate whether BrS should be classified as a cardiomyopathy.
Conflict of interest: none declared.
References
- 1. Monasky MM, Pappone C, Piccoli M, Ghiroldi A, Micaglio E, Anastasia L.. Calcium in Brugada syndrome: questions for future research. Front Physiol 2018;9:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano M, Vitale R, Cuko A, Giannelli L, Calovic Z, Conti M, Pozzi P, Natalizia A, Crisà S, Borrelli V, Brugada R, Sarquella-Brugada G, Guazzi M, Frigiola A, Menicanti L, Santinelli V.. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017;10:e005053.. [DOI] [PubMed] [Google Scholar]
- 3. Pappone C, Monasky MM, Ciconte G. Epicardial ablation in genetic cardiomyopathies: a new frontier. Firenze, Italy: Conoscere e Curare il Cuore, XXXV Congresso di Cardiologia del Centro per la Lotta contro l’Infarto Fondazione Onlus; 2018.
- 4. Fitzgerald DP, Das S, Malone MP, Holland BS, Schexnayder SM.. Seizures, Meds, and Vtach: a journey to a Brugada diagnosis. Pediatr Emerg Care 2018;doi:10.1097/PEC.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 5. Catalano O, Antonaci S, Moro G, Mussida M, Frascaroli M, Baldi M, Cobelli F, Baiardi P, Nastoli J, Bloise R, Monteforte N, Napolitano C, Priori SG.. Magnetic resonance investigations in Brugada syndrome reveal unexpectedly high rate of structural abnormalities. Eur Heart J 2009;30:2241–2248. [DOI] [PubMed] [Google Scholar]
- 6. Rudic B, Schimpf R, Veltmann C, Doesch C, Tülümen E, Schoenberg SO, Borggrefe M, Papavassiliu T.. Brugada syndrome: clinical presentation and genotype-correlation with magnetic resonance imaging parameters. Europace 2016;18:1411–1419. [DOI] [PubMed] [Google Scholar]
- 7. Papavassiliu T, Veltmann C, Doesch C, Haghi D, Germans T, Schoenberg SO, van Rossum AC, Schimpf R, Brade J, Wolpert C, Borggrefe M.. Spontaneous type 1 electrocardiographic pattern is associated with cardiovascular magnetic resonance imaging changes in Brugada syndrome. Heart Rhythm 2010;7:1790–1796. [DOI] [PubMed] [Google Scholar]
- 8. van Hoorn F, Campian ME, Spijkerboer A, Blom MT, Planken RN, van Rossum AC, de Bakker JMT, Wilde AAM, Groenink M, Tan HL.. SCN5A mutations in Brugada syndrome are associated with increased cardiac dimensions and reduced contractility. PLoS One 2012;7:e42037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abriel H, Rougier JS, Jalife J.. Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death. Circ Res 2015;116:1971–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNair WP, Sinagra G, Taylor MRG, Di Lenarda A, Ferguson DA, Salcedo EE, Slavov D, Zhu X, Caldwell JH, Mestroni L.. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol 2011;57:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orr N, Arnaout R, Gula LJ, Spears DA, Leong-Sit P, Li Q, Tarhuni W, Reischauer S, Chauhan VS, Borkovich M, Uppal S, Adler A, Coughlin SR, Stainier DYR, Gollob MH.. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat Commun 2016;7:11303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennett JS, Stroud DM, Becker JR, Roden DM.. Proliferation of embryonic cardiomyocytes in zebrafish requires the sodium channel scn5Lab. Genesis 2013;51:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cavalli M, Fossati B, Vitale R, Brigonzi E, Ricigliano VAG, Saraceno L, Cardani R, Pappone C, Meola G.. Flecainide-induced Brugada syndrome in a patient with skeletal muscle sodium channelopathy: a case report with critical therapeutical implications and review of the literature. Front Neurol 2018;9:385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tukkie R, Sogaard P, Vleugels J, de Groot IK, Wilde AA, Tan HL.. Delay in right ventricular activation contributes to Brugada syndrome. Circulation 2004;109:1272–1277. [DOI] [PubMed] [Google Scholar]
- 15. Zaklyazminskaya E, Dzemeshkevich S.. The role of mutations in the SCN5A gene in cardiomyopathies. Biochim Biophys Acta 2016;1863:1799–1805. [DOI] [PubMed] [Google Scholar]