Abstract
The worldwide incidence of coronavirus disease 2019 (COVID-19) infection is rapidly increasing, but there exists limited information on coronavirus disease 2019 in pregnancy. Here, we present our experience with 7 confirmed cases of coronavirus disease 2019 in pregnancy presenting to a single large New York City tertiary care hospital. Of the 7 patients, 5 presented with symptoms of coronavirus disease 2019, including cough, myalgias, fevers, chest pain, and headache. Of the 7 patients, 4 were admitted to the hospital, including 2 who required supportive care with intravenous hydration. Of note, the other 2 admitted patients who were asymptomatic on admission to the hospital, presenting instead for obstetrically indicated labor inductions, became symptomatic after delivery, each requiring intensive care unit admission.
Key words: COVID-19, novel coronavirus, pregnancy
Coronavirus disease 2019 (COVID-19) has led to the deadliest pandemic observed in more than 100 years. As of this writing (March 25, 2020), there are more than 367,457 confirmed cases and 16,113 deaths worldwide.1 Despite mounting international experience with COVID-19, little is known regarding the impact of the disease on pregnancy.2 , 3
Here, we report the first 7 confirmed cases of COVID-19 infection in pregnant women presenting to a single large New York City tertiary referral center (Table ). Of the 7 patients, 4 were admitted to the hospital, including 2 who required supportive care with intravenous hydration. The other 2 women (28.6% of this case series) required intensive care unit (ICU) admission, and both of these patients were asymptomatic upon presentation for indicated labor induction.
Table.
Patient characteristics and clinical details
| Case | Age (y) | BMI (kg/m2) | GA (wk+d) | MH | Chief complaint | Review of systems on admission |
Temperature, °C | WBC | Platelets | Dispo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Cough | Myalgias | Dyspnea | Chest pain | Headache | Diarrhea | Sick contacts | ||||||||||
| 1 | 38 | 38 | 37+0 | T2DM | Labor induction | N | N | N | N | N | N | N | N | 36.9–38.1 | 7.4 | 216 | ICU |
| 2 | 33 | 47 | 37+5 | T2DM, cHTN | Labor induction | N | N | N | N | N | N | N | N | 36.5–38.8 | 7.3 | 276 | ICU |
| 3 | 30 | 30 | 35+5 | None | Tachycardia (pulse 130 bpm) | N | N | N | N | Y | Y | N | Y | 36.7–38.3 | 4.5 | 185 | Admita |
| 4 | 32 | 29 | 32+5 | None | Fever, myalgias, cough | Y | Y | Y | N | N | N | N | Y | 36.6–37.6 | 6.5 | 180 | Admita |
| 5 | 27 | 31 | 26+3 | None | Chest pain | N | N | N | N | Y | N | N | N | 36.7–37.1 | 6.8 | 129 | Home |
| 6 | 38 | 34 | 28+0 | Asthma | Fever, myalgias, cough | Y | Y | Y | N | N | N | N | N | 36.9–37.0 | - | - | Home |
| 7 | 39 | 23 | 34+6 | None | Cough, HA, myalgias | N | Y | Y | N | N | Y | N | N | 36.4–37.2 | 5.4 | 255 | Home |
BMI, body mass index; cHTN, chronic hypertension; Dispo, initial disposition; GA, gestational age; HA, headache; ICU, intensive care unit; MH, medical history; N, No; T2DM, type 2 diabetes mellitus; WBC, white blood cell count; Y, Yes.
Breslin et al. Coronavirus disease 2019 in pregnancy. AJOG MFM 2020.
Cases 3 and 4 were each admitted for supportive care, and each was discharged home on hospital day 3.
Case Series
Case 1
A 38-year-old G3P2002 patient was admitted for labor induction on March 19, 2020, at 37 weeks’ gestation because of poorly controlled type 2 diabetes mellitus and intrahepatic cholestasis of pregnancy. She presented to the hospital with her husband, and both denied symptoms of fever, cough, shortness of breath, or sore throat before admission. Initial temperature at presentation to the hospital was 36.9°C (98.4°F).
During childbirth, the patient’s temperature increased to 38.5°C (101.3°F). Suspecting chorioamnionitis, ampicillin, gentamicin, and acetaminophen were administered, and she remained asymptomatic. The patient ultimately underwent cesarean delivery owing to arrest of descent. During hysterotomy closure, uterine atony was encountered, and blood loss reached 1.5 liters. Because of uncontrolled hemorrhage and maternal instability, she underwent general endotracheal intubation.
Approximately 1 minute after intubation, a rapid decline in tidal volumes and minimal end-tidal carbon dioxide were noted. Auscultation revealed wheezing with minimal air movement. Severe bronchospasm was suspected and medically managed. Hemostasis was obtained by the obstetrical care team.
Given the severe bronchospasm and reactive lung disease disproportionate to isolated intrapartum fever, the patient was evaluated for COVID-19 infection (SARS-CoV-2 PCR). Intraoperative chest radiograph revealed ill-defined hazy opacities in the right lower lobe and left basilar atelectasis (Figure ). After admission to the surgical intensive care unit (SICU), the SARS-CoV-2 test was conducted; the test result was positive. The patient received hydroxychloroquine therapy (600 mg twice daily for 1 day followed by 400 mg daily over the next 4 days); her respiratory status improved, and she was extubated 8 hours after SICU admission. She was discharged home on postpartum day 4 with a plan for telehealth follow-up. The result of initial neonatal SARS-CoV-2 PCR test was negative on day 1 of life.
Figure.
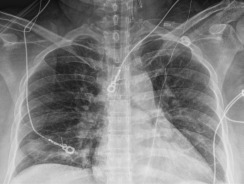
Case 1 chest radiograph
Chest radiograph of a recently delivered patient who experienced postpartum hemorrhage and instability, requiring intubation. Ventilation was complicated by severe bronchospasm and reactive lung disease, which prompted COVID-19 testing, and the test result was positive. This chest radiograph demonstrates left basilar atelectasis and ill-defined hazy opacities in the right lower lung. The endotracheal tube placement was low and subsequently repositioned by the anesthesiology team.
COVID-19, coronavirus disease 2019.
Breslin et al. Coronavirus disease 2019 in pregnancy. AJOG MFM 2020.
An estimated 15 healthcare providers were exposed to this patient before diagnosis, including during intubation, all with inadequate personal protective equipment (PPE). After the diagnosis of COVID-19, the patient was placed in a private room, and all healthcare workers donned PPE before entering her room.
Case 2
A 33-year-old G5P2022 patient was admitted for induction of labor at 37 weeks’ gestation on March 18, 2020, because of worsening chronic hypertension. Her medical history included mild intermittent asthma and type 2 diabetes mellitus. The patient presented to the hospital with her husband, and both denied symptoms of fever, cough, shortness of breath, or sore throat before admission. Initial temperature on presentation to the hospital was 36.5°C (97.7°F).
The patient underwent an uncomplicated primary cesarean delivery because of a failed induction, resulting in a vigorous liveborn neonate. The following day, approximately 25 hours after delivery, and 60 hours after presentation to the labor and delivery unit, the patient developed a cough that progressed to respiratory distress. Vital signs included temperature of 39.4°C (102.9°F), tachycardia (pulse 130 beats/min), and 88% oxygen saturation on room air, and she was dyspneic and diaphoretic. Coarse, wet breath sounds were auscultated bilaterally. Furosemide was administered owing to concern for pulmonary edema. Chest radiograph revealed mild pulmonary vascular congestion, with no consolidation or effusion. Five hours after collection, SARS-CoV-2 PCR test had a positive result. She received hydroxychloroquine with the same dosing regimen as case 1, along with azithromycin and ceftriaxone.
Although her respiratory status temporarily improved, the patient developed severe hypertension (blood pressure as high as 200/90 mm Hg) that ultimately required a nicardipine drip, and she was transferred to the ICU. By postoperative day 2, the patient was weaned off nicardipine. The patient currently remains hospitalized and is in postoperative day 5, with active issues including an ongoing oxygen supplementation requirement and acute kidney injury.
Following the patient’s COVID-19 diagnosis, her newborn was placed in an isolation nursery along with the infant from case 1. Initial neonatal COVID-19 test result was negative. An estimated 15 to 20 healthcare providers were exposed to this patient, again without adequate PPE before diagnosis. After the diagnosis, the patient was cared for in a private room, and all providers donned appropriate PPE before entering the room.
Discussion
This limited initial US experience suggested a need for immediate changes in obstetric clinical practice. Two of 7 (28.6%) confirmed COVID-19–positive patients in this early series were asymptomatic upon admission to the obstetrical service, and these same 2 patients ultimately required unplanned ICU admission. Importantly, their care before COVID-19 diagnosis involved exposure to multiple healthcare workers, all of whom lacked appropriate PPE. Furthermore, 5 of 7 confirmed COVID-19–positive women were afebrile on initial screening, and 4 did not initially report a cough (Table). Currently, COVID-19 screening and testing protocols vary by institution, but in some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.
It is reasonable to suspect that asymptomatic COVID-19 presentations are common and represent a substantial contribution to disease spread.4 , 5 Furthermore, data indicate that healthcare workers are a vulnerable population owing to the risk of viral transmission. This is undoubtedly because of their increased exposure to COVID-19–positive patients, both known and undiagnosed, as well as inadequate PPE supplies.
Obstetrical care providers are at particularly increased risk for occupational exposure because of long periods of interaction with patients during labor, multiple team members involved in patient care, and the unpredictable occurrence of sudden obstetrical emergencies with potential for unanticipated intubations in women undergoing labor and delivery. Given this risk, and without universal rapid viral testing, we must acknowledge that every admission and delivery present real risk for infection to our frontline healthcare workers. As such, ideal practice, if adequate supplies are available, would involve universal PPE including N95 masks for all COVID-19–positive deliveries, whether vaginal or cesarean, as well as for those with unknown COVID-19 status until disease status can be determined through testing. Surgical masks should also be provided for all team members on the inpatient service and for all patients presenting to labor units and worn at all times.6
Until adequate PPE supplies exist, we can reasonably expect our obstetrical and anesthesiology providers to become ill and exit the workforce at an accelerated rate. In just a week, 2629 Italian healthcare workers (8.3% of overall cases) had contracted the COVID-19 infection,7 with infections attributed to inadequate equipment and asymptomatic exposures. Without appropriate protection and rapid testing, we should expect institutions to take measures to safeguard their workforce that previously would have been inconceivable in modern society. Recently, the New York-Presbyterian Hospital made the difficult decision to announce a network-wide restriction prohibiting all visitors from attending deliveries, meaning that our patient’s partners will be unable to directly participate in the deliveries of their own children. Although this policy might seem Draconian, it should increase the protection of the mothers we care for, their infants, and the obstetrical care team, by recognizing what series such as this teach us: there is currently no easy way to clinically predict COVID-19 infection in asymptomatic people.
Footnotes
The authors report no conflict of interest.
Cite this article as: Breslin, N, Baptiste, C, Miller, R, et al. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM 2020;2:100111.
References
- 1.Center for Systems Science and Engineering, Johns Hopkins University Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering at JHU. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at:
- 2.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Liu Y., Liu L., Wang X., Luo N., Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-coronavirus-2 in Shenzhen, China. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa119. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghella V. Now! protection for obstetrical providers and patients. Am J Obstet Gynecol MFM. 2020;2:100109. doi: 10.1016/j.ajogmf.2020.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daily Beast Coronavirus is killing Italy’s doctors. The U.S. could be next. https://www.thedailybeast.com/covid-19-is-killing-italys-doctors-the-us-could-be-next?ref=scroll Available at:


