Abstract
Background
Strongyloides stercoralis has the ability to proliferate in its hosts for a long time. In most patients with a competent immune system, the infection remains asymptomatic.
Objectives
Herein, we report a case of concomitant infection of Strongyloides and Aspergillus. Similar cases reported previously were reviewed in the literature and discussed in terms of diagnosis, clinical presentation, and treatment.
Methods
The patient was a 55-year-old man who had a medical history of two masses in his lung and was treated with corticosteroids six months before the presentation.
Results
Using the parasitological methods, massive actively motile larvae of S. stercoralis were seen in the patient's faecal sample. Aspergillus infection was isolated from his fresh bronchoalveolar lavage (BAL) sample and confirmed by observing the septate, dichotomously branched hyphae in direct microscopic examination and also the isolation of the fungus from the culture medium. Molecular analysis revealed that the fungal species isolated from the patient are A. flavus and A. niger. Conclusion. The case highlights the features of concomitant infection of S. stercoralis and Aspergillus in immunocompromised patients and the importance of screening patients for strongyloidiasis before initiation of immunosuppressive therapy.
1. Background
Strongyloidiasis is a parasitic infection, mainly caused by Strongyloides stercoralis [1]. S. stercoralis is a globally distributed nematode that causes clinical symptoms in humans. It is estimated that around 370 million people, mainly in tropical and subtropical regions of the world, are infected with this nematode [2], and it is believed that the number of cases is increasing worldwide [3, 4].
The parasite has a complicated life cycle which includes a free-living cycle in soil and a parasitic phase in the host. Parasite transmission to humans occurs mainly through the entry of filariform larvae into the body via skin contact, although transmission may also occur through unusual ways, such as organ transplantation [5, 6]. After entering the body, the filariform larvae enter the lungs through the bloodstream and enter the gut through swallowing and become the parthenogenetic female.
S. stercoralis can survive for many years in the body without any clinical symptoms. However, disseminated disease or hyperinfection syndrome may develop as a result of immunocompromised states like administration of corticosteroids, tumor necrosis factor-antagonists, and transplantation, which carries a mortality rate of up to 87% [7]. Disseminated disease or hyperinfection syndrome may also occur in individuals coinfected with HTLV and alcoholics. Here, we describe the coinfection of S. stercoralis and Aspergillus in a patient who was treated with steroids for having two masses in his lung. Moreover, similar cases reported in the literature have been searched for and addressed in this report.
2. Case Report
A 55-year-old man originally from Behbahan (a county in Khuzestan Province in the south of Iran) was admitted to the Department of Internal Medicine, in Martyr Faghihi Hospital, Shiraz, Iran, in June 2019. He presented with chronic cough, shortness of breath, wheezing, chest pain, obvious weight loss, weakness, and loss of appetite but without chills or night sweats. He was a worker at the cement factory and had a history of picking mushrooms in the forest with bare feet for the last 8 months before the current admission. His previous medical record was indicating that he had two masses in his right lung for the past one year. Pantoprazole, beclomethasone, and atrovent therapy had been initiated for his chronic pneumonia before the current presentation. Moreover, methylprednisolone (600 mg/8 h; IV) and beclomethasone (two puffs, two times a day; each puff contains 40 μg of beclomethasone) were given to the patient before and during hospital stay.
A computed tomographic scan of his chest revealed two masses along with moderate to severe pleural effusion and evidence of pneumothorax.
Initial laboratory indices were as follows: hemoglobin concentration of 10.1 g/dL, white blood cells counts of 33.88 (normal, 4.0–10.0) × 109 cells/L with differentials of 31.81% neutrophil, 0.6% lymphocytes, 1.4% monocytes, zero count of eosinophil, and platelets count of 813 (normal, 150–450) × 109 cells/L. He had an increased C-reactive protein (CRP) level of 118 (normal, <10) mg/L and erythrocyte sedimentation rate (ESR) of 114 (normal 0–20) mm/hour. His creatinine concentration (3.7 mg/dL) was above the normal range (0.7–1.4 mg/dL).
Routine parasitological laboratory analysis of the fresh faecal specimen was requested, and massive (up to 10 larvae per microscopic field; 10X) actively motile larvae of S. stercoralis were identified in the wet mount preparation. These larvae are about 0.20–0.35 mm long with a round tip and elongated tail. They have a short buccal canal, an esophageal bulb, and a prominent genital primordium (Figure 1).
Figure 1.
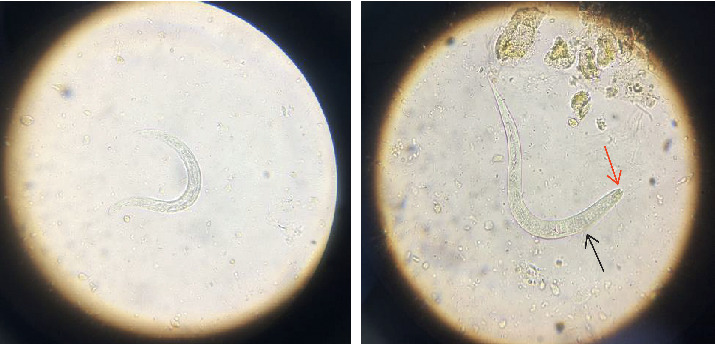
Larva of Strongyloides stercoralis in an unstained wet mount of stool, isolated from the patient in the current study (400x), where red arrow indicates short buccal canal and black arrow indicates rhabditoid esophagus.
Subsequently, bronchoalveolar lavage (BAL) and urine samples were subjected to direct microscopic examination in terms of S. stercoralis infection, but the samples were not positive for S. stercoralis. Surprisingly, a direct examination of BAL revealed septate hyphae with regular dichotomous branching. A potassium hydroxide-Calcofluor mount of BAL confirmed the presence of septate, dichotomously branched hyphae (Calcofluor white bind to chitin that is present in the cell walls of fungi and fluoresces when exposed to UV light) (Figure 2). The patient's BAL sample was cultured in a plate containing sabouraud dextrose agar, with 0.05% chloramphenicol. Two identical species of Aspergillus grew abundantly on the plate at the sample inoculation points (Figure 3).
Figure 2.
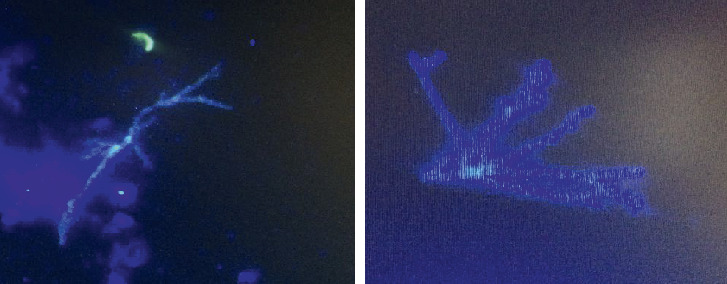
Fluorescent images of Calcofluor white-stained dichotomous branching hyphae in the BAL sample of the patient in the current study.
Figure 3.
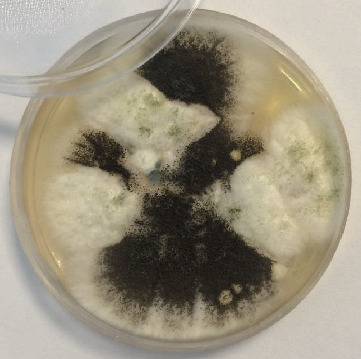
Culture of the BAL revealed the presence of Aspergillus species colonies (A. flavus and A. niger).
The fungal colonies were isolated from the plate, and molecular tests were performed for accurate identification of Aspergillus species, as previously described [8]. In brief, DNA was extracted from the isolates, using homogenization by a rapid mini-preparation method [9]. Polymerase chain reaction (PCR) amplification of the ITS1–5.8S–ITS2 rRNA region was performed, using a panfungal primer pair including ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR amplified an approximately 550–600 bp fragment in both of the tested Aspergillus species. Figure 4 shows the agarose gel electrophoresis of PCR products from the isolated Aspergillus species.
Figure 4.
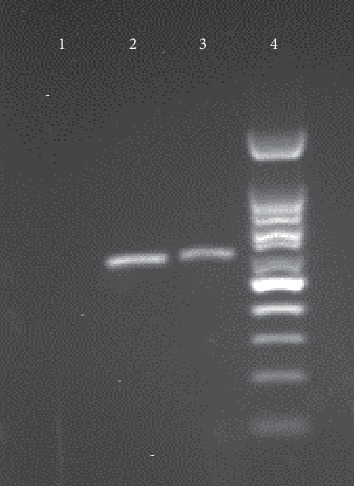
Agarose gel electrophoresis of ITS PCR products. Lane 1, negative control, lane 2, Aspergillus flavus, lane 3, Aspergillus niger, and lane 4, a 100 bp molecular size marker.
Sequencing was performed, using the forward primer (ITS1) as for the PCR, via an automated DNA sequencer (ABI PrismTM 3500 Genetic Analyzer, Genetic Group). The sequences were edited with Geneious software (http://www.geneious.com), and for final identification, the obtained consensus sequences were compared with those of available sequences in the GenBank (https://www.ncbi.nlm.nih.gov/pubmed/). The comparative DNA sequence analysis showed that the amplified sequence of one of the colonies had 99% identity with the ITS regions of A. niger with GenBank accession number MH055397.1 and another colony sequence had 100% identity with the sequence of A. flavus (GenBank accession number: MN179296.1). The consensus nucleotide sequence data determined in this study were deposited in the GenBank, under the accession numbers of MT197480 and MT197481.
Cytological evaluation of the pleural fluid sample also did not reveal any sign of malignancy. The patient continued to be in severe hypoxic and respiratory failure without improvement and eventually developed cardiac arrest and passed away 9 days after admission. Anthelmintic therapy (ivermectin; 200 µg/kg per day, orally) was started for the patient for one day, but due to his severe condition, the treatment was not continued. Postmortem examination was not allowed.
3. Discussion and Review of the Literature
Strongyloidiasis usually causes a symptom-free chronic disease that can remain undetected for a long time. However, in immunosuppression status, it may cause fatal hyperinfection syndrome or disseminated infection [10]. Accordingly, it has been recommended that in the endemic areas, patients undergoing immunosuppressive drug therapy need to be serologically tested for Strongyloides infection before starting treatment [11, 12], although serological diagnosis has its own limitations and is not widely available in every treatment center.
The most common concomitant infection in disseminated strongyloidiasis is Gram-negative bacteria that are carried by the parasite larvae from the gut to the bloodstream or lung [13, 14]. Coinfection of S. stercoralis and fungi, especially with Aspergillus, is rarely reported [15, 16]. Aspergillus is a worldwide distributed fungus that causes disease in immunocompromised patients and patients with underlying lung disease.
We performed a literature search using the terms “Strongyloides stercoralis AND Aspergillus,” “Strongyloidiasis AND Aspergillosis,” on PubMed, Scopus, and Google Scholar. Inclusion criteria were the diagnosis of S. stercoralis infection, disseminated or hyperinfection syndrome, confirmed by detection of larvae, and detection of Aspergillus by conventional laboratory methods (direct microscopic examination or culture). Exclusion criteria were lack of a parasitological or mycological confirmation. The study variables were age, gender, place of origin, underlying diseases, immunosuppressive therapy, clinical manifestations, eosinophilia, diagnostic evidence for S. stercoralis and Aspergillus, determination of Aspergillus species causing the infection, antiparasitic treatment, and the main outcomes. In total, 6 studies met the inclusion criteria, describing the coinfection of S. stercoralis and Aspergillus [15–20]. The clinical and laboratory details of each patient are given in Table 1.
Table 1.
Reported cases of coinfection of Strongyloides stercoralis and Aspergillus.
| Age/sex | Place of origin | Underlying disease | Immunosuppressive therapy | Clinical presentation | Eosinophilia | Positive sample for S. stercoralis | Positive sample for Aspergillus | Aspergillus species identified | Antiparasitic treatment | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 year/man | Behbahan | Two mass in the lung | Oral corticosteroid | Chronic cough, shortness of breath, wheezing, chest pain, obvious weight loss, weakness, and loss of appetite | 0% | Wet mount of faeces | Sputum direct examination and culture | A. flavus and A. niger | Ivermectin (only for one day) | Developed cardiac arrest and deceased | This study |
| 2 | 36 year/man | San Antonio, Texas | Acute lymphoblastic leukemia | Oral corticosteroid and chemotherapy | Dyspnea, malaise, fever, and hemoptysis | 18% eosinophilia | Sputum cytology | Sputum and lavage fluid cultures | A. flavus and A. terreus | Ivermectin | Discharged in stable condition | Shrestha et al. [17] |
| 3 | 66 year/man | Nicaragua | Ulcerative colitis | IV steroids | Watery diarrhea with occasional blood | Unknown | BAL and CSF direct examination | BAL direct examination and at autopsy, multiple foci of lung involvement by Aspergillus were seen | Unknown | Ivermectin | Developed bradycardia and asystole and deceased | Imperatore et al. [18] |
| 4 | 74 year/man | Unknown | Cough, shortness of breath after activity expectoration | Unknown | Weight loss, hemoptysis accompanied by fever, and poor appetite | 0.1% eosinophilia | BAL direct examination and wet mount of faeces | Several sputa and BAL cultures | Unknown | Albendazole | Discharged in stable condition | Guo et al. [15] |
| 5 | 73 year/man | Unknown | Non-Hodgkin's lymphoma, ischaemic heart disease, and chronic obstructive pulmonary disease | Oral corticosteroid and radiotherapy | General malaise, high fever, vomiting, and low blood pressure | 1% eosinophilia | Sputum direct examination | Sputum direct examination and culture | A. fumigatus | Did not receive | Deceased | Wagenvoort et al. [20] |
| 6 | 58-year/woman | Unknown | Nonallergic asthma | Aerosol corticosteroids | Progressive respiratory distress syndrome | 17.2% eosinophilia | Tracheal aspirations | High serum level of antibodies to Aspergillus | A. fumigatus | Ivermectin | Discharged in stable condition | Jacquemart et al. [19] |
| 7 | 59-year/man | Unknown | Chronic obstructive lung disease | Oral corticosteroid | Hematuria, hemoptysis, hematemesis, and respiratory failure | Unknown | Wet mount of faeces and sputum direct examination | Sputum culture and at autopsy demonstrated disseminated aspergillosis | A. fumigatus | Thiabendazole | Deceased | Tankanow et al. [16] |
Of these 6 reported cases, the majority (n = 5; 83.3%) were male and the median age was 61 years (ranged from 36 to 74). The age of our patient in the current study was in line with the age of the previously reported cases [16, 19].
Four (66.6%) of the reported cases, as well as our case, were admitted with a history of pulmonary abnormalities. Other underlying diseases that have been observed include acute lymphoblastic leukemia and ulcerative colitis [17, 18].
The use of corticosteroids is an important risk factor of strongyloidiasis. Prolonged corticosteroid therapy was the attributable cause of severe strongyloidiasis in 83.3% of the reported cases. Cases 2 and 5 (Table 1) had also received chemotherapy and radiotherapy, respectively, besides the use of the corticosteroid [17, 20]. Our patient had been given corticosteroids (methylprednisolone and beclomethasone) for 1 year due to his pulmonary mass. The administration of corticosteroids has long been a mainstay of therapy for inhibition of tumor growth [21]. Considering the comorbidity of strongyloidiasis with other conditions, a recent systematic review described a large number of severe strongyloidiasis cases (67%) as receiving steroids, having HIV infection (15%), or being transplant recipients (11.5%) before the onset of symptoms [22].
Clinical manifestations in our case included fever, a poor appetite, and weight loss, which is consistent with the findings of other reported cases [15–17, 19]. Only two (33.3%) cases presented with digestive disorders which is one of the main symptoms of hyperinfection in strongyloidiasis [18, 20].
Eosinophilia is usually defined as an eosinophil count of more than 5% of the circulating leukocytes [23]. Boulware et al. reported that almost 84% of strongyloidiasis cases have an eosinophil rate of >5% [24]. However, the immunocompromised patient with heavy S. stercoralis infection may have a normal or even a reduced eosinophil count [16]. Thus, a parasitic infection with normal or less than normal eosinophil levels is not unexpected. Our case had no eosinophilia which was similar to findings in cases 4 and 5 [15, 20].
In most of the reported cases, the initial diagnosis has not been strongyloidiasis, but after the evaluation of duodenal biopsy, bronchoalveolar lavage (BAL), and faecal or sputum samples, S. stercoralis infection has been confirmed [15–18, 20, 25]. In our case, we first did not think of a parasitic infection until we took the result of wet mount preparation of the fresh faecal sample.
In our case, S. stercoralis diagnosis was made by the unstained wet mount of the patient's stool sample. The most sensitive method for parasitological diagnosis of S. stercoralis is the agar plate culture, yet this method is mostly available in the research centers [26]. The direct wet mount microscopic examination is not the most adequate method for S. stercoralis diagnosis, since it has a low sensitivity. The fact that the larva was detected by this method in our case, by itself, already indicates that there was a high parasitic load.
Aspergillus species involvement in disease pathology must be carefully considered since these fungi have a universal distribution and are common laboratory contaminants. BAL, bronchial wash (BW), and sputum are the frequently used samples that are subjected to direct examination and culture for the confirmation of fungal infection. The criteria for diagnosis are important for distinguishing contaminants and pathogenic molds. The majority of studies stated at least three criteria which included identification of the mold in the sample by microscopy, isolation in culture, repeated isolation in culture, and inoculums counting [27]. Diagnostic evidence for Aspergillus infection in our case was the identification of septate, dichotomously branched hyphae in direct microscopic examination of fresh BAL sample and isolation of the fungus on chloramphenicol containing medium in at least 5 inocula. Studies have shown that isolation of Aspergillus from sputum in immunocompromised patients has a positive predictive value of 80–90% for the presence of invasive pulmonary aspergillosis (IPA) [28, 29]. IPA is caused by one of the four species of Aspergillus: A. fumigatus, A. flavus, A. niger, and A. terreus. We have isolated both A. flavus and A. niger species from the BAL sample in our case while as listed in Table 1, in three cases, A. fumigatus caused the infection [16, 19, 20]. This discrepancy may be due to the fact that in Iran, A. flavus is the dominant species of Aspergillus which has been isolated from clinical samples because of the abundance of the spores in the environment of this species compared to other species [30]. Although we have isolated both A. flavus and A. niger species from the patient's sample, we believe that the A. flavus is the main species of Aspergillus that caused pathogenesis in the patient and the possibility of environmental contamination by Aspergillus niger cannot be simply ruled out.
It should be noted that in our case, as the patient already had two lung masses and had a history of lung disease, the possibility of the infection with Aspergillus sp. occurring before the S. stercoralis infection cannot be ruled out.
Among the six reported cases in the medical literature, 3 (50%) cases have been discharged in stable condition, 1 (16.6%) case had fatal outcomes because of cardiac arrest, and 2 (33.3%) cases passed away without reporting the cause. It should be noted that among the six reported cases (Table 1) who passed away (n = 3, 42.8%), there were two cases who had not received any or proper antiparasitic treatment and one case who had taken a drug other than ivermectin (thiabendazole).
4. Conclusion
In this report, we have shown evidence that the use of corticosteroids may result in an immunosuppression status that makes the patients prone not only to common pathogen agents but also to more unusual pathogens, for instance, S. stercoralis and Aspergillus. It can be recommended that, in the endemic areas, before initiating any immunosuppressive therapy, routine faecal screening needs to be performed. Moreover, prophylactic therapy, when diagnostic techniques are unavailable, might be considered.
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
The study was approved by the ethics committee of Shiraz University of Medical Sciences (SUMS).
Consent
Consent for publication was obtained from the patient's relative.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
MM and BS designed the study. LH and MO collected the data. MM, LH, and MO performed the experiments. BS and MM were major contributors in writing the manuscript and analyzing the data. All authors have read and approved the submitted manuscript.
References
- 1.Barratt J. L., Lane M., Talundzic E., et al. A global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Neglected Tropical Diseases. 2019;13(9) doi: 10.1371/journal.pntd.0007609.e0007609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schär F., Trostdorf U., Giardina F., et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Neglected Tropical Diseases. 2013;7(7) doi: 10.1371/journal.pntd.0002288.e2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puthiyakunnon S., Boddu S., Li Y., et al. Strongyloidiasis—an insight into its global prevalence and management. PLoS Neglected Tropical Diseases. 2014;8(8) doi: 10.1371/journal.pntd.0003018.e3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majid M. S. A., Johani F. H., Ismail W. R. W., et al. Prevalence of strongyloidiasis in southeast Asia: a systematic review. International Journal of Public Health Research. 2018;8(2):1015–1024. [Google Scholar]
- 5.Nutman T. B. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263–273. doi: 10.1017/s0031182016000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J. H., Kim D. S., Yoon Y. K., Sohn J. W., Kim M. J. Donor-derived strongyloidiasis infection in solid organ transplant recipients: a review and pooled analysis. Transplantation Proceedings. 2016;48(7):2442–2449. doi: 10.1016/j.transproceed.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco-Tenza M. I., Ruiz-Maciá J. A., Navarro-Cots M., Gregori-Colomé J., Cepeda-Rodrigo J. M., Llenas-García J. Strongyloides stercoralis en un hospital comarcal del Levante español: una enfermedad no solo importada. Enfermedades Infecciosas Y Microbiología Clínica. 2018;36(1):24–28. doi: 10.1016/j.eimc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Zarei F., Mirhendi H., Motamedi M., et al. Black Aspergillus species isolated from clinical and environmental samples in Iran. Journal of Medical Microbiology. 2015;64(11):1454–1456. doi: 10.1099/jmm.0.000166. [DOI] [PubMed] [Google Scholar]
- 9.Liu D., Coloe S., Baird R., Pederson J. Rapid mini-preparation of fungal DNA for PCR. Journal of Clinical Microbiology. 2000;38(38):p. 471. doi: 10.1128/jcm.38.1.471-471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoshal U., Khanduja S., Chaudhury N., Gangwar D., Ghoshal U. C. A series on intestinal strongyloidiasis in immunocompetent and immunocompromised hosts. Tropical Gastroenterology. 2012;33(2):135–139. doi: 10.7869/tg.2012.31. [DOI] [PubMed] [Google Scholar]
- 11.Mejia R., Nutman T. B. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Current Opinion in Infectious Diseases. 2012;25(4):458–463. doi: 10.1097/qco.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Checkley A. M., Chiodini P. L., Dockrell D. H., et al. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. Journal of Infection. 2010;60(1):1–20. doi: 10.1016/j.jinf.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang W.-L., Zhang Q.-W., Tang S., Chen F., Zhang J.-B. Co-infection with Strongyloides stercoralis hyperinfection syndrome and Klebsiella in a nephrotic syndrome patient: a case report. Medicine. 2019;98(49) doi: 10.1097/md.0000000000018247.e18247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez J. B., Maque Y., Moquillaza M. A., Anicama W. E. E.coli meningitis presenting in a patient with disseminated Strongyloides stercoralis. Case reports in infectious diseases. 2013;2013:4. doi: 10.1155/2013/424362.424362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J., Sun Y., Man Y., et al. Coinfection of Strongyloides stercoralis and Aspergillus found in bronchoalveolar lavage fluid from a patient with stubborn pulmonary symptoms. Journal of Thoracic Disease. 2015;7(7):E43–6. doi: 10.3978/j.issn.2072-1439.2014.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tankanow L., Eichenhorn M. S. Disseminated Strongyloides stercoralis and Aspergillus fumigatus presenting as diffuse interstitial pneumonitis in a steroid-dependent chronic obstructive pulmonary disease patient. Henry Ford Hospital Medical Journal. 1988;36(1):41–43. [PubMed] [Google Scholar]
- 17.Shrestha P., O’Neil S. E., Taylor B. S., Bode-Omoleye O., Anstead G. M. Hemoptysis in the immunocompromised patient: do not forget strongyloidiasis. Tropical Medicine and Infectious Disease. 2019;4(1):p. 35. doi: 10.3390/tropicalmed4010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imperatore K., Basra P., Herring N., Omarzai Y., Vincentelli C. Fatal disseminated strongyloidiasis secondary to corticosteroid use: a report of two cases. Medical Microbiology Reports. 2017;1(1) [Google Scholar]
- 19.Jacquemart C., Firre E., Thiry A., Broux R. Allergic bronchopulmonary aspergillosis associated with strongyloidiasis. Revue medicale de Liege. 2008;63(7-8):469–473. [PubMed] [Google Scholar]
- 20.Wagenvoort J. H. T., Houben H. G. J., Boonstra G. L. M., Scherpbier J. Pulmonary superinfection with Strongyloides stercoralis in an immunocompromised retired coal miner. European Journal of Clinical Microbiology & Infectious Diseases. 1994;13(6):518–519. doi: 10.1007/bf01974648. [DOI] [PubMed] [Google Scholar]
- 21.McKay L. I., Cidlowski J. A. Corticosteroids in the treatment of neoplasms. Cancer Medicine. 2003;6 [Google Scholar]
- 22.Buonfrate D., Requena-Mendez A., Angheben A., et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infectious Diseases. 2013;13(1):p. 78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salas-Coronas J., Pérez-Arellano J. L., Ramírez-Olivencia G., Belhassen-García M., Carranza-Rodríguez C. Diagnosis and treatment of imported eosinophilia in travellers and immigrants: recommendations of the Spanish society of tropical medicine and international health (SEMTSI) Revista Espanola de Quimioterapia. 2017;30(1):62–78. [PubMed] [Google Scholar]
- 24.Boulware D. R., Stauffer W. M., Hendel-Paterson B. R., et al. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. The American Journal of Medicine. 2007;120(6):545 e1–545 e8. doi: 10.1016/j.amjmed.2006.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Sajee D. M., Al-Hamdani A. A case of gastric and duodenal strongyloidiasis. Sultan Qaboos University Medical Journal. 2010;10(2):p. 262. [PMC free article] [PubMed] [Google Scholar]
- 26.Buonfrate D., Formenti F., Perandin F., Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clinical Microbiology and Infection. 2015;21(6):543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Tarrand J. J., Lichterfeld M., Warraich I., et al. Diagnosis of invasive septate mold infections. American Journal of Clinical Pathology. 2003;119(6):854–858. doi: 10.1309/exbvyaupenbm285y. [DOI] [PubMed] [Google Scholar]
- 28.Zmeili O. S., Soubani A. O. Pulmonary aspergillosis: a clinical update. QJM. 2007;100(6):317–334. doi: 10.1093/qjmed/hcm035. [DOI] [PubMed] [Google Scholar]
- 29.Horvath J. A., Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. The American Journal of Medicine. 1996;100(2):171–178. doi: 10.1016/s0002-9343(97)89455-7. [DOI] [PubMed] [Google Scholar]
- 30.Roudbarmohammadi S., Salimi-Khorashad A., Forouzandeh-Moghadam M., Roudbary M. Investigating the presence of aspergillus fumigatus and A. Flavus using galactomannan enzyme assay and taqman real-time PCR technique. Jundishapur Journal of Microbiology. 2018;11(2) doi: 10.5812/jjm.13670. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


