Abstract
Artificial enzyme mimics have gained considerable attention for use in sensing applications due to their high stability and outstanding catalytic activity. We show that cerium oxide nanosheets (NSs) exhibit triple-enzyme mimetic activity. The oxidase-, peroxidase-, and catalase-like activities of the proposed nanoparticles are demonstrated using both colorimetric and electron paramagnetic resonance (EPR) spectroscopy. On the basis of the excellent catalytic activity of cerium oxide NSs toward hydrogen peroxide, an electrochemical approach for the high-throughput detection of H2O2 in living cells was established. This report presents an analytical microfluidic chip integrated with a cerium oxide NS mimic enzyme for the fabrication of a simple, sensitive, and low-cost electrochemical sensor. Three Au microelectrodes were fabricated on a glass substrate using photolithography, and the working electrode was functionalized using cerium oxide NSs. The operation of this biosensor is based on cerium oxide NSs and presents a high sensitivity over a wide detection range, between 100 nM and 20 mM, with a low detection limit of 20 nM and a high sensitivity threshold of 226.4 μA·cm–2·μM–1. This microfluidic sensor shows a strong response to H2O2, suggesting potential applications in monitoring H2O2 directly secreted from living cells. This sensor chip provides a promising platform for applications in the field of diagnostics and sensing.
1. Introduction
Since the first exciting discovery of ferromagnetic nanoparticles,1 various efficient nanomaterial-based mimic enzymes (nanozymes)2 have been developed over the past few decades. Such nanomaterials can catalyze specific redox-like-type reactions and exhibit great activity for oxidase-like,3,4 peroxidase-like,5−7 catalase-like,8,9 or superoxide dismutase-like (SOD) reactions.10,11 To date, there have been numerous works devoted to exploring nanomaterials’ enzyme mimetics from carbon- and metal-based nanomaterials.12,13 Recently, metal nanomaterials have become an area of increasing interest because of unique electronic and a larger variety of enzyme-like characteristics.14,15 These nanozymes are widely used in sensing and diagnosis applications.16 For example, Wang et al. developed a direct electrochemical assay for kanamycin detection based on the peroxidase-like activity of gold nanoparticles.17 AuNPs could catalyze the reaction between H2O2 and reduced thionine to produce oxidized thionine. This reaction exhibited a distinct reduction peak on gold electrode in differential pulse voltammetry (DPV) and could be utilized to quantify the concentration of kanamycin. Furthermore, Wang and his colleagues fabricated FePt–Au ternary metallic nanoparticles with powerful enzymatic mimic for H2O2 sensing.15 Among nanozymes, multiactivity nanozymes with two or more catalytic activities have attracted significant attention.18,19 Some nanozymes such as Co3O4,20 Ni–Pd NPs,21 CoMo hybrids,22 and V6O1323 have been reported with two or more catalytic activities. These kinds of nanozymes can have more effective applications in physiological and pathological processes.
Cerium oxide nanoparticles (nanoceria) have attracted enormous interest in recent years as nanocatalysts due to their unique physical and chemical properties. Nanoceria has been widely applied in various fields, such as catalysis, bioassays, and antioxidant therapy.24,25 This rare-earth oxide nanostructure shows high catalytic performance in various applications due to the presence of mixed valence states of Ce3+ and Ce4+, and the presence of oxygen vacancies. The key to this catalytic activity is that the redox couple can switch between each state in a CeO2 ↔ CeO2–x + x/2O2 (Ce4+ ↔ Ce3+) recycle process.26,27 The catalytic activity of nanoceria originates from the surface oxygen; thus, the active oxygen content on the surface must be increased to improve catalytic activities. As a result, increasing the Ce3+/(Ce3+ + Ce4+) ratio (shorted as “Ce3+ ratio”) enhances the surface oxygen defect in the structure, leading to improvement in catalytic properties.28,29
It is worth noting that H2O2 has a considerable impact on food production, textile industry, paper bleaching, pharmaceutical research, and environment pollution.30,31 It is a byproduct of various enzymatic reactions including glucose oxidase, cholesterol oxidase, glutamate oxidase, urate oxidase, lactate oxidase, alcohol oxidase, lysine oxidase, oxalate oxidase, and horseradish peroxidase.32 In living organisms, H2O2 regulates diverse biological processes such as immune cell activation, vascular remodeling, apoptosis, and root growth. The presence of excess H2O2 in living organisms also causes severe diseases like cancer and Parkinson’s disease.33,34 The determination of H2O2 in biological environments is of critical importance. Electrochemical methods have attracted great interest over competing H2O2 detection techniques such as chromatography,35 chemiluminescence,36 colorimetry,37 and fluorescence,38 due to their high sensitivity, fast response, low cost, and convenient operation.39,40
The microfluidic lab-on-a-chip (LOC) technology is recognized as one of the most promising tools to develop novel diagnostic platforms.41 Microfluidic chips can be applied as point-of-care (POC) devices for clinical diagnostics because of their inherent small size, portability, low cost, easy operation, and low amount of biological sample required.42,43 These devices include a set of microfluidic channels to control fluid flow throughout the chip, in which various procedures such as reagent mixing, affinity-based binding, and signal transduction can be implemented side-by-side.44 Sensors can be integrated within microfluidic devices to enable continuous measurement of single or multiple analytes in small sample volumes.45
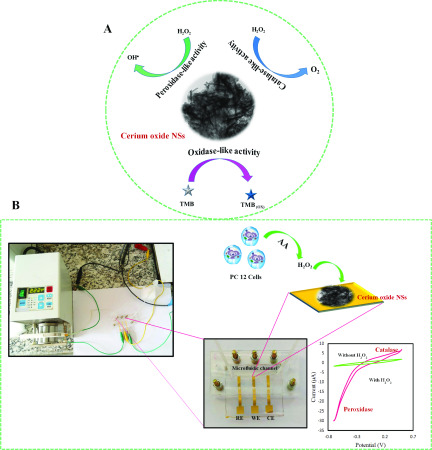
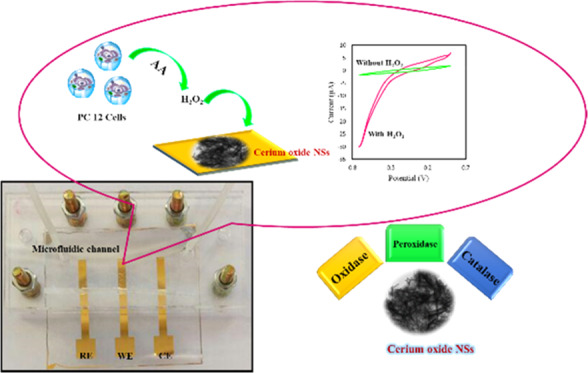
In this work, we present a microfluidic electrochemical LOC for the real-time detection of H2O2 using a cerium oxide nanosheet (NS)-modified Au working electrode. Cerium oxide NSs were synthesized via a simple hydrothermal route, and they simultaneously displayed oxidase-, peroxidase-, and catalase enzyme-like activities (Scheme 1). Cerium oxide NSs were integrated with a microfluidic platform for the effective detection of H2O2. This sensor is found to be highly selective and specific toward H2O2 with negligible interference from analytes such as glucose, dopamine, uric acid, glutathione, and ascorbic acid. Furthermore, cerium oxide NS-based LOC devices can find practical use in monitoring H2O2 inside living cells, which is indicative of their viability in real-world analysis applications.
Scheme 1. (A) Mimicking Three Different Enzymes Peroxidase, Catalase, and Oxidase by Individual Material Cerium Oxide, (B) Schematic Representation and Photograph of Lab-on-a-Chip Setup for the Electrocatalytic Reduction of H2O2.
2. Results and Discussion
2.1. Structure Characterization of Prepared Cerium Oxide
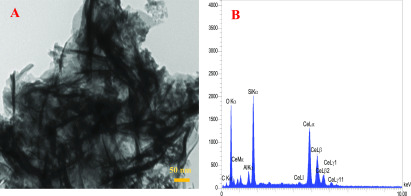
The morphology of cerium oxide was investigated by transmission electron microscopy (TEM). Figure 1A shows the wrinkled nanosheet structure of the prepared cerium oxide, which shows a large surface area for the reaction with H2O2. The energy-dispersive spectrometry (EDS) analysis of cerium oxide NSs revealed their elemental composition and corroborated the presence of Ce, C, and O in these nanostructures (Figure 1B).
Figure 1.
(A) TEM image and (B) EDS spectra of cerium oxide NSs.
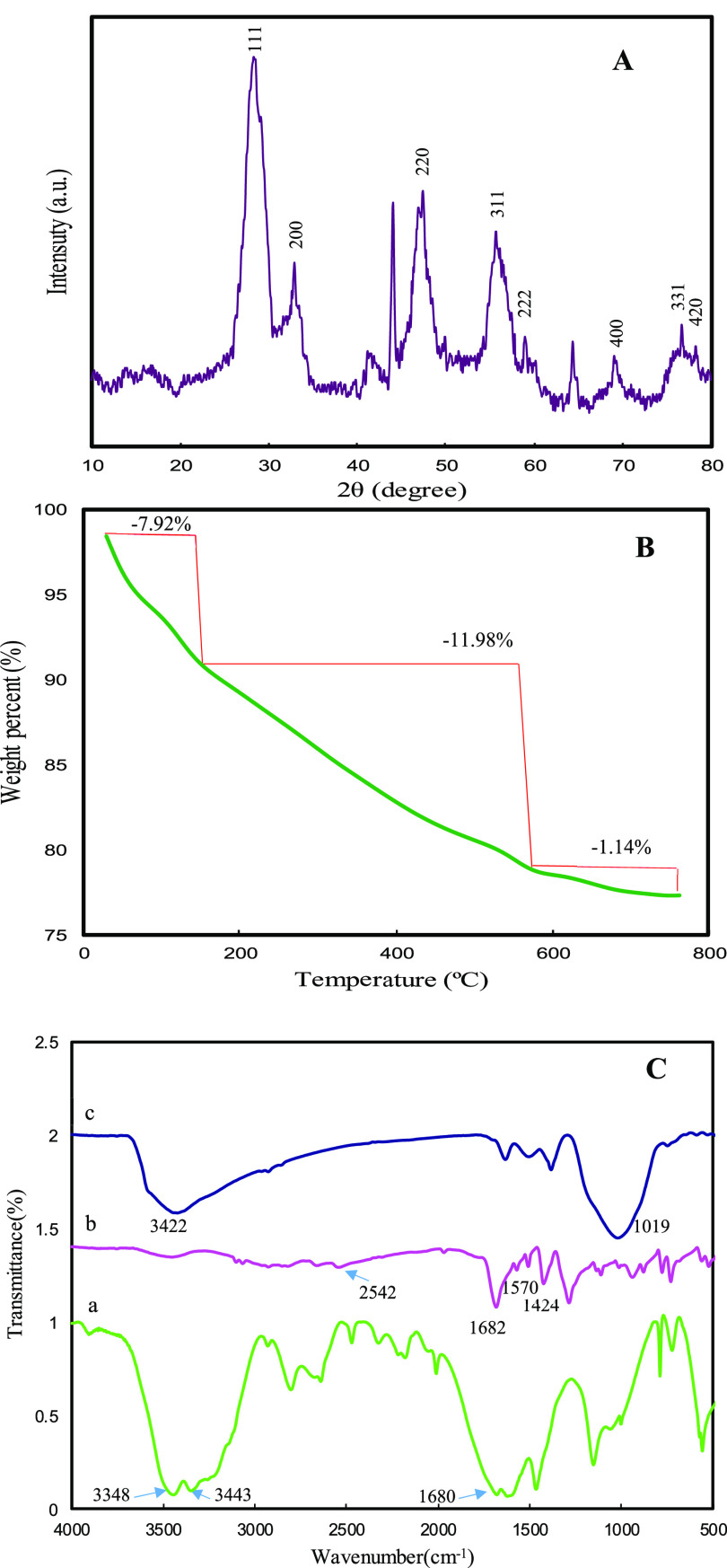
Figure 2A shows the X-ray diffraction (XRD) patterns of cerium oxide NSs. The XRD peaks are located at angles (2θ) of 28.6, 32.84, 47.27, 55.83, 59.09, 69.02, 76.74, and 78.64°, corresponding to the (111), (200), (220), (311), (222), (400), (331), and (420) planes of CeO2 in the face-centered cubic phase (JCPDS data card no: 34-0394).46 Peaks at angles 2θ = 44.12 and 64.42° can be assigned to Ce2O(CO3)2·H2O.47 The formation of Ce2O(CO3)2·H2O is due to the reaction of the Ce3+ ions from cerium nitrate hexahydrate with the CO32– and OH– ions from the hydrolysis of urea and terephthalic acid.48 Ce2O(CO3)2·H2O improves the Ce3+/(Ce3+ + Ce4+) ratio in the structure and promotes the formation of oxygen vacancy. Significantly, oxygen vacancies enhance the redox ability and allow easier surface reaction, which is in favor of catalytic reaction.29 The thermal stability of cerium oxide NSs was investigated through thermogravimetric analysis (TGA). As can be seen from Figure 2B, weight loss occurs by the following three steps: (1) release of physically adsorbed water, with 7.9% weight loss from room temperature to 160 °C; (2) decomposition of cerium carbonate to form ceria, with 12.0% weight loss from 160 to 470 °C, which is representative of the surface Ce3+/(Ce3+ + Ce4+) ratio; and (3) minimum weight loss above 470 °C, which can be attributed to the removal of captured CO2.49Figure 2C shows the Fourier transform infrared (FTIR) spectra of urea, terephthalic acid, and cerium oxide NSs. Urea has characteristic vibrational peaks at 3348 and 3443 cm–1 (NH2 group stretching) and at 1680 cm–1 (−C=O group stretching) (curve a).50 Peaks from terephthalic acid, at 2542, 1682, and 1570–1424 cm–1, are assigned to −COOH, −C=O, and an aromatic ring of the terephthalic acid, respectively. Concerning the IR spectrum of terephthalic acid (curve b) peaks in the 1285–1000 cm–1 region are fingerprints of −C–OH, −C=O, −C–CH, and −C–H bending modes, while the 700–800 cm–1 region contains the terephthalic acid aromatic ring bending mode.51 The IR spectrum of cerium oxide NSs (curve c) is different from its counterpart from reagents, in which most of the IR peaks are not present. Specifically, the broad IR absorption band 3422 cm–1 in the spectra of cerium oxide NSs is assigned to O–H stretching modes from residual water and Ce–OH, which is present in nanostructured cerium oxide because of its higher surface-to-volume ratio. Likewise, the same effect may be responsible for the absorption peak observed at 1019 cm–1, which can be attributed to C–O–Ce and is also not present in bulk cerium oxide.
Figure 2.
(A) XRD patterns. (B) TGA analysis. (C) FTIR spectra of (a) urea, (b) terephthalic acid, and (c) cerium oxide NSs.
2.2. Triple-Enzyme Catalytic Activity of Cerium Oxide NSs
To investigate the triple-enzyme catalytic activity of cerium oxide NSs and their oxidase-, peroxidase-, and catalase-like activities, a series of experiments were carried out as indicated in the following subsections.
2.2.1. Peroxidase-like Catalytic Activity of Cerium Oxide NSs
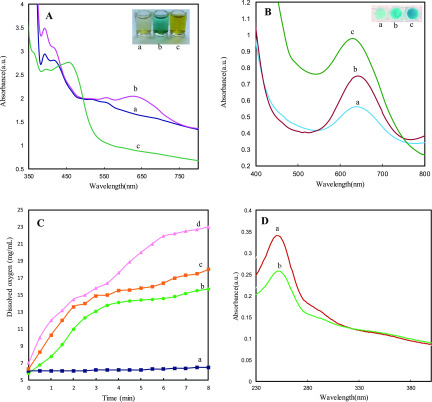
The peroxidase-like catalytic activity of cerium oxide NSs was investigated by catalyzing the oxidation of 3,3′,5,5 tetramethylbenzidine (TMB) in the presence of H2O2. As shown by curve a in Figure 3A, as-synthesized cerium oxide NSs show strong optoelectronic absorption in the visible region between 395 and 420 nm. After the addition of cerium oxide NSs to an aqueous solution of TMB + H2O2, an additional strong adsorption peak at 652 nm is observed, and the color of the solution turned blue (Figure 3A, curve b). After adding H2SO4, the adsorption peak at 652 nm disappears with the appearance of a peak at 450 nm and the color of the solution changed from blue to yellow (Figure 3A, curve c).
Figure 3.
(A) UV–vis spectra and photographs of (a) cerium oxide NSs solution, (b) cerium oxide NSs + TMB + H2O2, and (c) cerium oxide NSs + TMB + H2O2 + H2SO4 (concentration of enzyme: 2 mg·mL–1, reaction time: 5 min). (B) Effect of O2 concentration on the direct oxidation of TMB by cerium oxide without H2O2 ((a) N2, (b) air, (c) O2). (C) Dissolved oxygen generation catalyzed by cerium oxide NSs at different concentrations (a) 0 mg·mL–1, (b) 1 mg·mL–1, (c) 2 mg·mL–1, and (d) 4 mg·mL–1. (D) UV–vis spectra of (a) NBT and (b) NBT + cerium oxide NSs + H2O2.
The oxidation reaction was catalyzed
by peroxidase, but the catalytic
activity of cerium oxide could be stopped by H2SO4, leading to the cation radical of the TMB molecule, which further
lost another electron to form diamine.52 These changes could be expressed as follows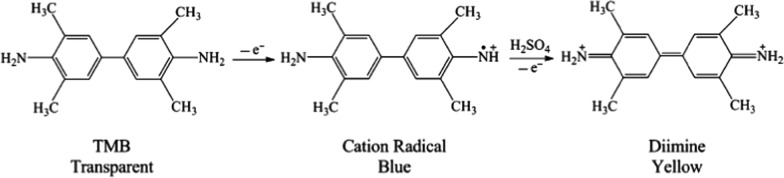
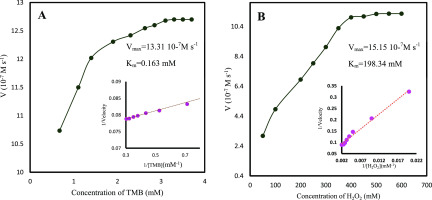
No color change was observed in the absence of cerium oxide NSs. This clearly shows that cerium oxide NSs showed peroxidase-like catalytic activity. Steady-state kinetic experiments were performed to further investigate the peroxidase-like catalytic property of cerium oxide NSs. As displayed in Figure 4, the initial rate versus TMB and H2O2 concentrations both followed with typical Michaelis–Menten behaviors in a certain range of substrate concentration. The Michaelis–Menten constant (Km) and maximum initial velocity (Vmax) were calculated using the Lineweaver–Burk plot (inserted Figure 4). It is known that the lower Km value reflects a higher affinity between enzymes and substrates.
Figure 4.
Steady-state kinetic analyses using the Michaelis–Menten model and Lineweaver–Burk model (insets) for cerium oxide NSs by (A) TMB as substrate and (B) H2O2 as substrate.
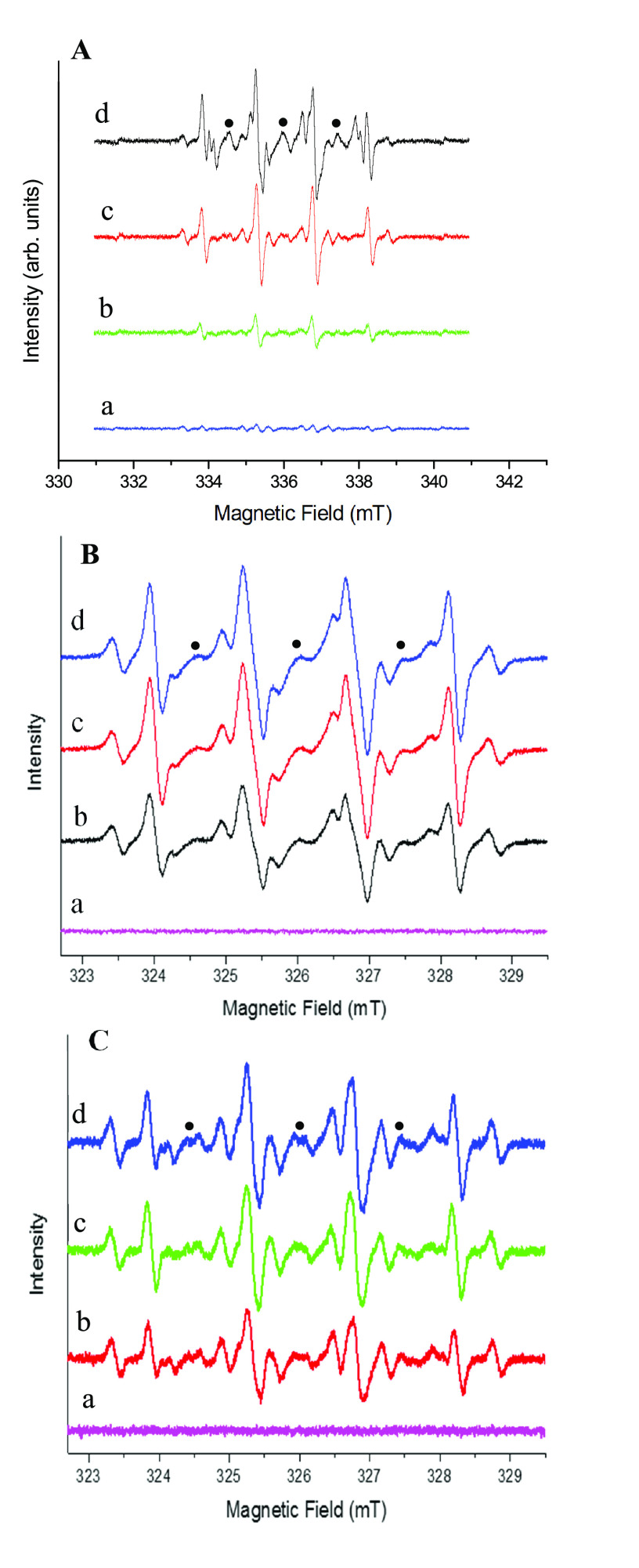
In a possible mechanism of peroxidase activity, which we are here proposing, cerium oxide NSs catalyze the decomposition of H2O2 to produce •OH radicals that oxidize the peroxidase substrate represented by TMB. This process is consistent with the observed color change of the solution from blue to yellow.53 Such a catalytic activity of the proposed nanozyme was demonstrated by monitoring the presence of •OH radicals from this reaction via electron paramagnetic resonance (EPR) spectroscopy. EPR is sensitive to short-living free radicals if spin traps or spin probes that prevent their rapid spin quenching can be used.54 To identify hydroxyl radicals generated in the catalytic cerium oxide NSs system, EPR was performed by adding DMPO as a spin trap to the solution, and a parametric study was performed, as demonstrated in Figure 5.
Figure 5.
Experimental EPR spectra recorded at room temperature after the reaction of cerium oxide NSs with H2O2 in the presence of DMPO spin trap. (The nitroxide degradation product of the spin trap is indicated by black dots.) EPR spectrum of the liquid phase separated from the (A) cerium oxide NSs at varying pH: (a) control (without cerium oxide NSs), (b) pH = 11, (c) pH = 7.0, and (d) pH = 3; (B) various cerium oxide NS concentrations: (a) 0 mg·mL–1, (b) 4 mg·mL–1, (c) 8 mg·mL–1, and (d) 12 mg·mL–1; and (C) various H2O2 concentrations: (a) 0%, (b) 4%, (c) 8%, and (d) 12%.
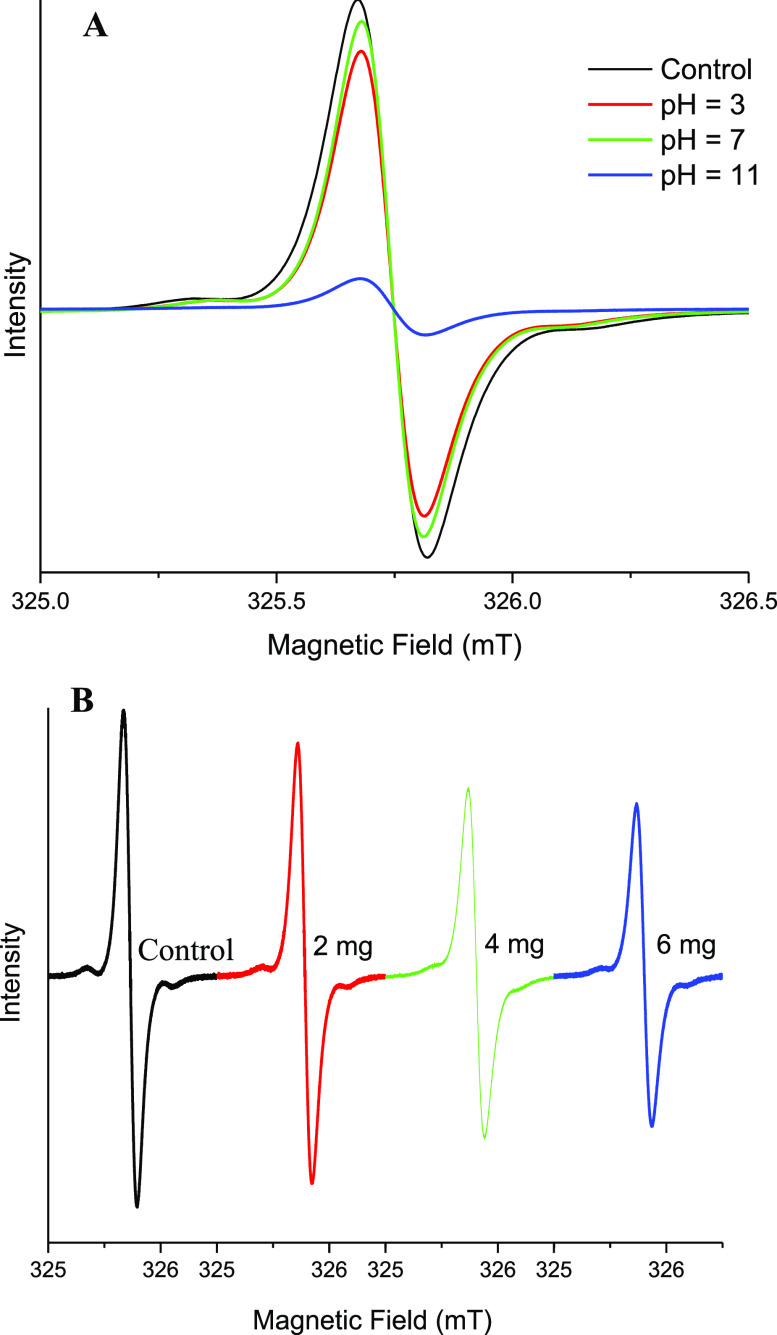
The EPR spectrum of DMPO–OH corresponds to line a in Figure 5A. The signal increases at decreasing pH, with increasing amounts of DMPO–•OH adducts being observed at lower pH when the reaction was performed in the presence of a constant amount of cerium oxide NSs. Conversely, no pH dependence of the EPR signal of DMPO was observed when the reaction was performed in the absence of the nanocomposite (Figure 5A, line a), which thus acts as a catalyst for the H2O2 decomposition. This model is in agreement with increasing enzyme activity at lower pH because more •OH radicals are produced under acidic conditions. These results confirmed the production of •OH radicals catalyzed by cerium oxide NSs. Furthermore, not only the concentration of generated •OH radicals but also the concentration of the cerium oxide NS catalyst is affected by the pH of the solution. The g-values were 2.00553, 2.00548, and 2.00557 at pH values of 11, 7, and 3, respectively, which are close to the values reported in previous studies.55 The EPR spectrum of DMPO–OH was contaminated by a triplet signal due to the nitrosyl radicals arising from the partial degradation of DMPO trap (black dots).56 In our measurement, the time between the spin labeling and the measurements was 5–10 min, so the additional hyperfine splitting could not attribute to the superoxide radicals (•O2–) because it is known that the •O2– adduct of DMPO (DMPO–OOH) is unstable (lifetime is 30–90 s) and it spontaneously decays into the DMPO–hydroxyl adduct.57,58 Furthermore, the reaction rates of DMPO with •O2– and •O2H are extremely smaller compared to that with OH radical. The rate constants of DMPO with •O2– and •O2H are 2–170 and 6.6 × 103 M–1·s–1, respectively, while that with •OH is reported to be 1.9–4.3 × 109 M–1·s–1.59,60 Therefore, the detection of •O2– with DMPO is not facile. Figure 5B shows that when the nanoparticle concentration is varied from 0 (line a) to 12 mg·mL–1 (line d), the catalytic activity also increases, consistent with an increase of the •OH signal up to 8 mg·mL–1 cerium oxide (line c) NP and a saturation of the effect at higher concentrations. The variation of H2O2 concentration from 0% (Figure 5C, line a) to 12% (line d) was also observed to improve the process effectiveness through the addition of more reagents, with an increase in the EPR signal intensity, indicating the generation of more DMPO–•OH(aq) adducts.
2.2.2. Oxidase-like Catalytic Activity of Cerium Oxide NSs
The prepared cerium oxide NSs could directly oxidize TMB, leading to blue color products even in the absence of H2O2 (Figure 3B). This indicates that cerium oxide NSs also exhibit oxidase-like catalytic activity. To further study the oxidation of the TMB chromogenic substrate by cerium oxide NSs, the effect of the oxidizing agent (dissolved oxygen) in the reaction system was investigated. Compared to bubbling an inert gas into the system of N2, the absorbance of oxidized TMB at 652 nm was significantly increased after saturation with O2. It is therefore concluded that increasing the concentration of oxygen as the electron acceptor in the oxidation of TMB can enhance the oxidase-like activity of cerium oxide NSs.
2.2.3. Catalase-like Catalytic Activity of Cerium Oxide NSs
To investigate the catalase-like activity of cerium oxide NSs, the concentration of dissolved oxygen in the system, consisting of cerium oxide NSs and H2O2, was recorded using a portable meter. The value of the dissolved oxygen concentration was monitored for 10 min as a function of cerium oxide NS concentration. As shown in Figure 3C, the concentration of dissolved oxygen increased proportionally to the concentration. This indicated that cerium oxide NSs can decompose H2O2 into O2 and provided strong evidence of the catalase-like activity. The production of O2 was also monitored by EPR by incorporating a spin probe (15N-PDT) in the system. The bimolecular combination of paramagnetic O2 and N-PDT results in shorter spin–spin relaxation times, broadening the EPR line widths with respect to the pristine EPR signal of the pure spin probe.61 As shown in Figure 6A, where the pH increased to 11 in the presence of cerium oxide NSs and H2O2, the EPR signal line width increased as well, with a consistent signal intensity decrease to account for a constant N-PDT concentration. The EPR line width also broadens with increasing cerium oxide NS concentration (Figure 6B). This also indicates increased oxygen formation due to the stronger catalase-like activity of more concentrated cerium oxide NSs.
Figure 6.
EPR spectra of 15N-PDT (A) in the presence of 2 mg of cerium oxide NSs and H2O2 (5%) at different pH values, and (B) in the presence of H2O2 (5%) and different concentration of cerium oxide NSs.
2.3. Electrocatalytic Activity of Cerium Oxide NSs
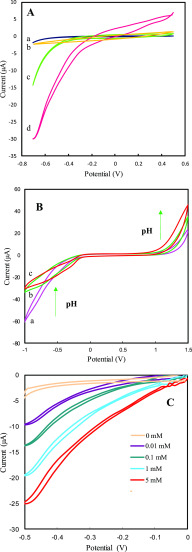
The electrocatalytic activity of cerium oxide NSs was tested by modifying the Au working electrode with cerium oxide NSs. A three-electrode microfluidic chip is used as the electrochemical cell for recording cyclic voltammograms (CV) and determining sensor performance. Cyclic voltammograms (CVs) were recorded for (a) bare Au electrode, (b) cerium oxide NS-modified Au electrode, (c) Au electrode + H2O2, and (d) cerium oxide NS-modified Au electrode + H2O2 in 0.1 M phosphate-buffered saline (PBS) (pH = 7.4) with a scan rate of 50 mV·s–1 (Figure 7A). The bare Au electrode and cerium oxide NS-modified Au electrode did not show any voltammetric response (curves a and b), and upon addition of H2O2, a redox process was detected on the Au bare electrode, indicating reduction of H2O2 (curve c). However, a large enhancement in the H2O2 redox response was observed for the nanoparticle-modified Au electrode (curve d). The oxidation and reduction responses of the electrode-modified cerium oxide NSs when reacting with H2O2 clearly show the catalase and peroxidase activities of cerium oxide NSs. The cerium oxide/Au electrode therefore can act as a mimetic catalase, where it significantly electrocatalyzes the decomposition of hydrogen peroxide (H2O2) to water (H2O) and molecular oxygen (O2) and also acts as a mimetic peroxidase to generate OH• radicals via decomposition of H2O2.30,62
Figure 7.
(A) Cyclic voltammograms of (a) bare Au electrode, (b) Au electrode/cerium oxide, (c) Au electrode + H2O2, and (d) Au electrode/cerium oxide + H2O2. (B) Cyclic voltammograms of Au electrode/cerium oxide + H2O2 at different pH values: (a) 5, (b) 7, and (c) 9. (C) Cyclic voltammograms of Au electrode/cerium oxide in the presence of varied H2O2 concentrations, recorded in N2-purged PBS (0.1 M) at a scan rate of 0.03 V·s–1.
2.4. Investigation of the Effect of pH on Cerium Oxide/Au Electrode Response
To understand the effect of pH on the electrochemical properties of the cerium oxide/Au electrode in the presence of H2O2, electrocatalytic studies were performed at three different pH values: 5.0, 7.0, and 9.0 in the presence of H2O2 (10 mM) using a N2-purged PBS (0.1 M) with a scan rate of 50 mV·s–1, and the results are shown in Figure 7B. At pH = 5.0, both oxidation and reduction were observed; however, the cathodic peak current is higher than the anodic peak, indicating that the peroxidase activity is dominant in cerium oxide NSs at lower pH (curve a). By increasing the buffer solution pH, the cathodic peak current decreases while the anodic peak current is increased relative to curve a, showing dominant catalase activity at higher pH values (curves b and c). These results demonstrate the pH switchability of the catalytic properties of cerium oxide NSs: at acidic pH (pH < 7), the peroxidase catalytic activity is dominant, while at basic pH (pH > 7), the catalase activity is dominant.
2.5. Analytical Performance of Cerium Oxide NS-Modified Au Electrode for Detection of H2O2
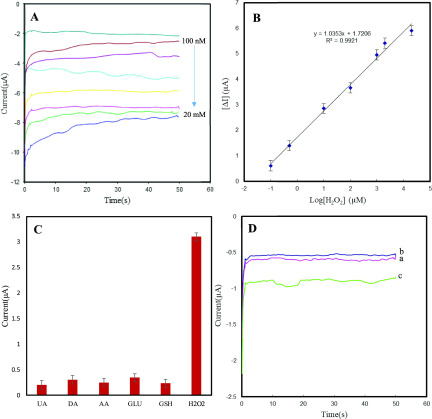
Figure 7C shows the CVs of cerium oxide NS-modified Au electrode in the presence of different concentrations of H2O2. As can be seen, with increasing H2O2 concentration, the reduction current increased, demonstrating the excellent catalytic activity of cerium oxide NSs toward the reduction of H2O2. Thereupon, the detection sensitivity of cerium oxide NS-modified electrodes to aqueous H2O2 was explored through a chronoamperometric study shown in Figure 8. The response for the different amounts of H2O2 is shown by the I–t curves collected at −0.5 V in Figure 8A, which indicates that the reduction currents increase gradually with higher concentrations of H2O2. The calibration plot indicates good linearity for the reduction current versus H2O2 concentrations in the range from 100 to 20 mM (Figure 8A). The linear regression equation generated for cerium oxide NS-modified electrodes was i (μA) = 1.03 logC (μM) + 1.72 μA with a correlation coefficient of R2 = 0.992 (Figure 8B). The lowest concentration of H2O2 that could be estimated by this microfluidic electrochemical sensor was 20 nM (S/N = 3), and the sensitivity was calculated to be 226.4 μA·cm–2·μM–1 based on this result. Compared to previously reported H2O2 sensors based on other nanomaterials or enzymes, this detection method based on microfluidic device outperforms the sensitivity and detection limit of other sensors, as shown in Table 1. The stability of the microfluidic device was examined after 2 weeks, with the result showing that the current through the device with 1 mM H2O2 exhibited only a small deviation over time with a relative standard deviation (RSD) of 3.04%. Six repeated measurements of 1 mM H2O2 led to a relative standard deviation of 2.9%, further showing good reproducibility of the biosensing interface. The selectivity of cerium oxide/Au electrode for H2O2 detection in PBS was also studied by evaluation of the interference effect of coexisting compounds such as ascorbic acid (AA), dopamine (DA), uric acid (UA) glutathione (GSH), and glucose on the electrode response. As illustrated in Figure 8C, there are negligible current responses when interfering molecules were used, confirming the good anti-interference ability of the biomimetic sensor for H2O2 detection.
Figure 8.
(A) Chronoamperometric responses of Au electrode/cerium oxide upon addition of different H2O2 concentrations. Applied potential: −0.5 V. (B) Logarithmic relationship between the concentrations of H2O2 (0.1, 0.5, 10, 100, 1000, 2000, and 20 000 μM). (C) Interference studies of cerium oxide-based lab-on-a-chip device on addition of 1 mM UA, DA, AA, GLU, GSH and 0.2 mM H2O2. (D) Chronoamperometric responses of Au electrode/cerium oxide for the reduction of H2O2 released from 106 PC 12 cells in 1 mL of 1 × PBS (pH = 7.4): (a) PC12 cells, (b) AA (4 μM), and (c) PC12 cells upon injection of 4 μM AA.
Table 1. Comparison of the Performance of Various Hydrogen Peroxide Sensors.
| electrode materials | linear range (μM) | detection limit (μM) | refs |
|---|---|---|---|
| MnO2 nanosheets | up to 454 | 0.005 | (63) |
| graphene/Pt nanocomposite | 0.5–3475 | 0.2 | (64) |
| Se/Pt nanocomposites | 10–15 000 | 3.1 | (65) |
| RGO–Au–PTBO | 5.0–25 362 | 0.2 | (66) |
| rGO@CeO2-AgNPs | 0.5–12 000 | 0.21 | (67) |
| TiO2@Cu2O | 1–15 mM | 0.15 | (68) |
| Au/GS/HRP/CS | 5–5130 | 1.7 | (69) |
| cerium oxide NSs | 0.1–20 000 | 0.01 | this work |
2.6. Real-Time Detection of H2O2 Released from Living Cells
To investigate the capability of the proposed system for real-time detection of H2O2, we chose the PC 12 cell as a model because it can release a trace amount of H2O2 under the stimulation of ascorbic acid (AA).63 The as-prepared 106 cells were suspended in 500 μL of PBS (pH = 7.4) for further use. As shown in Figure 8D, in the presence of PC 12 cells, the cathodic current increases to a higher platform after the addition of 4 μM AA (curve c), which corresponds to about 0.1 μM H2O2 released from the living cells, confirming that the trace amounts of H2O2 released from a living cell can be detected rapidly by the cerium oxide NSs. However, no changes in current are observed in the absence of either cells (curve b) or AA (curve a) under the same conditions, indicating that H2O2 is released from cells under the stimulation of AA. These results suggest that the fabricated microfluidic device is highly sensitive and reliable for the detection of H2O2 in living cells. So, compared to the advantages such as high activity, low detection limit, wide concentration range, and applicability of the presented sensor for measuring H2O2 release from cells, the limitation of the purposed sensor is negligible.
3. Conclusions
In summary, cerium oxide NSs were synthesized by a facile hydrothermal route and exhibited triple-enzyme mimetic activity: oxidase-, peroxidase-, and catalase-like activities. The enzyme mimic properties of cerium oxide NSs can be modulated by adjusting the pH. The peroxidase-like activity is predominant under acidic pH, while the catalase-like activity is prevalent under alkaline conditions. The underlying mechanisms of the catalytic processes involving cerium oxide NSs were investigated by means of EPR spectroscopy, which revealed that the peroxidase-like activity originates from the ability to produce hydroxyl (•OH) radicals. The catalase-like activity causes the decomposition of H2O2 to O2. The as-prepared nanocomposite was used for the electrochemical detection of H2O2 using LOC microfluidic devices. The chronoamperometric technique was utilized for the sensitive detection of H2O2. The linear range of this method was found to be between 100 nM and 20 mM, with a detection limit of 20 nM. The methods we developed have decisive advantages in terms of wide linear range, low detection limit, high sensitivity, easiness of operation, and good practicability. The developed methods were applied to the detection of H2O2 in living cells, which may be competitive with existing methods because of their low cost, simplicity, and reproducibility. We believe that our microfluidic sensors, along with electrochemical detection, may contribute to the growth of biosensing technologies toward practical applications in bioanalysis, food safety, and environmental diagnostics.
4. Experimental Section
4.1. Materials and Instruments
Cerium nitrate hexahydrate Ce(NO3)3·6H2O, terephthalic acid C6H4(CO2H)2, urea (CO(NH2)2), H2O2, 3,3′,5,5 tetramethylbenzidine (TMB), glucose, dopamine, uric acid, glutathione, and ascorbic acid and all other reagents were purchased from Merck or Fluka. All chemicals and reagents were of analytical grade and directly used without further purification. Deionized water produced from a Milli-Q Plus system (Millipore) was used in all experiments.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were obtained with a MIRA3 TESCAN HV: 20.0 kV instrument and a Philips EM 280 microscope, respectively. XRD patterns were recorded on a Bruker D8 Advance diffractometer equipped with a copper source and a general area detector diffraction system (GADDS). The FTIR and UV–vis spectra were recorded by a Vector-22 Bruker spectrophotometer and a SPECTROD 250-Analytik Jena spectrophotometer, respectively. Dissolved oxygen was monitored after the addition of H2O2 by a Handheld meter Oxi 330i/340i (WTW GmbH & Co. KG).
Electron paramagnetic resonance measurements were performed using a Jeol FA-200 EPR spectrometer operating in the X-band at 9.1 MHz and equipped with a cylindrical resonator. The hydroxyl radicals generated in the liquid phase were detected by applying the spin trapping technique. 5,5-Dimethyl-1-pyrroline N-oxide (DMPO) with a concentration of 20 mM was chosen as a suitable spin trap because of its high trapping ability and selectivity toward oxygen-centered radicals. In the spin trapping experiments, a predetermined amount of cerium oxide NSs was contacted with a mixture of 1 mL of H2O2/H2O and 10 μL of DMPO. The liquid sample (5 μL) was inserted into a quartz capillary tube with 1.0 mm inner diameter using a micropipette. The filled capillary was then sealed with parafilm and placed in a quartz glass EPR tube of 5 mm inner diameter (Wilmad LabGlass, 710-SQ-250M) and inserted in the microwave cavity, with all measurements recorded at room temperature unless otherwise stated.56
4.2. Synthesis of Cerium Oxide NSs
Cerium oxide NSs were prepared by a wet-chemical deposition precipitation method. Briefly, 0.25 g of Ce(NO3)3·6H2O was dissolved in 10 mL of distilled water. Subsequently, 0.02 g of urea was added to the solution under vigorous stirring. Then, 10 mL of terephthalic acid solution (60 μM) was added to the reaction solution. After stirring for 15 min, the precipitate product was transferred to a 40 mL Teflon-lined stainless steel autoclave and kept in an electric oven at 150 °C for 6 h. The autoclave was then taken out from the oven and left to cool to room temperature. The produced precipitate was collected via centrifugation, washed thoroughly with water and ethanol, and dried at 60 °C overnight.
4.3. Fabrication of Electrochemical Microfluidic Devices
All experiments were carried out in microfluidic chips made of polydimethylsiloxane (PDMS). The fabrication process can be broken down into three major steps: (1) fabrication of a three-electrode setup, (2) casting of PDMS, and (3) plasma bonding of PDMS over the prepared electrodes on the glass substrate. The electrochemical cell for detection comprised a set of three electrodes: a counter electrode (CE), a working electrode (WE), and a reference electrode (RE). Gold (Au) was chosen as the material for electrodes, and the electrochemical device was fabricated using standard photolithography techniques. The modified electrode was fabricated by drop-casting 5 μL of aqueous cerium oxide NSs (1 mg·mL–1) solution on the cleaned Au working electrode. For fabrication of microfluidic channels, a mixture of polydimethylsiloxane (PDMS) prepolymer and curing agent (10:1) was poured onto the preetched silicon mold followed by curing at 80 °C for 12 h. Then, the cured PDMS was peeled off and placed on the clean bare glass substrate. The width and depth of the channel were both 200 μm, and the surface area of the working electrode was 0.2 mm2. For better adhesion of PDMS channel to the glass, the sample was annealed at 80 °C for 2 h. Finally, it was treated with oxygen plasma before it was bonded to the patterned glass substrate. To ensure strong bonding between the PDMS channel and the glass substrate, the device was sandwiched between two plexiglass sheets. The width and depth of the channel were both 200 μm.
4.4. Electrochemical H2O2 Analysis by Microfluidic Electrochemical Devices
All electrochemical experiments were performed with a computer-controlled potentiostat, Autolab electrochemical analyzer model PGSTAT30 (Eco Chemie, Utrecht, the Netherlands) driven with GPES software (Eco Chemie) in conjunction with a personal computer for data storage and processing. A three Au electrode configuration was applied, in which the cerium oxide NS-modified Au electrode was used as the working electrode (WE) and placed into a microfluidic device. Peristaltic pumps (ISM834C) were connected to the inlet of the microfluidic device to injection of solution into the chip. The electrochemical sensing of H2O2 was carried out in 0.1 M PBS (pH = 7.4), and the PBS was degassed with N2 for 20 min before test. Cyclic voltammetric measurements for hydrogen peroxide were performed at a scanning rate of 50 mV·s–1. Chronoamperometry was performed at a constant applied potential of −0.5 V, and the resulting chronoamperograms were subjected to baseline correction before further analysis.
4.5. Detection of H2O2 in Real Sample
PC 12 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) solution containing 1% penicillin, 1% streptomycin, and 10% fetal bovine serum in a humidified atmosphere of 5% CO2 for 24 h at 37 °C in culture dishes. Then, the cells were removed from the Petri dish by trypsinization and washed three times with sterile buffer, followed by suspension in fresh DMEM. Upon the addition of AA (4 μM), the chronoamperometric current response flux of H2O2 in about 106 cells was recorded.
Acknowledgments
The authors acknowledge financial support from the Research Office of University of Kurdistan (grant number 4.1261) and Iranian Nanotechnology Initiative Funds for research on microfluidic-based sensors.
The authors declare no competing financial interest.
References
- Gao L.; Zhuang J.; Nie L.; Zhang J.; Zhang Y.; Gu N.; Wang T.; Feng J.; Yang D.; Perrett S.; Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577. 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- Nakamura E.; Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003, 36, 807–815. 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- Xiong Y.; Chen S.; Ye F.; Su L.; Zhang C.; Shen S.; Zhao S. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem. Commun. 2015, 51, 4635–4638. 10.1039/C4CC10346G. [DOI] [PubMed] [Google Scholar]
- Asati A.; Kaittanis C.; Santra S.; Perez J. M. pH-tunable oxidase-like activity of cerium oxide nanoparticles achieving sensitive fluorigenic detection of cancer biomarkers at neutral pH. Anal. Chem. 2011, 83, 2547–2553. 10.1021/ac102826k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh N.; Salimi A.; Hallaj R.; Fathi F.; Soleimani F. CuO/WO3 nanoparticles decorated graphene oxide nanosheets with enhanced peroxidase-like activity for electrochemical cancer cell detection and targeted therapeutics. Mater. Sci. Eng., C 2019, 99, 1374–1383. 10.1016/j.msec.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Ding Y.; Yang B.; Liu H.; Liu Z.; Zhang X.; Zheng X.; Liu Q. FePt-Au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sens. Actuators, B 2018, 259, 775–783. 10.1016/j.snb.2017.12.115. [DOI] [Google Scholar]
- Jain S.; Panigrahi A.; Sarma T. K. Counter Anion-Directed Growth of Iron Oxide Nanorods in a Polyol Medium with Efficient Peroxidase-Mimicking Activity for Degradation of Dyes in Contaminated Water. ACS Omega 2019, 4, 13153–13164. 10.1021/acsomega.9b01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. P.; Wu T. H.; Liu C. Y.; Chen K. C.; Chen Y. X.; Chen G. S.; Lin S. Y. Self-Supplying O2 through the Catalase-Like Activity of Gold Nanoclusters for Photodynamic Therapy against Hypoxic Cancer Cells. Small 2017, 13, 1700278 10.1002/smll.201700278. [DOI] [PubMed] [Google Scholar]
- Yamada M.; Yoshinari N.; Kuwamura N.; Saito T.; Okada S.; Maddala S. P.; Harano K.; Nakamura E.; Yamagami K.; Yamanaka K.; et al. Heterogeneous catalase-like activity of gold (I)–cobalt (III) metallosupramolecular ionic crystals. Chem. Sci. 2017, 8, 2671–2676. 10.1039/C6SC04993A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J.; Zhao X.; Li J.; Yang E.-C.; Zhao X.-J. Novel hierarchical NiO nanoflowers exhibiting intrinsic superoxide dismutase-like activity. J. Mater. Chem. B 2016, 4, 5217–5221. 10.1039/C6TB01390B. [DOI] [PubMed] [Google Scholar]
- Singh O.; Tyagi N.; Olmstead M. M.; Ghosh K. The design of synthetic superoxide dismutase mimetics: seven-coordinate water soluble manganese (II) and iron (II) complexes and their superoxide dismutase-like activity studies. Dalton Trans. 2017, 46, 14186–14191. 10.1039/C7DT03278A. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Ren J.; Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- Wei H.; Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Zhang X.; Huang L.; Zhang Z.; Dong S. One-pot synthesis of Fe3O4 nanoparticle loaded 3D porous graphene nanocomposites with enhanced nanozyme activity for glucose detection. ACS Appl. Mater. Interfaces 2017, 9, 7465–7471. 10.1021/acsami.6b16034. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Dong W.; Zhang X.; Chai H.; Huang Y. FeNPs@ Co3O4 hollow nanocages hybrids as effective peroxidase mimics for glucose biosensing. Sens. Actuators, B 2018, 263, 575–584. 10.1016/j.snb.2018.02.151. [DOI] [Google Scholar]
- Dong W.; Zhuang Y.; Li S.; Zhang X.; Chai H.; Huang Y. High peroxidase-like activity of metallic cobalt nanoparticles encapsulated in metal–organic frameworks derived carbon for biosensing. Sens. Actuators, B 2018, 255, 2050–2057. 10.1016/j.snb.2017.09.013. [DOI] [Google Scholar]
- Wang C.; Liu C.; Luo J.; Tian Y.; Zhou N. Direct electrochemical detection of kanamycin based on peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 936, 75–82. 10.1016/j.aca.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Dong J.; Song L.; Yin J.-J.; He W.; Wu Y.; Gu N.; Zhang Y. Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl. Mater. Interfaces 2014, 6, 1959–1970. 10.1021/am405009f. [DOI] [PubMed] [Google Scholar]
- Li J.; Liu W.; Wu X.; Gao X. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 2015, 48, 37–44. 10.1016/j.biomaterials.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Mu J.; Wang Y.; Zhao M.; Zhang L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. 10.1039/c2cc17013b. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Zhang L.; Shang C.; Zhang Z.; Dong S. Triple-enzyme mimetic activity of nickel–palladium hollow nanoparticles and their application in colorimetric biosensing of glucose. Chem. Commun. 2016, 52, 5410–5413. 10.1039/C6CC00194G. [DOI] [PubMed] [Google Scholar]
- Ding Y.; Wang G.; Sun F.; Lin Y. Heterogeneous Nanostructure Design Based on the Epitaxial Growth of Spongy MoS x on 2D Co (OH) 2 Nanoflakes for Triple-Enzyme Mimetic Activity: Experimental and Density Functional Theory Studies on the Dramatic Activation Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 32567–32578. 10.1021/acsami.8b10560. [DOI] [PubMed] [Google Scholar]
- Li H.; Wang T.; Wang Y.; Wang S.; Su P.; Yang Y. Intrinsic triple-enzyme mimetic activity of V6O13 nanotextiles: mechanism investigation and colorimetric and fluorescent detections. Ind. Eng. Chem. Res. 2018, 57, 2416–2425. 10.1021/acs.iecr.7b04821. [DOI] [Google Scholar]
- Alizadeh N.; Salimi A.; Hallaj R. Mimicking peroxidase activity of Co2 (OH) 2CO3-CeO2 nanocomposite for smartphone based detection of tumor marker using paper-based microfluidic immunodevice. Talanta 2018, 189, 100–110. 10.1016/j.talanta.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Li C.; Shi X.; Shen Q.; Guo C.; Hou Z.; Zhang J. Hot Topics and Challenges of Regenerative Nanoceria in Application of Antioxidant Therapy. J. Nanomater. 2018, 2018, 4857461 10.1155/2018/4857461. [DOI] [Google Scholar]
- Wu J.; Wang X.; Wang Q.; Lou Z.; Li S.; Zhu Y.; Qin L.; Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. 10.1039/C8CS00457A. [DOI] [PubMed] [Google Scholar]
- Alizadeh N.; Salimi A.; Hallaj R. Mimicking peroxidase-like activity of Co3O4-CeO2 nanosheets integrated paper-based analytical devices for detection of glucose with smartphone. Sens. Actuators, B 2019, 288, 44–52. 10.1016/j.snb.2019.01.068. [DOI] [Google Scholar]
- Ding H.; Yang J.; Ma S.; Yigit N.; Xu J.; Rupprechter G.; Wang J. Large Dimensional CeO2 Nanoflakes by Microwave-Assisted Synthesis: Lamellar Nano-Channels and Surface Oxygen Vacancies Promote Catalytic Activity. ChemCatChem 2018, 10, 4100–4108. 10.1002/cctc.201800784. [DOI] [Google Scholar]
- Huang Y.; Long B.; Tang M.; Rui Z.; Balogun M.-S.; Tong Y.; Ji H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal., B 2016, 181, 779–787. 10.1016/j.apcatb.2015.08.047. [DOI] [Google Scholar]
- Singh S.; Singh M.; Mitra K.; Singh R.; Gupta S. K. S.; Tiwari I.; Ray B. Electrochemical sensing of hydrogen peroxide using brominated graphene as mimetic catalase. Electrochim. Acta 2017, 258, 1435–1444. 10.1016/j.electacta.2017.12.006. [DOI] [Google Scholar]
- Vilian A. T. E.; Chen S.-M.; Lou B.-S. A simple strategy for the immobilization of catalase on multi-walled carbon nanotube/poly (L-lysine) biocomposite for the detection of H2O2 and iodate. Biosens. Bioelectron. 2014, 61, 639–647. 10.1016/j.bios.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Chen S.; Yuan R.; Chai Y.; Hu F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: a review. Microchim. Acta 2013, 180, 15–32. 10.1007/s00604-012-0904-4. [DOI] [Google Scholar]
- Chen W.; Cai S.; Ren Q.-Q.; Wen W.; Zhao Y.-D. Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst 2012, 137, 49–58. 10.1039/C1AN15738H. [DOI] [PubMed] [Google Scholar]
- Watt B. E.; Proudfoot A. T.; Vale J. A. Hydrogen peroxide poisoning. Toxicol. Rev. 2004, 23, 51–57. 10.2165/00139709-200423010-00006. [DOI] [PubMed] [Google Scholar]
- Hong J.; Maguhn J.; Freitag D.; Kettrup A. Determination of H2O2 and organic peroxides by high-performance liquid chromatography with post-column UV irradiation, derivatization and fluorescence detection. Fresenius’ J. Anal. Chem. 1998, 361, 124–128. 10.1007/s002160050847. [DOI] [Google Scholar]
- Lebiga E.; Fernandez R. E.; Beskok A. Confined chemiluminescence detection of nanomolar levels of H 2 O 2 in a paper–plastic disposable microfluidic device using a smartphone. Analyst 2015, 140, 5006–5011. 10.1039/C5AN00720H. [DOI] [PubMed] [Google Scholar]
- Chen S.; Hai X.; Chen X.-W.; Wang J.-H. In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal. Chem. 2014, 86, 6689–6694. 10.1021/ac501497d. [DOI] [PubMed] [Google Scholar]
- Yuan J.; Cen Y.; Kong X.-J.; Wu S.; Liu C.-L.; Yu R.-Q.; Chu X. MnO2-nanosheet-modified upconversion nanosystem for sensitive turn-on fluorescence detection of H2O2 and glucose in blood. ACS Appl. Mater. Interfaces 2015, 7, 10548–10555. 10.1021/acsami.5b02188. [DOI] [PubMed] [Google Scholar]
- Teymourian H.; Salimi A.; Khezrian S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. 10.1016/j.bios.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Salimi A.; Hallaj R.; Soltanian S.; Mamkhezri H. Nanomolar detection of hydrogen peroxide on glassy carbon electrode modified with electrodeposited cobalt oxide nanoparticles. Anal. Chim. Acta 2007, 594, 24–31. 10.1016/j.aca.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Yamada K.; Henares T. G.; Suzuki K.; Citterio D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. 2015, 54, 5294–5310. 10.1002/anie.201411508. [DOI] [PubMed] [Google Scholar]
- Alizadeh N.; Ghasemi F.; Salimi A.; Hallaj R.; Fathi F.; Soleimani F. Polymer nanocomposite film for dual colorimetric and fluorescent ascorbic acid detection integrated single-cell bioimaging with droplet microfluidic platform. Dyes Pigm. 2020, 173, 107875 10.1016/j.dyepig.2019.107875. [DOI] [Google Scholar]
- Alizadeh N.; Salimi A. Polymer dots as a novel probe for fluorescence sensing of dopamine and imaging in single living cell using droplet microfluidic platform. Anal. Chim. Acta 2019, 1091, 40–49. 10.1016/j.aca.2019.08.036. [DOI] [PubMed] [Google Scholar]
- Ben-Yoav H.; Dykstra P. H.; Bentley W. E.; Ghodssi R. A controlled microfluidic electrochemical lab-on-a-chip for label-free diffusion-restricted DNA hybridization analysis. Biosens. Bioelectron. 2015, 64, 579–585. 10.1016/j.bios.2014.09.069. [DOI] [PubMed] [Google Scholar]
- Riahi R.; Shaegh S. A. M.; Ghaderi M.; Zhang Y. S.; Shin S. R.; Aleman J.; Massa S.; Kim D.; Dokmeci M. R.; Khademhosseini A. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep. 2016, 6, 24598 10.1038/srep24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampaiah D.; Reddy T. S.; Kandjani A. E.; Selvakannan P.; Sabri Y. M.; Coyle V. E.; Shukla R.; Bhargava S. K. Fe-doped CeO2 nanorods for enhanced peroxidase-like activity and their application towards glucose detection. J. Mater. Chem. B 2016, 4, 3874–3885. 10.1039/C6TB00422A. [DOI] [PubMed] [Google Scholar]
- Cho Y. J.; Jang H.; Lee K.-S.; Kim D. R. Direct growth of cerium oxide nanorods on diverse substrates for superhydrophobicity and corrosion resistance. Appl. Surf. Sci. 2015, 340, 96–101. 10.1016/j.apsusc.2015.02.138. [DOI] [Google Scholar]
- Ikuma Y.; Oosawa H.; Shimada E.; Kamiya M. Effect of microwave radiation on the formation of Ce2O (CO3) 2· H2O in aqueous solution. Solid State Ionics 2002, 151, 347–352. 10.1016/S0167-2738(02)00538-6. [DOI] [Google Scholar]
- Heidari F.; Irankhah A. Effect of surfactants and digestion time on nano crystalline cerium oxide characteristics synthesized by differential precipitation. Ceram. Int. 2014, 40, 12655–12660. 10.1016/j.ceramint.2014.04.112. [DOI] [Google Scholar]
- Lin Z.; Waller G.; Liu Y.; Liu M.; Wong C. P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. 10.1002/aenm.201200038. [DOI] [Google Scholar]
- Varaprasad K.; Pariguana M.; Raghavendra G. M.; Jayaramudu T.; Sadiku E. R. Development of biodegradable metaloxide/polymer nanocomposite films based on poly-ε-caprolactone and terephthalic acid. Mater. Sci. Eng., C 2017, 70, 85–93. 10.1016/j.msec.2016.08.053. [DOI] [PubMed] [Google Scholar]
- Cui L.; Wu J.; Li J.; Ju H. Electrochemical sensor for lead cation sensitized with a DNA functionalized porphyrinic metal–organic framework. Anal. Chem. 2015, 87, 10635–10641. 10.1021/acs.analchem.5b03287. [DOI] [PubMed] [Google Scholar]
- Su L.; Qin W.; Zhang H.; Rahman Z. U.; Ren C.; Ma S.; Chen X. The peroxidase/catalase-like activities of MFe2O4 (M = Mg, Ni, Cu) MNPs and their application in colorimetric biosensing of glucose. Biosens. Bioelectron. 2015, 63, 384–391. 10.1016/j.bios.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Iravani S.; Soofi G. J.. Measurement of Oxidative Stress Using ESR Spectroscopy. In Electron Spin Resonance Spectroscopy in Medicine; Springer, 2019; pp 73–81. [Google Scholar]
- Kurake N.; Tanaka H.; Ishikawa K.; Takeda K.; Hashizume H.; Nakamura K.; Kajiyama H.; Kondo T.; Kikkawa F.; Mizuno M.; Hori M. Effects of •OH and •NO radicals in the aqueous phase on H2O2 and generated in plasma-activated medium. J. Phys. D: Appl. Phys. 2017, 50, 155202 10.1088/1361-6463/aa5f1d. [DOI] [Google Scholar]
- Sobańska K.; Pietrzyk P.; Sojka Z. Generation of reactive oxygen species via electroprotic interaction of H2O2 with ZrO2 gel: ionic sponge effect and pH-switchable peroxidase-and catalase-like activity. ACS Catal. 2017, 7, 2935–2947. 10.1021/acscatal.7b00189. [DOI] [Google Scholar]
- Finkelstein E.; Rosen G. M.; Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: Practical aspects. Arch. Biochem. Biophys. 1980, 200, 1–16. 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Buxton G. V.; Greenstock C. L.; Helman W. P.; Ross A. B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O–) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. 10.1063/1.555805. [DOI] [Google Scholar]
- Buettner G. R.; Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem. Biophys. Res. Commun. 1978, 83, 69–74. 10.1016/0006-291X(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Hawkins C. L.; Davies M. J. Detection and characterization of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta, Gen. Subj. 2014, 1840, 708–721. 10.1016/j.bbagen.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Wen T.; He W.; Chong Y.; Liu Y.; Yin J.-J.; Wu X. Exploring environment-dependent effects of Pd nanostructures on reactive oxygen species (ROS) using electron spin resonance (ESR) technique: implications for biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 24937–24943. 10.1039/C5CP04046A. [DOI] [PubMed] [Google Scholar]
- Alizadeh N.; Salimi A.; Hallaj R.; Fathi F.; Soleimani F. Ni-hemin metal–organic framework with highly efficient peroxidase catalytic activity: toward colorimetric cancer cell detection and targeted therapeutics. J. Nanobiotechnol. 2018, 16, 93 10.1186/s12951-018-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y.; Xu J.; Chen J.; Xu Q.; Xiao X.; Jin D.; Pang H.; Hu X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators, B 2017, 252, 72–78. 10.1016/j.snb.2017.05.124. [DOI] [Google Scholar]
- Zhang Y.; Bai X.; Wang X.; Shiu K.-K.; Zhu Y.; Jiang H. Highly sensitive graphene–Pt nanocomposites amperometric biosensor and its application in living cell H2O2 detection. Anal. Chem. 2014, 86, 9459–9465. 10.1021/ac5009699. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhang J.-J.; Xuan J.; Jiang L.-P.; Zhu J.-J. Fabrication of a novel nonenzymatic hydrogen peroxide sensor based on Se/Pt nanocomposites. Electrochem. Commun. 2010, 12, 777–780. 10.1016/j.elecom.2010.03.031. [DOI] [Google Scholar]
- Chang H.; Wang X.; Shiu K.-K.; Zhu Y.; Wang J.; Li Q.; Chen B.; Jiang H. Layer-by-layer assembly of graphene, Au and poly (toluidine blue O) films sensor for evaluation of oxidative stress of tumor cells elicited by hydrogen peroxide. Biosens. Bioelectron. 2013, 41, 789–794. 10.1016/j.bios.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Yao Z.; Yang X.; Wu F.; Wu W.; Wu F. Synthesis of differently sized silver nanoparticles on a screen-printed electrode sensitized with a nanocomposites consisting of reduced graphene oxide and cerium (IV) oxide for nonenzymatic sensing of hydrogen peroxide. Microchim. Acta 2016, 183, 2799–2806. 10.1007/s00604-016-1924-2. [DOI] [Google Scholar]
- Li Z.; Xin Y.; Zhang Z. New photocathodic analysis platform with quasi-core/shell-structured TiO2@ Cu2O for sensitive detection of H2O2 release from living cells. Anal. Chem. 2015, 87, 10491–10497. 10.1021/acs.analchem.5b02644. [DOI] [PubMed] [Google Scholar]
- Zhou K.; Zhu Y.; Yang X.; Luo J.; Li C.; Luan S. A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochim. Acta 2010, 55, 3055–3060. 10.1016/j.electacta.2010.01.035. [DOI] [Google Scholar]