Abstract
Dental plaque is a biofilm composed of a complex oral microbial community. The accumulation of plaque in the pit and fissures of dental elements often leads to the development of tooth decay (dental caries). Here, potent anti-biofilm materials were developed by incorporating zinc methacrylates or di-n-butyl-dimethacrylate-tin into the light-curable sealant and their physical, mechanical, and biological properties were evaluated. The data revealed that 5% di-n-butyl-dimethacrylate-tin (SnM 5%) incorporated sealant showed strong anti-biofilm efficacy against various single-species (Streptococcus mutans or Streptococcus oralis or Candida albicans) and S. mutans-C. albicans cross-kingdom dual-species biofilms without either impairing the mechanical properties of the sealant or causing cytotoxicities against mouse fibroblasts. The findings indicate that the incorporation of SnM 5% in the experimental pit and fissure self-adhesive sealant may have the potential to be part of current chemotherapeutic strategies to prevent the formation of cariogenic oral biofilms that cause dental caries.
Keywords: pit and fissure sealants, metallic monomer, antibiofilm, Streptococcus mutans, Candida albicans
Introduction
Dental caries is one of the most prevalent chronic diseases, compromising the health and wellness of children and adults (Dye et al. 2007). It is a multifactorial disease, yet microbial biofilm formation on the tooth surface is considered to be a key virulence factor (Selwitz et al. 2007; Koo et al. 2017). For example, biofilm formation on restorative materials often leads to the onset of tooth decay (Busscher et al. 2010; Song et al. 2015). In particular, pit and fissures are the most frequent sites affected by dental caries due to their structural irregularity and morphological complexity (Weintraub 2001).
Streptococcus mutans, a member of the oral microbial community, is highly acidogenic and aciduric, which significantly contributes to the pathogenesis of dental caries by modulating the transition from non-pathogenic to highly cariogenic biofilms (Loesche 1986). S. mutans actively utilizes dietary sucrose to synthesize exopolysaccharides (EPS) via glucosyltransferase (Bowen & Koo 2011). These EPS, prime building blocks of cariogenic biofilms, enhance cell adhesion and cohesion that promote both microbial accumulation onto a surface and the development of densely packed cell aggregates, resulting in a highly structured and adherent biofilm (Hall-Stoodley et al. 2004; Klein et al. 2015; Peterson et al. 2015). Furthermore, the highly structured biofilm matrix protects the bacteria from antibiotics, making it extremely difficult to treat (Hwang et al. 2014; Lebeaux et al. 2014). Thus, the prevention of biofilm formation/development continues being the best approach against dental caries (Simonsen 2011; Hwang, Koltisko, et al. 2017).
Traditional pit and fissure sealants have been used to prevent the invasion of microorganisms and plaque accumulation for patients with a high occlusal caries risk (Mickenautsch & Yengopal 2016). For instance, pit and fissure sealant with fluoride, the mainstay of caries prevention, reduce the demineralization of tooth enamel by enhancing its remineralization (Kühnisch et al. 2012). However, it does not offer complete disease protection since the antibacterial activity of fluoride compounds is limited (Featherstone & Doméjean 2012). In addition, it is necessary to improve the application of pit and fissure sealants to simplify clinical procedures (Han et al. 2007) or to avoid potential pitfalls, such as plaque accumulation on sealants (Papageorgiou et al. 2017). Therefore, the search for alternative anti-biofilm agents that can more effectively block biofilm formation and arrest progression of caries lesions on pit and fissure is warranted.
Previous studies have shown that metal cross-linked monomers, such as zinc methacrylate, di-n-butyldimethacrylate-tin, and silver methacrylate, in a dental adhesive formulation exhibit antibacterial activity against mutans streptococci (Henn et al. 2011; Rubin Cocco et al. 2018). Although zinc methacrylate exhibited antibacterial activity (Henn et al. 2011) and organotin compounds showed both antifungal (Dylg et al. 2010) and antibacterial (Salam et al. 2012; Rubin Cocco et al. 2018) activities, to the best of the authors’ knowledge, the anti-biofilm efficacy of a pit and fissure sealant containing these monomers has not been evaluated against cariogenic biofilm models, particularly cross-kingdom biofilms that cause severe dental caries.
Here, self-adhesive resin-based pit and fissure sealants containing zinc methacrylate (ZnM) or di-n-butyldimethacrylate-tin (SnM) were developed to achieve easy manipulation/application and potent anti-biofilm activity. The physical and mechanical properties and aesthetics (translucency) of sealants were characterized to comprehensively assess their potential for dental application. Furthermore, their anti-biofilm activity was assessed by employing single-species (S. mutans/Streptococcus oralis/Candida albicans) or S. mutans/C. albicans cross-kingdom biofilm models. The data revealed that the SnM-based sealant exhibited an outstanding anti-biofilm effect without negatively affecting the mechanical properties, compared with the control. Furthermore, no cytotoxicity was observed from the SnM-based sealant against mice L929 fibroblast cells. Collectively, the anti-biofilm properties combined with the easy application of this material may serve as a platform to further develop restorative dental materials.
Materials and methods
Preparation of experimental self-adhesive resin-based sealant
Self-adhesive resin-based pit and fissure sealant were formulated using a resin matrix containing triethylene glycol dimethacrylate, bisphenol A glycidyl methacrylate, glycerol dimethacrylate phosphate and water (Esstech, Essington, PA). Then, phenylbis (2,4,6-trimethylbenzoyl)-phosphine oxide and diphenyliodonium hexafluorophosphate were added as a photoinitiator and catalyst, respectively (Sigma-Aldrich, Saint Luis, MO). Finally, nanometric silica Aerosil® OX80 (Evonik, Essen, Germany) was added to the resin matrix. Zinc methacrylate or di-n-butyl-dimethacrylate-tin (Gute Chemie, Karlsruhe, Germany) (Figure 1) were added to form four experimental groups: ZnM 2.5 %; ZnM 5 %; SnM 2.5 %; SnM 5 %. A sealant without the incorporation of metallic monomers was used as a control.
Figure 1.

Chemical structure of zinc methacrylate and di-n-butyl-methacrylate-tin.
Degree of C=C conversion
The degree of C=C conversion (DC) was evaluated using real-time Fourier transformed infrared spectroscopy (Prestige21; Shimadzu, Tokyo, Japan) with an attenuated total reflectance device incorporating a horizontal diamond crystal (GladiATR, PIKE Technologies, Madison, WI, USA). Each sealant was placed in the total reflectance cell and a preliminary reading for the uncured material (monomer) was taken using 24 co-added scans and 4 cm−1 resolutions. Then, the material was light-activated for 20 s using an LED light source (Bluephase N®, Ivoclar Vivadent) at an incident irradiance of 1,400 mW cm−1, and readings before and after the direct irradiation were carried out (n=3). The percentage of the degree of conversion was calculated as previously described (Ogliari et al. 2006).
Translucency parameter
The translucency parameter (TP) of each sealant was measured using a spectrophotometer (SP60; X-Rite, Grand Rapids, MI, USA). Unpolymerized sealants were placed into silicon molds (diameter 7 mm, thickness 1 mm) placed on a glass slide covered by a polyester strip. A second strip and a glass slide were used to cover the mold. Light-activation was carried out for 20 s on top and bottom surfaces. The specimens were stored in distilled water at 37 °C (n=5). The CIEL*a*b* color coordinates were measured after 24 h. Color readings were taken over white (L*=93.07, a*=1.28, b*=5.25) and black (L*=27.94, a*=0.01, b*=0.03). The TP for each specimen was calculated using the formula: TP=[(L*W-L*B)2 + (a*W-a*B)2 + (b*W-b*B)2]1/2, where W and B refer to the color coordinates measured on the white and black backgrounds (Johnston & Reisbick 1997; Kim et al. 2009).
Microshear bond strength
For the microshear bond strength (μSBS) test, an elastomer mold with two orifices of 1.5 mm in diameter and 0.5 mm in thickness was positioned over the enamel surface. The sealant was inserted into the orifices and light-activated for 20 s. After the matrix was gently removed, resultant specimens with two cylindrical restorations at the enamel surface were stored for 24 h in distilled water at 37 °C (n=10). Then, the specimens were positioned into a universal testing machine, where a thin wire was looped around the sealant cylinder. The specimens were submitted to μSBS test at a crosshead speed of 1 mm min−1 and the results were expressed in MPa. A scheme of the μSBS test protocol is demonstrated elsewhere (Münchow et al. 2013).
Flexural strength
Specimens were obtained with the following dimensions 10.0 mm × 2.0 mm × 2.0 mm (length, height, and width respectively). The samples were irradiated for 20 s on both sides (n=10). After 24 h of storage in distilled water at 37 °C, specimens were submitted to a three-point bending test on a mechanical testing machine (DL500; EMIC, São José dos Pinhais, PR, Brazil). The load was applied centrally on the bar at a crosshead speed of 0.5 mm min−1 until failure (López-Torres et al. 2011).
Depth of cure
The depth of cure was analyzed by the scraping method (ISO 6874 2015). The materials were filled into a cylindrical metallic mold (4.0 mm diameter, 6.0 mm height) and irradiated through a polyester strip for 20 s. The sealant was pushed out from the mold and the uncured material (if any) was removed. The maximum thickness of the cured material was measured with a digital caliper (n=3).
Single- and dual-species biofilm model
The experimental self-adhesive sealants were tested against single- or dual-species biofilm model to confirm their antibacterial and antifungal effects. Streptococcus mutans UA159, a well-established virulent cariogenic bacterium (Xiao et al. 2012), and Streptococcus oralis ATCC 35307, one of the most commonly detected early colonizers of the tooth surface (Fujiwara et al. 2000), were chosen as model oral bacteria. In addition, Candida albicans SC5314 was used as a model fungus, which is frequently detected with S. mutans from children with severe dental caries (Yang et al. 2012; Xiao et al. 2016; Xiao et al. 2018).
Single- or dual-species biofilm was formed following the previously reported protocol with modification (Koo et al. 2010; Xiao et al. 2012; Hwang, Liu, et al. 2017). Firstly, the anti-biofilm efficacy of each sealant material was screened using a S. mutans single-species biofilm model. Briefly, each of the experimental sealants was applied and polymerized on hydroxyapatite (HA) discs (1.25 cm in diameter, surface area of 2.7 ± 0.2 cm2, Clarkson, Chromatography Products, Inc., South Williamsport, PA), followed by coating with filter-sterilized human whole saliva to mimic the pellicle-coated tooth enamel. Then, each sealant-saliva-coated HA disc was vertically-suspended and inoculated with actively growing 105 CFU ml−1 of S. mutans in ultra-filtered (10-kDa cutoff; Millipore, Billerica, MA) tryptone-yeast extract (UFTYE) medium containing 1% (w/v) sucrose. Biofilms were grown at 37 °C in 5% CO2 and the medium was changed twice daily (8 am and 6 pm) until the end of the experimental period (42 h). After 42 h, the biomass (dry weight) of biofilms and the viability of the microbial cells (colony forming units, CFU) in biofilms were analyzed, and the pH of the biofilm culture supernatant was measured.
For visualization, only half of the HA disc was coated with SnM 5% sealant and S. mutans biofilms were cultured as detailed above. After 42 h, bacteria were stained with Syto 9 (485/498 nm; Molecular Probes) as described previously (Koo et al. 2010; Xiao et al. 2012). Then, confocal imaging (Zeiss LSM 800 upright laser scanning microscope) was performed using a 20× (numerical aperture = 1.0) water immersion objective. To minimize the autofluorescence signal from the sealant, spectral imaging and linear unmixing methodology were applied. Biofilms were scanned in ten 20-nanometer wavebands from 450 to 650 nanometers. Then, linear unmixing was performed using the Zeiss ZEN software by defining reference spectra from biofilms and biofilm-uncoated SnM 5% surface. Unmixed images were assembled and false-colored using Zeiss ZEN software.
Next, other single-species (S. oralis or C. albicans) and dual-species biofilm (S. mutans/C. albicans) models were employed to further assess the anti-biofilm activity. Here, only the SnM 5% sealant was tested since it presented the highest efficacy against S. mutans single-species biofilm. S. oralis or C. albicans single-species biofilm was formed by inoculating actively growing 105 CFU ml−1 of S. oralis or 104 CFU ml−1 of C. albicans in UFTYE medium supplemented with 1% (w/v) sucrose, and cultured as described above. On the dual-species model, a defined microbial population of S. mutans (106 CFU ml−1) and C. albicans (104 CFU ml−1) was inoculated to mimic cross-kingdom plaque-biofilm concept as detailed elsewhere (Hwang, Liu, et al. 2017). The medium was changed twice daily (8 am and 6 pm) until the end of the experimental period (42 h). Then, biofilm biomass, CFU, and the pH of the biofilm culture supernatant were determined as described above.
Scanning electron microscopy (SEM)
To check the effect of the SnM 5% on biofilm morphology, S. mutans single-species biofilms were formed on HA discs with or without SnM 5% coating as described above and performed SEM analysis (Liu et al. 2016). Briefly, after harvesting biofilms at 42 h, the biofilms were fixed using 2.5% glutaraldehyde and 2.0% paraformaldehyde for 4 h. Then, the biofilms were washed three times in ultrapure water, from a Milli-Q system (18.2 MΩ cm, Millipore, USA), and serially dehydrated with 50%, 70%, 80%, 90% and 100% ethanol for 10 min each. The discs were then dried with mixtures of ethanol:hexamethyldisilazane (1:1 and 1:4) (Polysciences, Inc., Warrington, PA, USA), and 100% of hexamethyldisilazane for 10 min each. The discs were dried for 1 h at room temperature, and then they were mounted on a microscope holder. Finally, the interface between discs and holder was coated with Colloidal Silver-liquid (Industry Road, Hatfield, PA, USA). Biofilms on each disc were observed by scanning electron microscopy (SEM™) (Quanta FEG 250, FEI, Hillsboro, OR).
Cytotoxicity assay
A cytotoxicity screening assay was performed following the ISO standard method using L929 mice fibroblasts to facilitate reproducibility (ISO 10993–5 2009). Briefly, DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS), 2% L-glutamine, penicillin (100 U ml−1) and streptomycin (100 mg ml−1) was used as the cell culture medium. Mouse fibroblasts of the L929 immortalized cell line were maintained in DMEM and incubated at 37 °C in a humidified atmosphere of 5% CO2 until confluence. After this, the cells were maintained in DMEM medium in 96-well plates for 24 h.
Specimens (n=6) of each experimental material were made using a silicone mold (5 mm diameter and 1 mm depth). Specimens were placed in 24-well plates with 1 ml of DMEM at 37 °C, pH 7.2. After 24 h, 200 μl of the eluate were transferred to the 96-well plates previously prepared and incubated for 24 h. WST-1 (Roche Applied Science, Germany) was applied to assess cell metabolic function by mitochondrial dehydrogenase activity, and the absorbance at 450 nm was measured via a microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA). The cytotoxicity assay was repeated twice.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics 20 Software (Armonk, NY. USA). The degree of conversion, the translucency parameter, the shear bond strength, and the flexural strength and depth of cure results were analyzed using a one-way analysis of variance ANOVA and Tukey’s post hoc. Biological results were analyzed using the Kruskal-Wallis test (nonparametric and based on ranks) for two-group comparisons. Statistical differences were considered if p < 0.05.
Results and discussion
Recent studies are reported in the literature regarding the application of antibacterial monomers for the synthesis of potent dental restorative materials (De Carvalho et al. 2014; Kitagawa et al. 2014; Hirose et al. 2016; Rubin Cocco et al. 2018). Among these, metallic monomers such as di-n-butyl-dimethacrylate-tin exhibited excellent bactericidal effects on planktonic cells when incorporated into the experimental adhesive system (Rubin Cocco et al. 2018). To test whether these metallic monomers can disrupt the formation and development of various oral biofilms under cariogenic conditions (in the presence of sugar), experimental self-adhesive pit and fissure sealants were formulated which incorporated these metallic monomers.
Self-adhesive materials are highly attractive for dental materials, especially in pediatric dentistry, since the protocol does not require pretreatment of tooth surfaces, thereby representing a less time-consuming technique (Poitevin et al. 2013). In this study, self-adhesive pit and fissure sealants incorporating zinc methacrylate or di-n-butyl-dimethacrylate-tin monomers (Figure 1) were successfully prepared via a simple and easy method using the monomer mixture as described in the Materials and methods. Table 1 summarizes the characteristics of the materials, including the degree of conversion, the translucency parameter, shear bond strength, flexural strength, and the depth of cure. The data revealed that there were no significant differences in the degree of conversion and translucency parameter among the composites tested in this study. As the incorporation of large amounts of antimicrobials can potentially harm the mechanical properties of the surface (Xue et al. 2015) and dental materials are subject to dynamic cyclic loading, it is critical to check whether the addition of biologically active components affects the mechanical strength of the composites. Interestingly, incorporation of zinc methacrylate or di-n-butyl-dimethacrylate-tin monomers did not compromise the shear bond strength, flexural strengths, nor the depth of cure (Table 1), indicating the high compatibility of these materials for dental application.
Table 1.
Degree of Conversion (DC), Translucency Parameter (TP), Shear Bond Strength, Flexural Strength, Elastic modulus and Depth of Cure of each group. Values in parenthesis indicate standard deviation. There are no significant differences in each parameter among groups.
| Group | Degree of Conversion (%) (n=3) | TP (n=5) | Shear bond strength (MPa) (n=10) | Flexural Strength MPa (n=10) | Elastic modulus GPa (n=10) | Depth of Cure (n=3) |
|---|---|---|---|---|---|---|
| ZnM 2.5% | 55.60 (0.7) | 70.7 (4.0) | 11.7 (4.4) | 59.24 (16.4) | 1.5 (0.2) | 5.8 (0.01) |
| ZnM 5% | 59.91 (1.9) | 59.4 (8.3) | 10.9 (3.3) | 48.71 (14.6) | 1.4 (0.1) | 5.8 (0.05) |
| SnM 2.5% | 58.77 (1.0) | 61.3 (4.5) | 12.4 (4.1) | 56.53 (10.9) | 1.4 (0.1) | 5.7 (0.1) |
| SnM 5% | 57.16 (2.1) | 70.7 (4.5) | 15.42 (4.1) | 49.98 (12.3) | 1.3 (0.1) | 5.8 (0.02) |
| Control | 50.24 (8.7) | 70.2 (4.5) | 10.6 (2.8) | 61.21 (6.9) | 1.4 (0.2) | 5.6 (0.1) |
Then, the anti-biofilm efficacy of the composite surfaces was tested using a virulent cariogenic pathogen, the well-characterized EPS-matrix producing, biofilm-forming, and acidogenic/aciduric strain S. mutans (Xiao et al. 2012). Figure 2 shows the anti-biofilm activity of the sealants against S. mutans biofilms. The data showed that ZnM 2.5% surface did not exhibit sufficient antibacterial efficacy against S. mutans biofilm (both CFU and dry weight (DW)), while ZnM 5% showed a significant improvement in the antibacterial activity (~40% reduction in CFU) with minimal reduction in the DW, compared with the control (Figure 2). Interestingly, further reductions in the CFU of S. mutans were observed from both SnM 2.5% and 5% surfaces (~70% reduction vs the control). However, SnM 5% exhibited a substantially lower DW than SnM 2.5% (>2-fold reduction). To evaluate the overall anti-biofilm efficacy of the sealants coated disc, a new factor reflecting both CFU and DW changes was introduced by multiplying fold reductions in the CFU and DW (Supplemental material Figure S1). As shown in Figure S1, the fold change in the CFU*DW of SnM 5% was close to 0 (compared with the control), indicating the outstanding anti-biofilm activity of the SnM 5%. Next, the anti-biofilm efficacy of the SnM 5% was visualized using confocal microscopy. To directly contrast the anti-biofilm effect, a half-portion of HA disc coated with SnM 5% sealant (another half-portion left without coating; bare HA) was utilized. The data showed that only the SnM 5% coated area (white-colored) effectively hampered S. mutans microcolony formation, while sizeable microcolonies were formed on the uncoated area (bare HA disc) (Supplementary material Figure S2). Representative SEM images of 42 h S. mutans biofilm formed on SnM 5% sealant also revealed that only small scattered bacterial clusters with a minimal amount of EPS were observed across the surface (Figure 3 top panels). In contrast, large dense bacterial clusters with an abundant EPS matrix were observed on the control surface (Figure 3 bottom panels). Collectively, the data indicate that SnM 5% has the capability to effectively inhibit biofilm development by reducing the number of bacteria and the total biomass. However, the mechanism of action of SnM 5% regarding biomass reduction is as yet unclear, ie, whether it suppresses EPS production per bacterium or whether it is simply due to the low number of active bacterial cells in the biofilms.
Figure 2.
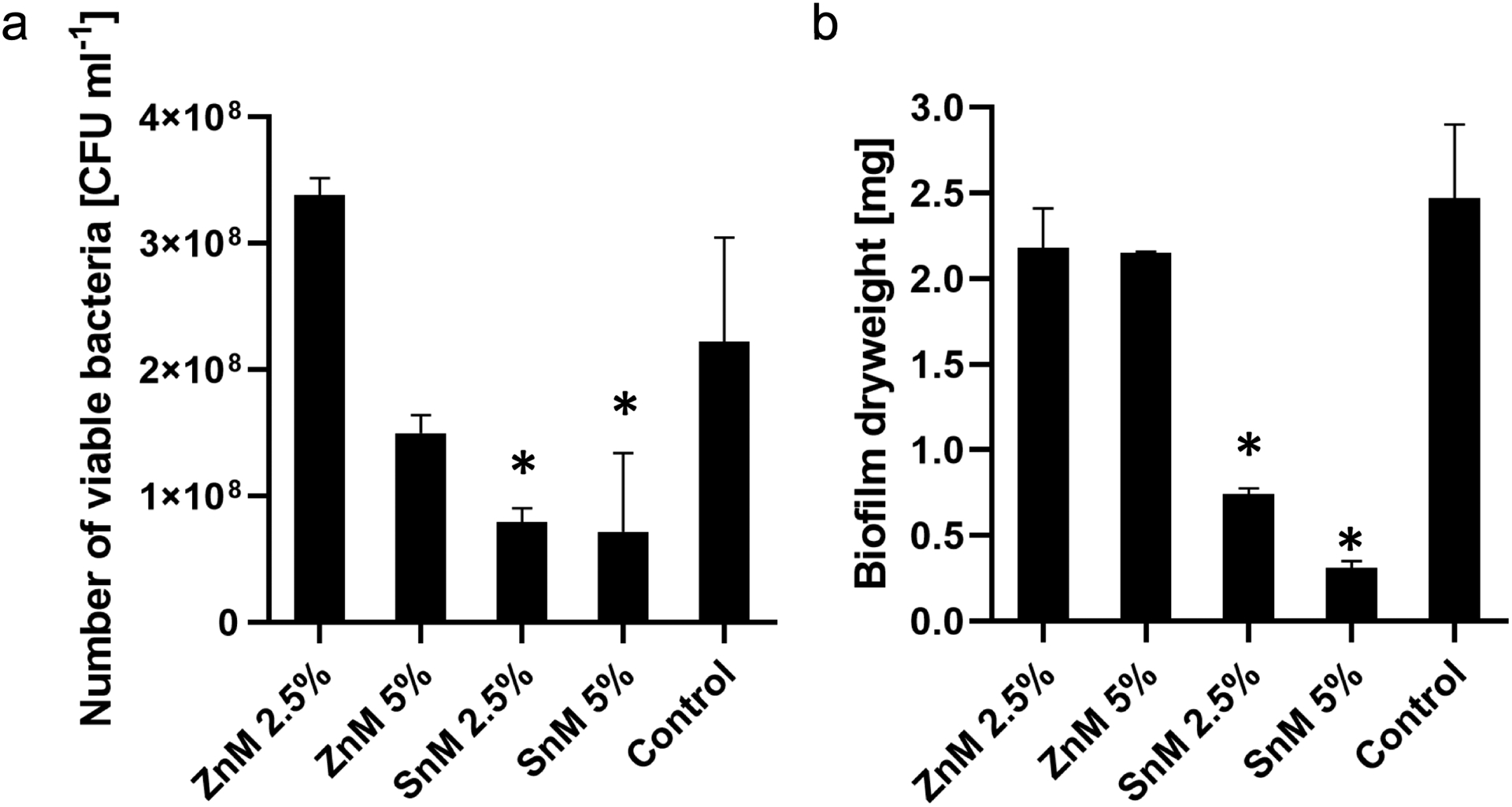
Microbiological data for 42 h old S. mutans biofilms formed on each surface. (a) The number of viable bacteria [CFU ml−1] and (b) the total dry weight [mg]. * indicates that the values are significantly different from those of the control surface (p < 0.05) (n=4).
Figure 3.
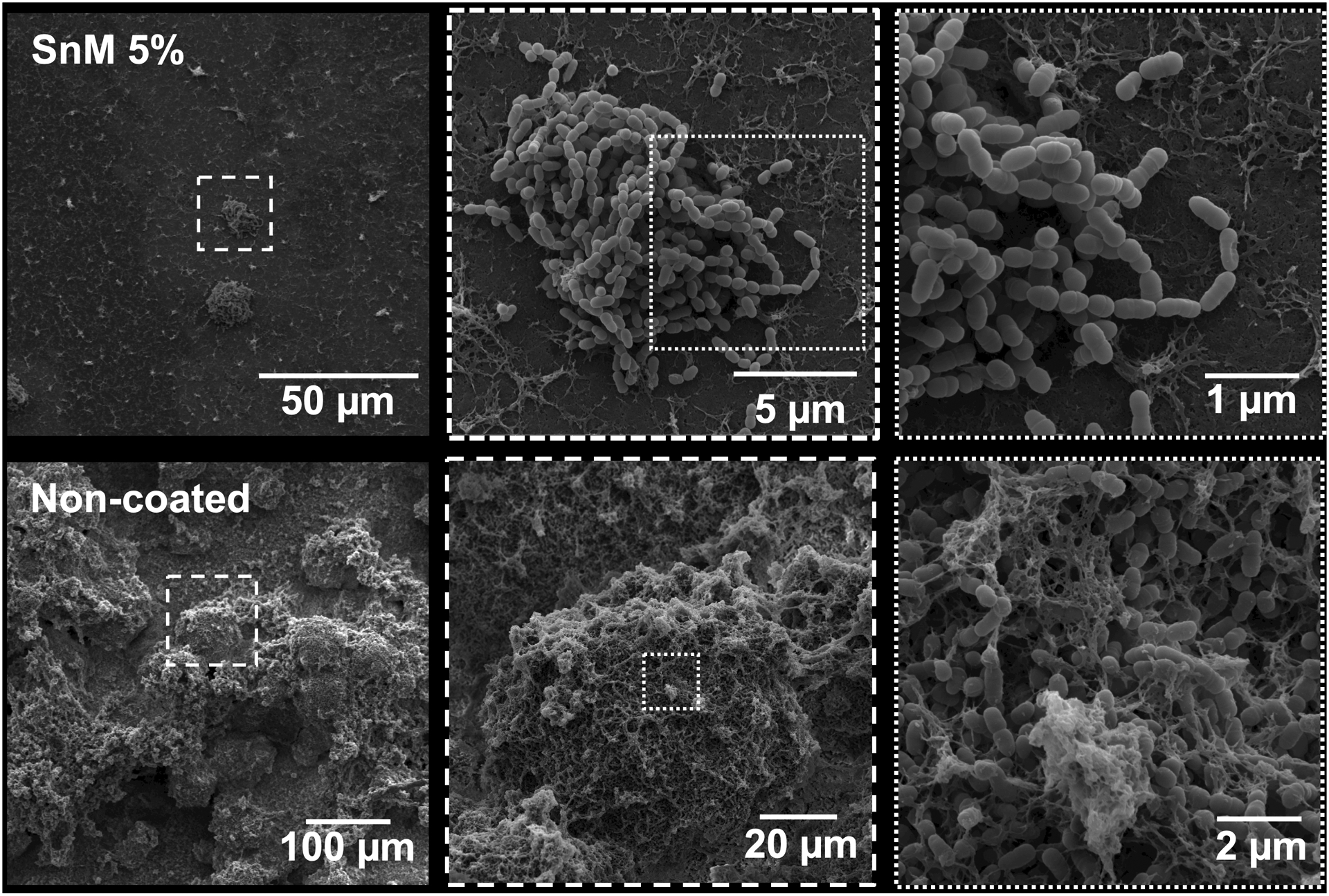
Representative SEM images of 42 h old S. mutans single-species biofilms formed on SnM 5% (top panels) and control surfaces (bottom panels).
In addition, the pH of the culture medium after biofilm harvesting (42 h) was examined (Table 2). There was no difference in the pH of the biofilm culture supernatant between control and ZnM groups, exhibiting an acidic pH (~ 4.5). Although ZnM 5% caused an ~ 40% reduction in the CFU, a similar acidic pH was detected from the culture supernatant of ZnM 5% as observed from the control. In contrast, the pH of the biofilm culture supernatant from SnM 2.5% was just slightly lower than the critical pH (5.5) that can cause tooth demineralization. Surprisingly, when the biofilm was cultured on SnM 5%, the pH of the culture supernatant did not drop but kept at neutral pH values close to 7. It is possible that either a low number of total bacteria or retarded metabolic activity of individual bacteria in biofilms formed on the SnM 5% surface may lessen the total amount of acids produced, thereby maintaining the pH of biofilm culture supernatant as neutral.
Table 2.
The average and standard deviation of the pH of the biofilm culture medium after harvesting (42 h).
| Groups | Single-species (n =4) | Dual-species (n=4) | ||
|---|---|---|---|---|
| Streptococcus mutans | Streptococcus oralis | Candida albicans | Streptococcus mutans and Candida albicans | |
| ZnM 2.5% | 4.55 (0.03) | - | - | - |
| ZnM 5% | 4.65 (0.05) | - | - | - |
| SnM 2.5% | 5.44 (0.09)* | - - |
- | - |
| SnM 5% | 6.68 (1.0)* | 6.5 (0.4)* | 6.8 (0.1) | 6.6 (0.3)* |
| Control | 4.54 (0.01) | 5.7 (0.9) | 6.8 (0.1) | 4.4 (0.1) |
indicates significant differences (p<0.05).
As broad-spectrum antibacterial (Salam et al. 2012; Rubin Cocco et al. 2018) and antifungal (Dylg et al. 2010) activities of organotin (IV) compounds have been reported, the anti-biofilm activities of the SnM 5% on another oral bacterium and fungal cell were assessed to further evaluate the potential clinical application of the SnM 5% sealant. As another oral bacterium, S. oralis, an early colonizer and acidogenic strain (Fujiwara et al. 2000) was chosen and C. albicans, an opportunistic fungal pathogen associated with severe early childhood caries (ECC) (Yang et al. 2012; Xiao et al. 2016; Xiao et al. 2018) was selected as a fungal organism. S. oralis or C. albicans single-species biofilm on control or SnM 5% surface was formed and microbiological assays (CFU and DW) were performed. As observed from the S. mutans biofilm experiments, a substantial reduction in the viable microorganisms and total biomass of S. oralis or C. albicans single-species biofilm formed on SnM 5% was observed (vs the control; Figure 4). Also, the pHs of the biofilm culture supernatant from SnM 5% (either S. oralis or C. albicans) were significantly higher than those from the control (Table 2). The data indicate the broad-spectrum antibacterial and antifungal activities of the SnM 5% sealant.
Figure 4.
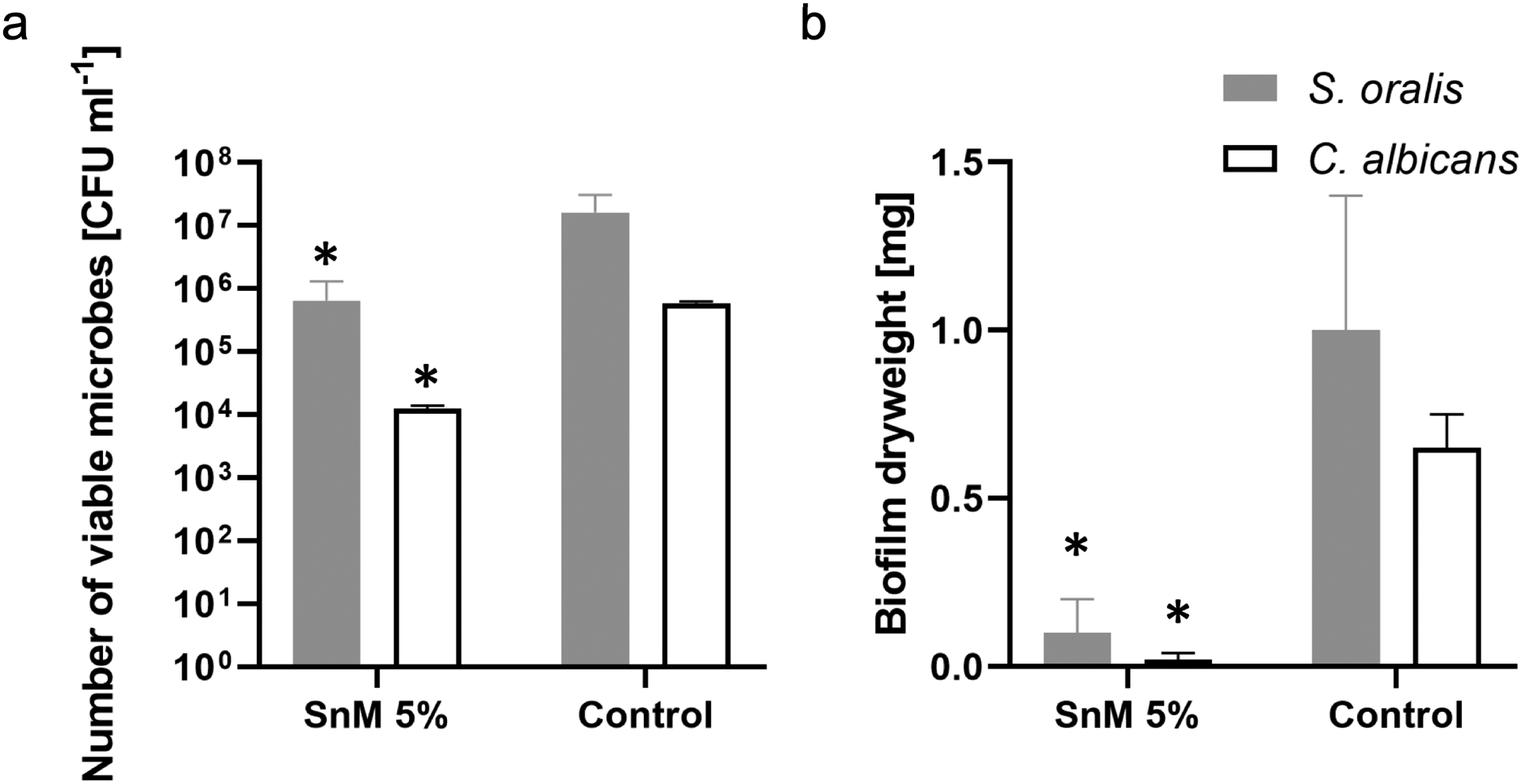
Microbiological data for 42 h old S. oralis or C. albicans single-species biofilms formed on SnM 5% or the control surface. (a) The number of viable microbial cells [CFU ml−1] and (b) the total dry weight [mg]. * indicates that the values are significantly different from those of the control surface (p < 0.05) (n=4).
By recognizing the importance of bacterial-fungal interaction in the context of ECC, the anti-biofilm efficacy of SnM 5% against S. mutans-C. albicans dual-species biofilm was assessed. As observed in previous studies (Falsetta et al. 2014; Hwang, Liu, et al. 2017), the co-culture of S. mutans and C. albicans resulted in a significant increase in the CFU and the DW in dual-species biofilms, compared with S. mutans single-species biofilms (Figure 5). Surprisingly, the SnM 5% successfully disrupted the cross-kingdom biofilm formation and development, showing log-reductions of microbial cells, substantial reductions in the DW (Figure 5), and elevation of the pH of the biofilm culture supernatant close to neutral pH (Table 2). In summary, such strong anti-biofilm activity of SnM 5% against various single-species and polymicrobial biofilms reveals its potential for clinical application.
Figure 5.
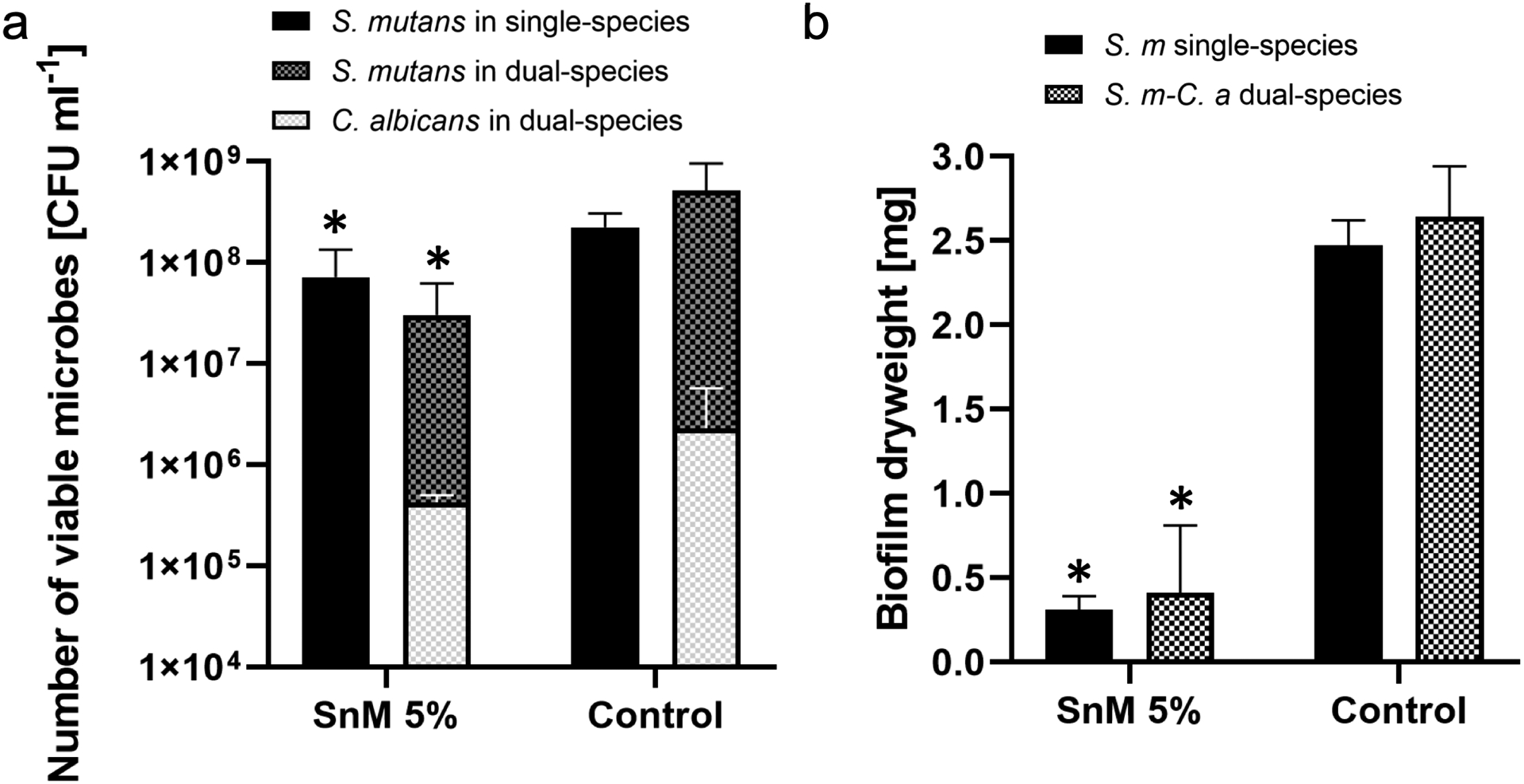
Microbiological data for 42 h old S. mutans single-species or S. mutans-C. albicans dual-species biofilms formed on SnM 5% or the control surface. (a) The number of viable microbial cells [CFU ml−1] and (b) the total dry weight [mg]. * indicates that the values are significantly different from those of the control surface (p < 0.05) (n=4).
A number of mechanisms concerning the biological action of organotin derivatives have been proposed. For example, the mode of action of organotin (IV) compounds was suggested whereby a hydrogen bond is formed with the active centers of the bacterial cell constituents, thereby interfering with normal cell processes such as enzyme production (Baul 2008). Furthermore, those bacterially-produced enzymes normally require free sulfhydryl groups (-SH) for their activity, while it can be easily deactivated by ions of the Sn (Baul 2008). Thus, it is conceivable that the production and/or function of enzymes associated with the metabolic activity of streptococci (S. mutans or S. oralis) might be severely disrupted by SnM 5% sealant. With respect to antifungal activity, it was proposed that crossing over of an organotin compound through the cytoplasmic membrane of yeast cells might be involved in the biological activities (White & Tobin 2004; Buck-Koehntop et al. 2006). Additionally, study of the structure-activity relationships revealed a connection between the antimicrobial activity and the coordination environment of the tin atom such as the type and/or the number of organic fragments (Menezes et al. 2008). In this study, di-n-butyldimethacrylate-tin exhibited a superior biofilm inhibition effect, possibly due to the fact that the butyl group is lipophilic which can enhance the penetration of the compounds through the cell membrane, thereby reaching the target sites efficiently (López-Torres et al. 2011; Hadi et al. 2019). However, a complete understanding is still elusive and there are many factors affecting the biological function of organotin compounds, therefore further studies on the mechanism of action need to be performed.
Finally, it is important to emphasize that the incorporation of metallic monomers into the self-adhesive pit and fissure sealants did not show cytotoxicity against fibroblast cells (Figure 6). Other studies also found similar results when 1% Sn methacrylate or 5% Zn methacrylate were added to adhesive systems (Henn et al. 2011; Rubin Cocco et al. 2018). However, additional studies must be performed to better evaluate the effects of the experimental materials under more clinically relevant conditions using other cell lines, such as human gingival fibroblasts, dental pulp fibroblasts/stem cells, and gingival epithelial cells.
Figure 6.
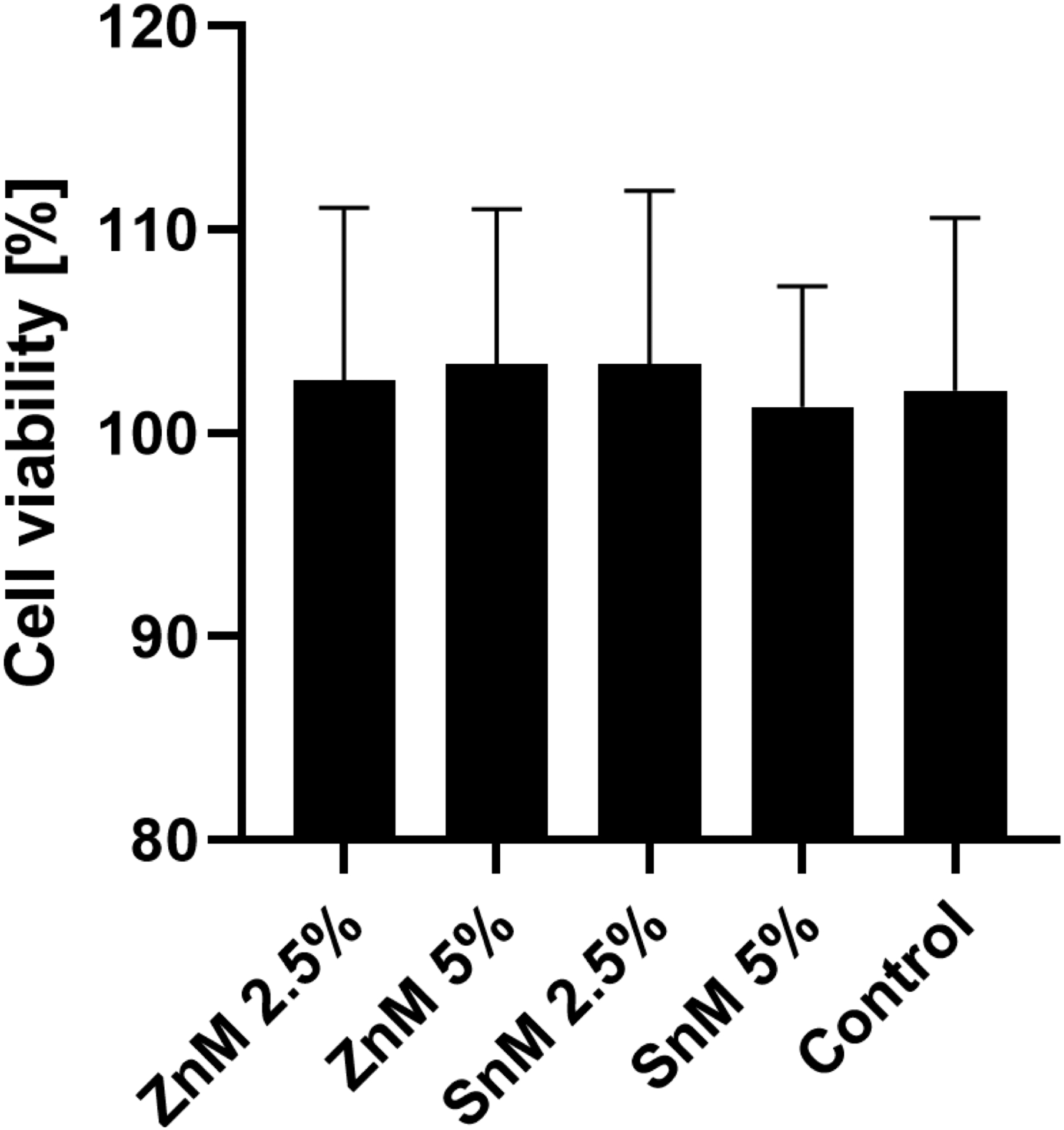
Cell viability of L929 fibroblasts on each surface. There are no significant differences among groups (n=6).
Conclusion
Self-adhesive pit and fissure sealants incorporating zinc methacrylate or di-n-butyldimethacrylate-tin were developed. The sealant modified with 5% di-n-butyldimethacrylate-tin (SnM 5%) presented adequate physical and mechanical properties without causing cytotoxicity so as to be an effective dental restorative material. SnM 5% containing sealant also demonstrated strong antibacterial and antifungal activities against various single-species and cross-kingdom dual-species oral biofilms. Di-n-butyldimethacrylate-tin (SnM 5%) may be considered as a potent agent that can be added to the current chemotherapeutic strategies to prevent the formation of cariogenic biofilms that cause tooth decay.
Supplementary Material
Figure S1. Fold changes of CFU*DW of 42 h old S. mutans biofilms formed on each surface. * indicates that the values are significantly different from those of the control surface (p < 0.05) (n=4).
Figure S2. Representative confocal images of 42 h old S. mutans single-species biofilms formed on hydroxyapatite (HA) disc with or without SnM 5% coating. There are no visible biofilms formed on SnM 5% coated area (white), while sizeable microcolonies were observed on the non-coated bare HA disc surface.
Acknowledgments
The authors thank Dr. Hyun (Michel) Koo for suggestions and discussion.
Funding
This study was supported in part by the National Institutes for Dental and Craniofacial Research (NIDCR) grant (DE027970 (GH)). The authors would like to thank CAPES (Higher Education Personnel Improvement Coordination) for granting a scholarship to the first author. Also, the authors thank the Brazilian National Council for Scientific and Technological Development (CNPq) grants (310879/2015-9 and 309848/2017-2), and the study and project funding agency (FINEP), grant (01.10.0709.05) for financial support.
References
- Baul TSB. 2008. Antimicrobial activity of organotin(IV) compounds: a review. Appl Organomet Chem. 22:195–204. [Google Scholar]
- Bowen WH, Koo H. 2011. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck-Koehntop BA, Porcelli F, Lewin JL, Cramer CJ, Veglia G. 2006. Biological chemistry of organotin compounds: interactions and dealkylation by dithiols. J Organomet Chem. 691:1748–1755. [Google Scholar]
- Busscher HJ, Rinastiti M, Siswomihardjo W, Van der Mei HC. 2010. Biofilm formation on dental restorative and implant materials. J Dent Res. 89:657–665. [DOI] [PubMed] [Google Scholar]
- De Carvalho F, Puppin-Rontani J, Dos Santos R, Carlo H, Bonan P, Garcia-Godoy F, Puppin-Rontani R. 2014. In vitro effect of S. mutans biofilm on fluoride/MDPB-containing adhesive system bonded to caries-affected primary dentin. Am J Dent. 27:227–232. [PubMed] [Google Scholar]
- Dye B a, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltrán-Aguilar ED, Horowitz AM, Li C-H. 2007. Trends in oral health status: United States, 1988–1994 and 1999–2004. National Center for Health Statistics. Vital Health Stat 11(248). 2007 [place unknown]. [PubMed] [Google Scholar]
- Dylg M, Pruchnik H, Pruchnik F, Majkowska-Skrobek G, Ułaszewski S. 2010. Antifungal activity of organotin compounds with functionalized carboxylates evaluated by the microdilution bioassay in vitro. Med Mycol. 48:373–383. [DOI] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, Koo H. 2014. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 82:1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone JD, Doméjean S. 2012. The role of remineralizing and anticaries agents in caries management. Adv Dent Res. 24:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Hoshino T, Ooshima T, Sobue S, Hamada S. 2000. Purification, characterization, and molecular analysis of the gene encoding glucosyltransferase from Streptococcus oralis. Infect Immun. 68:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi AG, Jawad K, Ahmed DS, Yousif E. 2019. Synthesis and biological activities of organotin (IV) carboxylates: a review. Syst Rev Pharm. 10:26–31. [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2:95–108. [DOI] [PubMed] [Google Scholar]
- Han L, Okamoto A, Fukushima M, Okiji T. 2007. Evaluation of physical properties and surface degradation of self-adhesive resin cements. Dent Mater J. 26:906–914. [DOI] [PubMed] [Google Scholar]
- Henn S, Nedel F, De Carvalho RV, Lund RG, Cenci MS, Pereira-Cenci T, Demarco FF, Piva E. 2011. Characterization of an antimicrobial dental resin adhesive containing zinc methacrylate. J Mater Sci Mater Med. 22:1797–1802. [DOI] [PubMed] [Google Scholar]
- Hirose N, Kitagawa R, Kitagawa H, Maezono H, Mine A, Hayashi M, Haapasalo M, Imazato S. 2016. Development of a cavity disinfectant containing antibacterial monomer MDPB. J Dent Res. 95:1487–1493. [DOI] [PubMed] [Google Scholar]
- Hwang G, Klein MI, Koo H. 2014. Analysis of the mechanical stability and surface detachment of mature Streptococcus mutans biofilms by applying a range of external shear forces. Biofouling. 30:1079–1091. [DOI] [PubMed] [Google Scholar]
- Hwang G, Koltisko B, Jin X, Koo H. 2017. Nonleachable imidazolium-incorporated composite for disruption of bacterial clustering, exopolysaccharide-matrix assembly, and enhanced biofilm removal. ACS Appl Mater Interfaces. 9:38270–38280. [DOI] [PubMed] [Google Scholar]
- Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. 2017. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 13:e1006407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 10993–5. 2009. Biol Eval Med devices — Part 5 Tests Vitr Cytotox [Internet]. https://www.iso.org/standard/36406.html
- ISO 6874. 2015. Dent — Polym pit Fiss sealants [Internet]. https://www.iso.org/obp/ui/#iso:std:iso:6874:ed-3:v1:en
- Johnston WM, Reisbick MH. 1997. Color and translucency changes during and after curing of esthetic restorative materials. Dent Mater. 13:89–97. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Son HH, Cho BH, Lee IB, Um CM. 2009. Translucency and masking ability of various opaque-shade composite resins. J Dent. 37:102–107. [DOI] [PubMed] [Google Scholar]
- Kitagawa R, Kitagawa H, Izutani N, Hirose N, Hayashi M, Imazato S. 2014. Development of an antibacterial root canal filling system containing MDPB. J Dent Res. 93:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MI, Hwang G, Santos PHS, Campanella OH, Koo H. 2015. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. 2017. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 15:740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Xiao J, Klein MI, Jeon JG. 2010. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 192:3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnisch J, Mansmann U, Heinrich-Weltzien R, Hickel R. 2012. Longevity of materials for pit and fissure sealing - results from a meta-analysis. Dent Mater. 28:298–303. [DOI] [PubMed] [Google Scholar]
- Lebeaux D, Ghigo J-M, Beloin C. 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 78:510–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kamesh AC, Xiao Y, Sun V, Hayes M, Daniell H, Koo H. 2016. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials. 105:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 50:353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Torres E, Zani F, Mendiola MA. 2011. Antimicrobial activity of organotin(IV) complexes with the ligand benzil bis(benzoylhydrazone) and 4,4′-bipyridyl as coligand. J Inorg Biochem. 105:600–608. [DOI] [PubMed] [Google Scholar]
- Menezes DC, Vieira FT, De Lima GM, Wardell JL, Cortés ME, Ferreira MP, Soares MA, Vilas Boas A. 2008. The in vitro antifungal activity of some dithiocarbamate organotin(IV) compounds on Candida albicans - a model for biological interaction of organotin complexes. Appl Organomet Chem. 22:221–226. [Google Scholar]
- Mickenautsch S, Yengopal V. 2016. Caries-preventive effect of high-viscosity glass ionomer and resin-based fissure sealants on permanent teeth: a systematic review of clinical trials. PLoS One. 11:e0146512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münchow EA, Bossardi M, Priebe TC, Valente LL, Zanchi CH, Ogliari FA, Piva E. 2013. Microtensile versus microshear bond strength between dental adhesives and the dentin substrate. Int J Adhes Adhes. 46:95–99. [Google Scholar]
- Ogliari FA, de Sordi MLT, Ceschi MA, Petzhold CL, Demarco FF, Piva E. 2006. 2,3-Epithiopropyl methacrylate as functionalized monomer in a dental adhesive. J Dent. 34:472–477. [DOI] [PubMed] [Google Scholar]
- Papageorgiou SN, Dimitraki D, Kotsanos N, Bekes K, van Waes H. 2017. Performance of pit and fissure sealants according to tooth characteristics: a systematic review and meta-analysis. J Dent. 66:8–17. [DOI] [PubMed] [Google Scholar]
- Peterson BW, He Y, Ren Y, Zerdoum A, Libera MR, Sharma PK, van Winkelhoff AJ, Neut D, Stoodley P, van der Mei HC, Busscher HJ. 2015. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev. 39:234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitevin A, De Munck J, Van Ende A, Suyama Y, Mine A, Peumans M, Van Meerbeek B. 2013. Bonding effectiveness of self-adhesive composites to dentin and enamel. Dent Mater. 29:221–230. [DOI] [PubMed] [Google Scholar]
- Rubin Cocco A, de Oliveira da Rosa WL, Luque Peralta S, Timm Maske T, da Silva AF, Andrade Hartwig C, Foster Mesko M, Piva E, Guerra Lund R. 2018. New adhesive system based in metals cross-linking methacrylate. J Mech Behav Biomed Mater. 77:519–526. [DOI] [PubMed] [Google Scholar]
- Salam MA, Affan MA, Saha R, Ahmad FB, Sam N. 2012. Synthesis, characterization and in vitro antibacterial studies of organotin(IV) complexes with 2-hydroxyacetophenone-2- methylphenylthiosemicarbazone (H 2 dampt). Bioinorg Chem Appl. 2012:698491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwitz RH, Ismail AI, Pitts NB. 2007. Dental caries. The Lancet. 369:51–59. [DOI] [PubMed] [Google Scholar]
- Simonsen RJ. 2011. From prevention to therapy: minimal intervention with sealants and resin restorative materials. J Dent. 39:S27–S33. [DOI] [PubMed] [Google Scholar]
- Song F, Koo H, Ren D. 2015. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 94:1027–1034. [DOI] [PubMed] [Google Scholar]
- Weintraub JA. 2001. Pit and fissure sealants in high-caries-risk individuals. J Dent Educ. 65:1084–1090. [PubMed] [Google Scholar]
- White JS, Tobin JM. 2004. Role of speciation in organotin toxicity to the yeast Candida maltosa. Environ Sci Technol. 38:3877–3884. [DOI] [PubMed] [Google Scholar]
- Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, Castillo DA, Campbell F, Davis J, Herzog K, et al. 2018. Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res. 52:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, Heydorn A, Koo H. 2012. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 8:e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Moon Y, Li L, Rustchenko E, Wakabayashi H, Zhao X, Feng C, Gill SR, McLaren S, Malmstrom H, et al. 2016. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PLoS One. 11:e0164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Xiao H, Zhang Y. 2015. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int J Mol Sci. 16:3626–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XQ, Zhang Q, Lu LY, Yang R, Liu Y, Zou J. 2012. Genotypic distribution of Candida albicans in dental biofilm of Chinese children associated with severe early childhood caries. Arch Oral Biol. 57:1048–1053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Fold changes of CFU*DW of 42 h old S. mutans biofilms formed on each surface. * indicates that the values are significantly different from those of the control surface (p < 0.05) (n=4).
Figure S2. Representative confocal images of 42 h old S. mutans single-species biofilms formed on hydroxyapatite (HA) disc with or without SnM 5% coating. There are no visible biofilms formed on SnM 5% coated area (white), while sizeable microcolonies were observed on the non-coated bare HA disc surface.


