Abstract
Background
Cancer patients are regarded as a highly vulnerable group in the current Coronavirus Disease 2019 (COVID-19) pandemic. To date, the clinical characteristics of COVID-19-infected cancer patients remain largely unknown.
Patients and methods
In this retrospective cohort study, we included cancer patients with laboratory-confirmed COVID-19 from three designated hospitals in Wuhan, China. Clinical data were collected from medical records from 13 January 2020 to 26 February 2020. Univariate and multivariate analyses were carried out to assess the risk factors associated with severe events defined as a condition requiring admission to an intensive care unit, the use of mechanical ventilation, or death.
Results
A total of 28 COVID-19-infected cancer patients were included; 17 (60.7%) patients were male. Median (interquartile range) age was 65.0 (56.0–70.0) years. Lung cancer was the most frequent cancer type (n = 7; 25.0%). Eight (28.6%) patients were suspected to have hospital-associated transmission. The following clinical features were shown in our cohort: fever (n = 23, 82.1%), dry cough (n = 22, 81%), and dyspnoea (n = 14, 50.0%), along with lymphopaenia (n = 23, 82.1%), high level of high-sensitivity C-reactive protein (n = 23, 82.1%), anaemia (n = 21, 75.0%), and hypoproteinaemia (n = 25, 89.3%). The common chest computed tomography (CT) findings were ground-glass opacity (n = 21, 75.0%) and patchy consolidation (n = 13, 46.3%). A total of 15 (53.6%) patients had severe events and the mortality rate was 28.6%. If the last antitumour treatment was within 14 days, it significantly increased the risk of developing severe events [hazard ratio (HR) = 4.079, 95% confidence interval (CI) 1.086–15.322, P = 0.037]. Furthermore, patchy consolidation on CT on admission was associated with a higher risk of developing severe events (HR = 5.438, 95% CI 1.498–19.748, P = 0.010).
Conclusions
Cancer patients show deteriorating conditions and poor outcomes from the COVID-19 infection. It is recommended that cancer patients receiving antitumour treatments should have vigorous screening for COVID-19 infection and should avoid treatments causing immunosuppression or have their dosages decreased in case of COVID-19 coinfection.
Key words: cancer, COVID-19, retrospective case study, severe clinical events
Introduction
In December 2019, a cluster of pneumonias caused by an unknown pathogen was first reported in Wuhan, a city within the central part of China.1 , 2 Earlier cases were linked to a large seafood and live animal market selling different wild animal species.3 The causative agent of the pneumonia was later identified as a novel coronavirus and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 Genetic analysis of the full-length genome sequences revealed SARS-CoV-2 to be most closely related to a known bat coronavirus termed BatCoV RaTG13, suggesting bats as the likely origin.3 This suggested the high possibility of animal-to-human transmission. Afterwards, human-to-human transmission was confirmed in 15 health care workers, who were all infected by one patient with the novel coronavirus infection.4 Identification of the pathogen and transmission pattern have led to the implementation of top-level preventive and control measures by the Chinese government and the World Health Organization (WHO), who consequently declared Coronavirus Disease 2019 (COVID-19) a public health emergency of international concern.
Before December 2019, six coronaviruses strains were known to infect humans, including two highly pathogenic strains, SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), causing SARS and MERS, respectively, and four other strains causing respiratory illnesses ranging from a self-resolving cold to severe pneumonia.5 SARS-CoV, which emerged in China in 2002, caused an epidemic infecting 8098 individuals that resulted in 774 deaths, with a case fatality rate of ∼10%. Subsequently, MERS-CoV emerged in the Middle East, causing a persistent epidemic since 2012 and killing 34% of the infected people between 2012 and 2019 (2494 cases and 858 deaths). Like the other coronaviruses, SARS-CoV-2 primarily causes respiratory tract infections. An initial prospective study of the clinical features of 41 laboratory-confirmed cases in Wuhan demonstrated a severe illness that was clinically similar to SARS.6 According to the Chinese Center for Disease Control and Prevention (CDC) report on 44 672 laboratory-confirmed cases nationwide between the initial outbreak and 11 February 2020, the overall case fatality rate of COVID-19 was 2.3%.7 Although deaths from COVID-19 were less frequent compared with patients infected by SARS-CoV or MERS-CoV, COVID-19 was far more transmissible, with each new infected case producing an average of 2.68 new secondary cases.8 By 26 February 2020, the ongoing outbreak had caused a total of 78 497 confirmed infected cases and 2744 deaths in China, subsequently leading to a pandemic involving more than 70 countries.5
In the COVID-19 crisis, cancer patients are regarded as a highly vulnerable group. A recent investigation of 18 patients who had been previously diagnosed with cancer, from a nationwide cohort of 2007 COVID-19 cases, found that patients with cancer had a higher risk of severe clinical events than those without cancer.9 The case fatality rate reached 5.6% among cancer patients compared with 2.3% in the general population.7 However, with a relatively small sample size, limited clinical information, and high heterogeneity of the course of the disease, many critical issues concerning treatment principles of COVID-19-infected cancer patients remain unclear. There is an urgent need to answer the following questions, including whether COVID-19-infected cancer patients will have distinct clinical courses and worse outcomes, such as death from the infection or severe pneumonia, and whether cancer patients should receive antitumour treatments as usual in epidemic areas. Therefore, we aimed to explore these issues by conducting an urgent retrospective case study on critical COVID-19-infected cancer patients.
Methods
Study design and participants
A retrospective case study was carried out in three hospitals designated for COVID-19 patients in Wuhan, namely, Tongji Sino-French New Town Hospital, Union Red Cross Hospital, and Union West Hospital, all affiliated with the Tongji Medical College of Huazhong University of Science and Technology. Hospitalised cancer patients diagnosed with COVID-9 infection were identified between 13 January 2020 and 26 February 2020. Patients previously diagnosed with solid cancer and had a laboratory-confirmed SARS-CoV-2 infection were enrolled. Nasal and/or pharyngeal swabs were collected and tested for SARS-CoV-2 RNA with RT-PCR assay as previously described.6 Clinical retrospective data were retrieved from the medical records, including demographic features, clinical features, laboratory findings, and chest computed tomography (CT) images. Two physicians (LZ and MZ) independently reviewed the data.
This study was approved by the Ethics Committee of the Tongji Medical College of Huazhong University of Science and Technology (No. TJ-IRB20200210). The requirement for informed patient consent was waived by the Ethics Committee due to the rapid emergence of this infectious disease.
Study definitions
COVID-19 was diagnosed based on the criteria published by the WHO and confirmed by RT-PCR assay of nasal and/or pharyngeal specimens. Acute respiratory distress syndrome (ARDS) was defined according to the interim guidance of WHO for COVID-19.10 Hospital-related transmission was suspected if a cluster of hospitalised patients in the same ward became infected in a certain period and, under such circumstances, possible sources of infection were traced.11 Severe clinical events (a composite end point) were defined as a condition requiring admission to an intensive care unit (ICU), the use of mechanical ventilation, or death.12
Statistical analysis
For descriptive analysis, continuous variables were presented as the mean with standard deviation or as median with interquartile range (IQR), as appropriate. Categorical variables are presented as number (%). The Shapiro–Wilk test was used to test the normality of data distribution. The Kaplan–Meier method was used for time-to-event data to estimate the median time and its corresponding 95% confidence interval (CI). To explore potential factors of COVID-19-infected cancer patients developing severe clinical events, the hazard ratio (HR) and the corresponding 95% CIs from the Cox proportional hazards model were calculated. All statistical analysis was carried out using SPSS Statistics version 26.0 (IBM, New York, NY). A two-side P-value <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
We retrospectively enrolled 28 patients with cancer of the 1276 patients (2.2%) admitted to three designated hospitals for quarantine and treatment of COVID-19 between 13 January 2020 and 26 February 2020. Demographic and clinical features of these patients are summarised in Table 1 . The median (IQR) age was 65.0 (56.0–70.0) years; 17 (60.7%) of them were males. All patients were local residents of the three main districts of Wuhan and most patients (67.9%) were from Hankou, the starting point of the outbreak, where the Huanan market is located.
Table 1.
Demographic and baseline clinical characteristics of COVID-19-infected cancer patients
| Characteristic | Patients (N = 28) |
|---|---|
| Median age (interquartile range), years | 65.0 (56.0–70.0) |
| Male sex | 17 (60.7) |
| Residential district | |
| Wuchang | 6 (21.4) |
| Hankou | 19 (67.9) |
| Hanyang | 3 (10.7) |
| Patients' hospital | |
| Tongji Sino-French New Town Hospital | 14 (50) |
| Union West Hospital | 2 (7.1) |
| Union Red Cross Hospital | 12 (42.9) |
| Tumour diagnosis | |
| Lung cancer | 7 (25.0) |
| Oesophagus cancer | 4 (14.3) |
| Breast cancer | 3 (10.7) |
| Laryngocarcinoma | 2 (7.1) |
| Liver cancer | 2 (7.1) |
| Prostatic cancer | 2 (7.1) |
| Cervical cancer | 1 (3.6) |
| Gastric cancer | 1 (3.6) |
| Colon cancer | 1 (3.6) |
| Rectum cancer | 1 (3.6) |
| Nasopharynx cancer | 1 (3.6) |
| Endometrial cancer | 1 (3.6) |
| Ovarian cancer | 1 (3.6) |
| Carcinoma of testis | 1 (3.6) |
| Tumour stage | |
| Stage I/II/III | 18 (64.3) |
| Stage IV | 10 (35.7) |
| History of prior treatment | |
| Operationa | 21 (75.0) |
| Chemo/radiotherapya | 25 (89.3) |
| Target/immunotherapya | 6 (21.4) |
| Chemotherapy (<14 days)b | 3 (10.7) |
| Radiotherapy (<14 days)b | 1 (3.6) |
| Target therapy (<14 days)b | 2 (7.1) |
| Immunotherapy (<14 days)b,c | 1 (3.6) |
| Source of infection | |
| In community | 20 (71.4) |
| Nosocomial transmission | 8 (28.6) |
| Comorbidities | |
| Diabetes | 4 (14.3) |
| Chronic cardiovascular and cerebrovascular disease (including hypertension and coronary heart disease) | 4 (14.3) |
| Chronic pulmonary disease (including chronic obstructive pulmonary disease and asthma) | 1 (3.6) |
| Chronic liver disease (including chronic hepatitis B and cirrhosis) | 2 (7.1) |
| Symptoms and signs at on admission | |
| Fever | 23 (82.1) |
| Cough | 22 (78.6) |
| Fatigue | 18 (64.3) |
| Dyspnoea | 14 (50) |
| Myalgia | 4 (14.3) |
| Diarrhoea | 3 (10.7) |
| Chest pain | 2 (7.1) |
| Fever time, days | 7 (0–30) |
| Fever to dyspnoea time, days | |
| Lung cancer | 1.0 (0.0–3.5) |
| Non lung cancer | 5.0 (4.0–7.0) |
Data are presented as n (%) unless noted otherwise.
COVID-19, Coronavirus Disease 2019.
Treatment after the diagnosis of cancer.
Time from last antitumour treatment to diagnosis of COVID-19.
One patient received treatment combining chemotherapy and immunotherapy.
Among the cancer patients, lung cancer was the most frequent type of cancer (n = 7, 25.0%), followed by oesophageal cancer (n = 4, 14.3%) and breast cancer (n = 3, 10.7%). Ten patients (35.7%) were diagnosed with stage IV cancer. Baseline information on cancer history of all the cases are summarised in supplementary Table S1, available at Annals of Oncology online. All the patients had a history of antitumour therapy. Within 14 days of COVID-19 diagnosis, six (21.4%) patients had received at least one kind of antitumour therapy such as chemotherapy (n = 3, 10.7%), targeted therapy (n = 2, 7.1%), radiotherapy (n = 1, 3.6%), immunotherapy (n = 1, 3.6%; one of them received treatment combining chemotherapy and immunotherapy) (Table 1). There were two main clusters of patients: eight (28.6%) patients who developed COVID-19 while undergoing antitumour therapy in hospitals and 20 (71.4%) patients in their communities. In addition to cancer, 11 (39.2%) patients had at least one or more coexisting chronic diseases (Table 1). The most common symptoms on admission were fever (n = 23, 82.1%), dry cough (n = 22, 81%), and fatigue (n = 18, 64.3%); 14 (50.0%) patients developed dyspnoea and 4 (14.3%) patients had a resting respiratory rate of >30 breaths per minute.
Laboratory and radiologic findings
Laboratory findings on admission are presented in supplementary Table S2, available at Annals of Oncology online. The blood count results showed anaemia in 21 (75%) patients, leucopaenia in 9 (32.1%) patients, and lymphopaenia in 23 (82.1%) patients. Low levels of serum albumin (31.1, 28.6–34.8 g/L) were observed in 25 (89.3%) patients and high levels of serum globulin (32.1, 27.9–37.1 g/L) in 11 (39.3%) patients. High levels of lactate dehydrogenase (262.9, 168.5–508.0 U/L) were found in 10 (50%) patients, highly sensitive C-reactive protein levels in 23 (82.1%) patients, and elevated erythrocyte sedimentation rate in 16 (57.1%) patients. Most patients (92.9%) had normal serum levels of procalcitonin. D-Dimer was elevated in 11 (39.3%).
Radiologic features on chest CT on admission are also shown in supplementary Table S2, available at Annals of Oncology online. All patients had abnormal findings on chest CT: 22 patients (78.6%) had bilateral involvement, whereas the remaining 6 (21.4%) patients had unilateral focal involvement. Ground-glass opacity, the predominant CT imaging pattern, was observed in 21 (75%) patients. Patchy consolidation was the second most common finding in 13 (46.3%) patients. Interstitial abnormalities, including reticular appearance, fibrous strips, and interlobular septal thickening, were found in four (14.3%) patients. Follow-up CT was carried out 7–14 days after admission and showed improvement in 13 patients (46.4%), unchanged appearance in 5 patients (17.9%), and deterioration in 6 patients (21.4%). Four patients (14.3%) did not obtain imaging data due to critical illness or death. Notably, in our study, CT images in seven (17.9%) lung cancer patients showed reduced lung volume due to tumour volume, co-existing with features of pneumonia. Figure 1 demonstrates typical CT findings of two patients.
Figure 1.
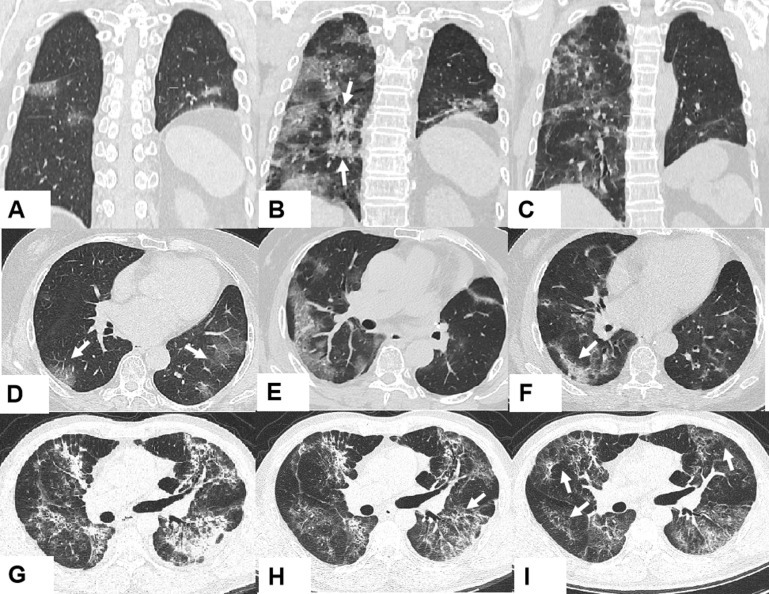
Representative images of the chest computed tomography (CT) scan at different times throughout the disease course.
(A–C) Axial CT scanning and (D–F) coronal scanning images are from a 70-year-old woman who was diagnosed with adenocarcinoma and received left upper lobectomy in 2010. As her adenocarcinoma recurred in 2012, she has so far received four courses of chemotherapy and targeted therapy (gefitinib). (A and D) Day 1 after symptom onset: left lung with reduced lung volume after left upper lobectomy and multifocal ground-glass opacities in the bilateral inferior lung lobes (arrows). (B and E) Day 10 after symptom onset: progressively diffused ground-glass opacities and consolidation (arrows) in bilateral subpleural regions. (C and F) Day 25 after symptom onset: improvement of ground-glass opacities and little fibrous stripe in the right lower lung (arrow). (G–I) Coronal CT scanning images are from a 47-year-old man who was diagnosed with nasopharyngeal carcinoma in 2016. Radiotherapy adjuvant chemotherapy was carried out. (G) Day 21 after symptom onset: diffused ground-glass opacities, obvious consolidation, mixed with reticular appearance in bilateral lungs. (H) Day 28 after symptom onset: decreased ground-glass opacity, consolidation, and interlobular septal thickening (arrow). (I) Day 32 after symptom onset: further improvement in appearance with predominant reticular patterns (arrows).
Treatment and complications
Treatment options of patients are presented in Table 2 . A total of 22 patients (78.6%) received oxygen therapy. Ten (35.7%) patients were put on invasive mechanical ventilation, with two (7.1%) requiring endotracheal intubation and invasive ventilation because of progressive hypoxia. Significantly more severe cases were subjected to mechanical ventilation (noninvasive: 53.3% versus 0%, P < 0.001; invasive: 13.3% versus 0%, P < 0.001) as compared with nonsevere ICU cases. The median period of mechanical ventilation for noninvasive ventilation was 2.5 (IQR 1.0–5.0) days and for invasive this was 2.5 days. None of the severe patients received extracorporeal membrane oxygenation in our study. In the COVID-19 outbreak, antiviral agents are largely empirical, without evidence from randomised controlled trials. As many as 20 (71.4%) patients were prescribed at least one antiviral agent, such as arborol (n = 14, 50%), lopinavir/ritonavir (n = 10, 35.7%), ganciclovir (n = 9, 32.1%), and ribavirin (n = 1, 3.6%), whereas 9 patients (32.1%) were administered combinations of antiviral agents. Empirical antibiotics were administered to 23 patients (82.1%). Systemic corticosteroids were administered to 15 patients (53.6%). Administration of corticosteroids was more frequent in patients with severe events (12/15, 80%) than those with nonsevere events (3/13, 23.1%). Seven of eight ARDS patients were administered systemic corticosteroids. The dosage and period of corticosteroids in the severe cases were higher than nonsevere cases [dose (mg/kg/d): 1.0 versus 0.6, period (days): 3.0 (2.0–4.8) versus 5.0], but the difference was not significant. Moreover, intravenous immunoglobin was prescribed to 12 patients (35.7%).
Table 2.
Treatment and clinical outcome of COVID-19-infected cancer patients
| Treatment | No. (%), median (interquartile range) |
|---|---|
| Physiotherapy | |
| Oxygen therapy | 22 (78.6) |
| Mechanical ventilation | 10 (35.7) |
| Noninvasive/severe, days | 8/15 (53.3), 2.5 (1.0–5.0) |
| Invasive/severe | 2 /15 (13.3), 2.5 (NA) |
| Extracorporeal membrane oxygenation | 0 |
| Admission to intensive care unit | 6 (21.4) |
| Medicine therapy | |
| Antibiotic treatment | 23 (82.1) |
| Antiviral treatment (dose/day) | 20 (71.4) |
| Lopinavir/ritonavir (400/100 mg, p.o. b.i.d.) | 10 (35.7) |
| Arbidol (200 mg, p.o. t.i.d.) | 14 (50.0) |
| Ganciclovir (500 mg, i.v. drip b.i.d.) | 9 (32.1) |
| Ribavirin (500 mg, i.v. drip b.i.d.) | 1 (3.6) |
| Combination (>1 drug) | 9 (32.1) |
| Systemic corticosteroids | 15 (53.6) |
| Intravenous immunoglobin, days | 10.0 (35.7), 3.0 (1.0–3.0) |
| Time from symptoms to hospitalisation, days | 6.0 (3.0–10.0) |
| Complications | |
| ARDS | 8 (28.6) |
| Septic shock | 1 (3.6) |
| Pulmonary embolism suspecteda | 2 (7.1) |
| AMIa | 1 (3.6) |
| Severe events | 15 (53.6) |
| Time from diagnosis to severe events | 7.0 (5.0–15.0) |
| Occurrence of severe events | |
| Stage IV vs non-stage IV | 7/10 (70.0) versus 8/18 (44.4) |
| Antitumour ≤14 versus >14 daysb | 5/6 (83.3) versus 10/22 (45.5) |
| Antitumour ≤30 versus >30 daysb | 5/12 (41.7) versus 10/16 (62.5) |
| Patchy consolidation vs no patchy consolidationc | 11/13 (84.6) versus 4/15 (26.7)∗ |
| Computed tomography scan evaluation | |
| Improvement | 13 (46.4) |
| Unchanged appearance | 5 (17.9) |
| Deterioration | 6 (21.4) |
| NA | 4 (14.3) |
| Clinical symptoms evaluation | |
| Improvement | 14 (50.0) |
| Stable | 3 (10.7) |
| Worse | 11 (39.3) |
| Clinical outcomes | |
| Staying in hospital | 10 (35.7) |
| Discharge from hospital | 10 (35.7) |
| Death | 8 (28.6) |
| Hospital stay (patients staying in hospital), days | 19.0 (16.0–28.5) |
| Hospital stay (patients discharge), days | 13.5 (10.8–17.8) |
| Time from diagnosis of infection to death, days | 16.0 (9.0–22.3) |
| Cause of death | |
| ARDS | 5 (62.5) |
| Septic shock | 1 (12.5) |
| Pulmonary embolism suspected | 1 (12.5) |
| AMI | 1 (12.5) |
AMI, acute myocardial infarction; ARDS, acute respiratory distress syndrome; b.i.d., biduum two days; COVID-19, Coronavirus Disease 2019; i.v., intravenous; NA, not available; p.o., by mouth; t.i.d., (ter die sumendum) three times a day.
∗P < 0.05.
One patient had ARDS by co-incidence.
Time from last antitumour treatment to diagnosis of COVID-19.
Computed tomography scan on admission.
The clinical outcome of patients is also shown in Table 2. As of 26 February 2020, 15 (53.6%) patients developed severe clinical events, 6 (21.4%) patients were admitted to ICU, 10 (35.7%) patients had life-threatening complications, and 8 (28.6%) of the patients died. Of the 10 stage IV cancer patients, 7 (70%) had developed severe events, whereas 44.4% of the non-stage IV patients had such events. Among six cancer patients who received antitumour treatment within 14 days of being diagnosed with COVID-19, five (83/%) developed severe events. In addition, 84.6% of patients (11/13) with patchy consolidation on CT on admission had developed severe events.
The most common complication was ARDS (n = 8, 28.6%), followed by septic shock (n = 1, 3.6%), and acute myocardial infarction (n = 1, 3.6%). Two patients (7.1%) were suspected to have pulmonary embolism. A total of 10 (35.7%) of 28 patients had been discharged with a median hospital stay of 13.5 days (IQR 10.8–17.8); 10 (35.7%) cases were inpatients with a median stay of 19.0 days (IQR 16.0–28.5). Of the 28 patients, 8 (28.6%) died, with a median time of 16.0 days (IQR 9.0–22.3) from admission to death. The cause of death included ARDS (5/8, 62.5%), followed by pulmonary embolism (1/8, 12.5%), septic shock (1/8, 12.5%), and acute myocardial infarction (1/8, 12.5%).
Risk factors for developing severe event
The association of clinical factors with severe events is summarised in supplementary Table S3, available at Annals of Oncology online, which was evaluated using the univariate Cox proportional hazards model. Compared with those who did not receive antitumour treatment within 14 days, cancer patients who received antitumour treatment within 14 days before COVID-19 diagnosis, including chemotherapy (n = 3, 10.7%), radiotherapy (n = 1, 3.6%), targeted therapy (n = 2, 7.1%), and immunotherapy (n = 1, 3.6%, combined with chemotherapy), had a higher risk of developing severe events with borderline statistical significance. Moreover, patchy consolidation on the first CT on admission suggested an elevated risk of developing severe events than those cases without consolidation (HR = 5.000, 95% CI 1.576–15.861, P = 0.006).
Similar results were observed in the multivariate-adjusted Cox proportional hazards model after being adjusted for age and sex (Table 3 ). Cancer patients who had received last antitumour treatment within 14 days had a statistically significant increased risk of developing severe events (HR = 4.079, 95% CI 1.086–15.322, P = 0.037). Furthermore, cancer patients with patchy consolidation on CT on admission had a higher risk of developing severe events (HR = 5.438, 95% CI 1.498–19.748, P = 0.010). The adjusted survival curve of severe events showed that cancer patients who underwent antitumour treatment in the past 14 days or had patchy consolidation in CT on admission had significantly higher severe events (Figure 2 A and B).
Table 3.
Multivariate analysis for the risk of severe events
| Clinical factors | HR | 95% CI | P∗ |
|---|---|---|---|
| Sex | 0.574 | 0.162–2.038 | 0.390 |
| Age | 1.455 | 0.478–4.430 | 0.509 |
| Antitumour ≤14 daysa | 4.079 | 1.086–15.322 | 0.037 |
| Patchy consolidationb | 5.438 | 1.498–19.748 | 0.010 |
CI, confidence interval; COVID-19, Coronavirus Disease 2019; HR, hazard ratio.
A two-sided P < 0.05 was considered statistically significant.
Time from last antitumour treatment to diagnosis of COVID-19.
Computed tomography scan on admission.
Figure 2.
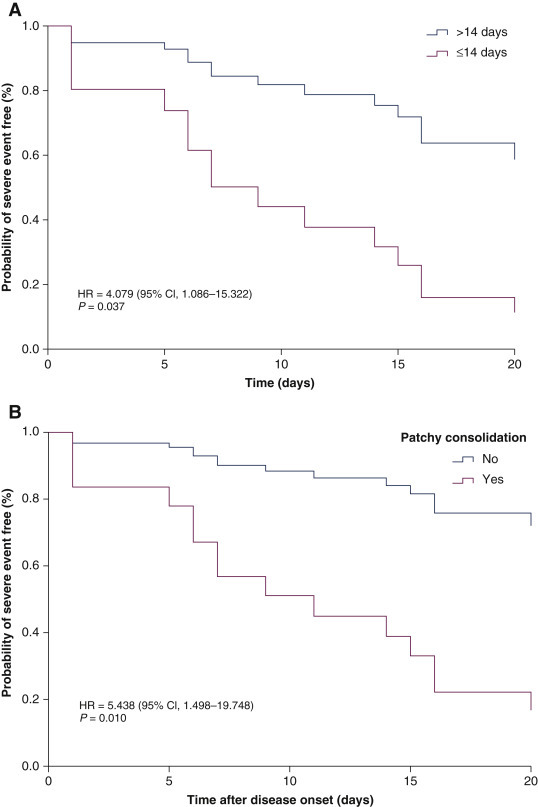
Kaplan–Meier curve of risk factors for developing severe events, adjusted by age and sex.
(A) Cancer patients who received antitumour treatment within 14 days before Corona Disease 2019 (COVID-19) diagnosis or >14 days after its diagnosis. (B) Patchy consolidation in the first computed tomography scan on admission or its absence on admission.
Discussion
The clinical characteristics of 28 cancer patients with laboratory-confirmed COVID-19 from three designated hospitals in Wuhan, China, as of 26 February 2020, are described. As much as 53.6% of the patients developed severe events, 21.4% were admitted to ICU, 35.7% had life-threatening complications, and 28.6% of the patients died.
Our results showed the following clinical features of COVID-19-infected cancer patients: typical symptoms of fever, dry cough, fatigue, and dyspnoea, along with blood lymphopaenia and high levels of highly sensitive C-reactive protein. Cancer patients present with clinical features similar to those in the general population, except for anaemia and hypoproteinaemia, which were frequently found in this cohort. Anaemia and hypoproteinaemia were considered to be a major consequence of nutritional deterioration in cancer patients, which may adversely affect immunocompetence and increase the susceptibility to respiratory pathogens. In our cohort, the symptom of dyspnoea was found to occur much earlier from the onset of COVID-19 infection in lung cancer patients as compared with the general population [1.0 (0.0–3.5) versus 8.0 (5.0–13.0) days],11 and earlier as compared with other cancer patients [1.0 (0.0–3.5) versus 5.0 (4.0–7.0) days]. Patients with lung cancer, with worse baseline lung function and endurance, are more likely to develop more severe anoxia and progress more rapidly with COVID-19, indicating an urgent and increased need to treat COVID-19-infected cancer patients, with special emphasis on patients with lung cancer.
In this study, the severe events were defined as the admission to ICU, requiring mechanical ventilation, or death. In the study population, 53.6% of the cancer patients developed severe events, with 28.6% of the patients dying. In the general COVID-19-infected population, 4.7% of confirmed cases reached clinically critical status, and nearly half of the critical cases (2.3%) were fatal.7 Patients with cancer are particularly susceptible to respiratory pathogens and severe pneumonia, because they are at an immunosuppressive state due to malignancy and antitumour therapy. It was found that within 14 days, antitumour therapies were significantly associated with the occurrence of severe clinical events in COVID-19 infection. Previous investigations by Liang et al.9 showed a lower percentage of cancer patients [7 (39%) of 18 patients] developing severe events. The main reasons for the discrepancy can be attributed to variation in the definition of severe events and the study populations. Liang et al.9 defined clinically severe events as the patients' admission to the ICU requiring invasive ventilation or death. Their cohort narrowed the scope of severe cases to patients under invasive ventilation, which is different from all the mechanical ventilation cases enrolled in our study. All the cases in our cohort came from Wuhan, whereas the cases of Liang et al.9 were from the entire nation. Wuhan faced a dire shortage of medical resources to cope with the influx of patients at the early stage of the outbreak, and some patients were not admitted to the hospital in time; hence, it is presumed that delayed admission contributed to increased mortality.
We also found that the CT feature of patchy consolidation on admission is a risk factor associated with severe events. Ground-glass opacity and patchy consolidation were both common CT findings in COVID-19-infected cancer patients, similar to the features in the general population.13 Shi et al.13 analysed the timing of emergence and persistence of those features on CT. They found that ground-glass opacity appeared first, even before symptom onset, then increased during the following 2 weeks, and decreasing gradually in the third week. Patchy consolidation usually appeared in the first to second weeks after symptom onset. It can rapidly evolve into bilateral extensive consolidation, with a white lung appearance on CT, leading to poor prognosis. Therefore, CT on admission showing patchy consolidation may imply that admission time of these cases would be at least 1 to 2 weeks after the onset of illness. The delayed admission time for cancer patients may be a reasonable explanation for the poor outcome of some cases in our cohort.
In our cohort, 71.4% of the patients were prescribed at least one antiviral agent. About one-third of patients received more than one antiviral agent. However, currently there is no drug that has proven to be effective against SARS-CoV-2. Systemic steroids remain controversial in the treatment of viral pneumonia. Usage of steroids has been considered to slow virus clearance due to its immunosuppressive effect, which was often associated with an increased risk of opportunistic infections, especially in patients who required mechanical ventilation. In our observations, even though more than half of patients received steroids treatment, we could not demonstrate reduced incidence of severe events in our cancer patient cohort.
It was also noted that 28.6% of our patients had developed COVID-19 infection during hospitalisation and nosocomial transmission of SARS-CoV-2 was suspected. Hospital-related transmission has been reported in both patients and health care workers. In a retrospective case study with 138 patients, 41.3% of the patients were reported to have acquired COVID-19 infection during hospitalisation, and of these, 5 patients were from the oncology department.11 Nationwide statistics of the Chinese CDC confirmed COVID-19 transmission within patients in health care settings.7 Human-to-human transmission has also been previously confirmed in familial clusters or travel-related clusters.14 , 15 SARS-CoV and MERS-CoV had also been confirmed to occur through nosocomial transmission. Therefore, health care facilities need to re-emphasise the importance of basic infection control measures to combat the spread of contagious pathogen via respiratory droplets.
Some cancer patients are also shown to have acquired COVID-19 infection on receiving antitumour treatment during hospitalisation. However, delaying antitumour treatment cannot be recommended as a reasonable choice to reduce the infection risk in the ongoing pandemic. Cancer patients should receive antitumour treatment in the setting of vigorous screening for COVID-19, including chest CT scan and nucleic acid testing, and the same should be extended to their companions. Treatment strategies likely to cause immunosuppression should be avoided or have dosages decreased, and patients who are generally in poor condition should not receive such treatments. In addition, at least 7 days before antitumour treatment, cancer patients should stay in the observation ward and in isolation from other patients. Stronger personal protection, including protection mechanisms for their families, should be made for cancer patients.
Our findings support the vulnerability of cancer patients in the current pandemic. However, our findings are also based on some study limitations. First, the study was retrospective, nonrandomised, and based on a small sample size. The tumour types were diverse, and heterogeneity could not be avoided. Second, some important confounders were not able to be included in the multivariate analyses, such as tumour stage. In the descriptive analyses, we found that 70% of stage IV patients developed severe events. Although the univariable analyses showed no statistically significant associations, we still suggest that the stage of cancer may affect the clinical course of COVID-19-infected cancer patients. However, we could not include tumour stage in the multivariable Cox model analysis due to the high correlation between stage and antitumour treatment within 14 days (correlation coefficient r = −0.518, P = 0.005). Third, due to an urgent and retrospective descriptive study design, we only reported crude rates of complications and fatality in cancer patients with COVID-19 infection. The comparisons between cancer and noncancer patients with COVID-19 infection could reveal more useful information, as would comparisons of less severe cases not included in our study population. Thus, future studies with larger sample sizes and prospective study designs are warranted to further explore the risk factors and severe events in COVID-19-infected cancer patients.
Acknowledgements
We thank all patients involved in the study. English editing work by D & A Research and Consultancy of Pan Africa Science Journal (https://www.panafricajournal.com) is also appreciated.
Funding
This work was funded by the research grants from the National Natural Science Foundation of China (No. 81700032, No. 81570348, and No. 81572934) and Huazhong University of Science and Technology COVID-19 Rapid Response Call China (No. 2020kfyXGYJ0).
Disclosure
All authors have declared no conflicts of interest.
Supplementary data
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):40–42. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;5791(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) WHO; Geneva: 2020. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected. Interim Guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at. [Google Scholar]
- 11.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada P., Buathong R., Phuygun S. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Euro Surveill. 2020;25(8) doi: 10.2807/1560-7917.ES.2020.25.8.2000097. ES.2020.25.8.2000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


