Abstract
Pathogenic enterotoxigenic Escherichia coli (ETEC) has been considered a major cause of diarrhea which is a serious public health problem in humans and animals. This study was aimed at examining the effect of γ-aminobutyric acid (GABA) supplementation on intestinal secretory immunoglobulin A (SIgA) secretion and gut microbiota profile in healthy and ETEC-infected weaning piglets. A total of thirty-seven weaning piglets were randomly distributed into two groups fed with the basal diet or supplemented with 40 mg·kg−1 of GABA for three weeks, and some piglets were infected with ETEC at the last week. According to whether ETEC was inoculated or not, the experiment was divided into two stages (referred as CON1 and CON2 and GABA1 and GABA2). The growth performance, organ indices, amino acid levels, and biochemical parameters of serum, intestinal SIgA concentration, gut microbiota composition, and intestinal metabolites were analyzed at the end of each stage. We found that, in both the normal and ETEC-infected piglets, jejunal SIgA secretion and expression of some cytokines, such as IL-4, IL-13, and IL-17, were increased by GABA supplementation. Meanwhile, we observed that some low-abundance microbes, like Enterococcus and Bacteroidetes, were markedly increased in GABA-supplemented groups. KEGG enrichment analysis revealed that the nitrogen metabolism, sphingolipid signaling pathway, sphingolipid metabolism, and microbial metabolism in diverse environments were enriched in the GABA1 group. Further analysis revealed that alterations in microbial metabolism were closely correlated to changes in the abundances of Enterococcus and Bacteroidetes. In conclusion, GABA supplementation can enhance intestinal mucosal immunity by promoting jejunal SIgA secretion, which might be related with the T-cell-dependent pathway and altered gut microbiota structure and metabolism.
1. Introduction
Postweaning is a critical stage in swine husbandry, because inappropriate management procedures in this stage may cause health problems in the swine industry and lead to significant economic losses [1]. Weaning piglets are quite vulnerable to a variety of environmental stressors [2] and pathogens (e.g., enterotoxigenic Escherichia coli (ETEC)) [3], which could induce severe diarrhea and pose great threat to the health of weaning piglets. To deal with these problems, antibiotics have been used extensively to prevent pathogen infections. However, the widespread use of antibiotics in farm animals has been proved to cause severe problems like pathogenic drug resistance [4]. Thus, it is necessary to develop effective nutritional regulatory strategies to enhance intestinal immunity and prevent intestinal infection in weaning piglets.
Immunoglobulin A (IgA) secreted by plasma cells is the most abundant immunoglobulin in the body and is of critical importance in intestinal mucosal immunity [5]. The function of SIgA in intestinal mucosal immunity includes immune exclusion, antigen presentation, and interaction with gut commensals [6–10]. Therefore, it is clear that SIgA plays a critical role in maintaining intestinal mucosal immunity and preventing intestinal infection. A previous study reported that the fecal SIgA concentration in piglets reached the peak within a few days after birth [11]. After that, it constantly decreased to a relatively low level in about 10 days [11]. From then on, the fecal SIgA concentration in piglets remained low until at least 50 days of age [11]. This suggests that lack of SIgA might be an underlying reason why weaning piglets are so susceptible to numerous stressors and pathogens.
Current studies revealed that dietary amino acid supplementation, such as glutamine, arginine, and leucine, is an effective way to promote intestinal immunity and health [12–16]. Gamma-aminobutyric acid (GABA) is a well-known neurotransmitter generated through the decarboxylation of glutamic acid (Glu) catalyzed by glutamic acid decarboxylase (GAD) and also has critical roles in the immune system [17, 18]. Recent years have witnessed a growing interest in the application of GABA in animal husbandry. For instance, dietary GABA supplementation reduced the negative influences of weaning stress on weanling piglets by reducing aggressive behavior and regulating endocrine hormones [19]. For chicks under beak trimming stress, the supplementation of GABA significantly improves the immune response of chicks [20]. Our previous study also revealed that GABA supplementation can modulate the intestinal functions, including intestinal immunity, intestinal amino acid profiles, and gut microbiota in weanling piglets [21]. Furthermore, recent studies reported that GABA could alleviate intestinal pathogenic infection through attenuating epithelial cell apoptosis and promoting host Th17 responses [22, 23]. Moreover, a previous study found that intestinal microbiota-derived GABA also could increase intestinal IL-17 expression by activating mechanistic target of rapamycin complex 1- (mTORC1-) ribosomal protein S6 kinase 1 (S6K1) signaling in the context of ETEC or Citrobacter rodentium infection and drug-induced intestinal inflammation [24]. These studies have raised the possibility that GABA supplementation has great prospect in improving intestinal immunity and preventing intestinal infection through metabolism of intestinal microbiota in weanling piglets.
Therefore, this study is mainly aimed at examining the effects of dietary GABA supplementation on the growth performance, intestinal SIgA secretion, gut microbiota profiles, and metabolism in the normal and ETEC-infected weanling piglets. In total, we confirmed that the increased SIgA production is likely to be related to the activation of the T-cell-dependent pathway and altered intestinal microbial metabolism.
2. Materials and Methods
2.1. Bacterial Strain
An enterotoxigenic Escherichia coli F4-producing strain W25K (O149:K91, K88ac; LT, STb, EAST), which was isolated from a piglet with diarrhea [25], was used in the present study.
2.2. Piglets and Experiment Design
All procedures adopted in this experiment were approved by the Animal Welfare Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences. A total of thirty-seven Duroc × Landrace × Yorkshire weanling piglets (5.82 ± 0.86 kg) were enrolled in the experiment at 21 d of age. The piglets were housed individually in an environmentally controlled nursery with hard plastic slatted flooring. All animals had free access to drinking water. The room temperature was maintained at 25 ± 2°C throughout the whole experiment. The composition and nutrient levels of the diets met the nutrient requirements for weanling piglets according to recommendations of the NRC (2012). The experiment lasted for three weeks and was divided into two stages. The first two weeks are the first stage, and the last week is the second stage. At the beginning of the experiment, all piglets were randomly distributed into two groups: (1) control group (CON, basal diet, n = 18) and (2) GABA group (GABA, basal diet with 40 mg·kg−1 of GABA supplementation, n = 19). On the 14th day of the experiment, 6 pigs in each group were slaughtered for sampling, and the remaining piglets were fed with ETEC to construct the infection model. Seven days after the modeling, 6 pigs were slaughtered for sampling in each group. According to whether or not ETEC was inoculated, it was divided into two stages: uninfected and infected (referred as CON1 and CON2 and GABA1 and GABA2). At the last day of every stage, six piglets from each group were randomly selected and sacrificed after anesthesia. Before being sacrificed, 10 mL blood was taken from the anterior vena cava and serum samples were obtained by centrifugation at 2000 × g for 10 min at 4°C and stored at -80°C. The liver, kidney, spleen, heart, and lung were obtained and weighed for calculating the relative weight of each organ. Samples from the same positions of the jejunum, ileum, colon, and feces were collected and immediately snap-frozen in liquid nitrogen and stored at −80°C for RNA extraction, determination of cytokine concentration, microbiota, and metabolite analysis.
2.3. Growth Performance and Organ Indices
Body weight and feed intake were recorded at the end of the first stage and the end of the whole experiment. The average daily gain and average daily feed intake were calculated with the ratio of total bodyweight gain to experimental days and the ratio of feed intake to experimental days. The feed conversion ratio is referred to as the ratio of the feed intake to the body weight gain.
2.4. Serum Amino Acid Analysis
Serum free amino acids were analyzed by high-performance liquid chromatography (HPLC) according to the manufacturer's instructions. The preprocessing of the samples was conducted as following description. In brief, firstly, 2 mL of serum samples was centrifuged at 3000 rpm for 5 minutes. Then 1 mL of supernatants was mixed with 0.8% sulfosalicylic acid solution. After being incubated at 4°C for 15 minutes, the mixtures were then centrifuged at 10,000 rpm for 10 minutes and filtered by a 0.22 μm filter membrane before being analyzed by HPLC.
2.5. Serum Biochemical Analysis
Serum biochemical parameters were determined by the Biochemical Analytical Instrument (Beckman CX4) according to the instructions of manufacturer. And corresponding kits were bought from Roche (Shanghai, China).
2.6. Immunohistochemistry Analysis
The jejunum, ileum, and colon samples were fixed in 4% buffered paraformaldehyde for 24 hours at room temperature and embedded in paraffin and sectioned at a thickness of 3 mm. After being heated at 60°Cfor 30 to 60 minutes, the sections were dewaxed in xylene (10 min, twice) and then rehydrated in a descendent ethanol scale (100%, 95%, 85%, and 75%, 5 min every time). After being washed by distilled water for 5 minutes, the sections were soaked in a 0.01 M citrate buffer (pH = 6.0) and heated to boiling by a microwave oven for 25 minutes. When it had been cooled down and washed by PBS, 3% H2O2 was added into the buffer to inactivate the endogenous enzymes. The treated slides were incubated with primary antibodies (IgA, ab112746, Abcam), diluted at the ratio of 1 : 100, overnight at 4°C. Washed in PBS, the slides were incubated in 50~ 100 μL biotinylated secondary antibodies and HRP-conjugated peroxidase at 37°C for 30 minutes. The chromogen was 3,39-diaminobenzidine free base (DAB).
2.7. ELISA
Equal amounts of samples were applied to examine IgA levels of the jejunum, ileum, and colon, as well as IFN-γ, IL-1β, IL-10, IL-13, IL-17, IL-4, and TNF-α levels of the jejunum by commercially available ELISA kits (Cusabio Biotech Company Limited, Wuhan, China) in accordance with the manufacturer's instructions.
2.8. Real-Time Quantitative PCR
Total RNA of ground jejunum tissue was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The synthesis of complementary DNA was accomplished with the PrimeScript RT reagent kit with gDNA Eraser (Takara Bio Inc., Qingdao, China). RT-PCR was performed in duplicate with an ABI 7900 PCR system (ABI Biotechnology, MD, USA). Primers for the selected genes were designed using the Oligo 5.0 software (Molecular Biology Insights, Inc., USA) and Primer 6.0 software (PRIMER-e, New Zealand) and listed in Supplementary Table 1. β-Actin was used as an internal control to normalize target gene transcript levels. Relative expression of target genes was calculated by the 2-ΔΔCt method [21]. The relative gene expression was expressed as a ratio of the expression of the GABA group to the controls.
2.9. Gut Microbiota Analysis
16S rRNA sequencing and general data analyses were performed by a commercial company (Novogene, Beijing, China). In brief, total genome DNA from samples was extracted using the CTAB/SDS method. The V3-V4 regions were amplified using the specific primer with the barcode. All PCR reactions were carried out in 30 μL reactions with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of the forward and reverse primers, and about 10 ng template DNA. Thermal cycling consisted of the initial denaturation at 98°C for 1 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, and elongation at 72°C for 30 s, and finally 72°C for 5 min. PCR products were mixed in equidensity ratios. Then, mixture PCR products were purified with the GeneJET™ Gel Extraction Kit (Thermo Scientific). Sequencing libraries were generated using Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific) following the manufacturer's recommendations. The library quality was assessed on the Qubit 2.0 Fluorometer (Thermo Scientific). At last, the library was sequenced on an Ion S5TM XL platform and 400 bp/600 bp single-end reads were generated. After the quality control, clean reads were obtained from single-end reads. Sequences analyses were performed by Uparse software (Uparse v7.0.1001). Sequences with ≥97% similarity were assigned to the same OTUs. Representative sequence for each OTU was screened for further annotation. Alpha indices (ACE, Chao1, observed species, Shannon, and Simpson) are applied in analyzing complexity of species diversity for a sample. Principal Coordinate Analysis (PCoA) was performed to get principal coordinates and visualize from complex, multidimensional data.
2.10. Metabolite Profiling Analysis
Untargeted metabolomics of piglet feces was performed by a commercial company (Novogene, Beijing, China). Preparation of samples could be briefly concluded as follows: dry completely in a vacuum concentrator without heating; add 60 μL methoxyamination hydrochloride (20 mg/mL in pyridine) incubated for 30 min at 80°C; and add 80 μL of the N,O-bis(trimethylsilyl)trifluoroacetamide reagent (1% trimethylchlorosilane, v/v) to the sample aliquots, incubated for 1.5 h at 70°C. All samples were analyzed by a gas chromatograph system coupled with a Pegasus HT time-of-flight mass spectrometer (GC-TOF-MS). GC-TOF-MS analysis was performed using an Agilent 7890 gas chromatograph system coupled with a Pegasus HT time-of-flight mass spectrometer. The system utilized a DB-5MS capillary column coated with 5% diphenyl cross-linked with 95% dimethylpolysiloxane (30 m × 250 μm inner diameter, 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA). A 1 μL aliquot of the analyte was injected in splitless mode. Helium was used as the carrier gas, the front inlet purge flow was 3 mL min−1, and the gas flow rate through the column was 1 mL min−1. The initial temperature was kept at 50°C for 1 min, then raised to 310°C at a rate of 20°C min−1, then kept for 6 min at 310°C. The injection, transfer line, and ion source temperatures were 280, 280, and 250°C, respectively. The energy was -70 eV in electron impact mode. The mass spectrometry data were acquired in full-scan mode with the m/z range of 50-500 at a rate of 12.5 spectra per second after a solvent delay of 4.78 min. Chroma TOF 4.3X software of LECO Corporation and LECO-Fiehn Rtx5 database were used for raw peak exacting, data baseline filtering and calibration of the baseline, peak alignment, deconvolution analysis, peak identification, and integration of the peak area. Both mass spectrum match and retention index match were considered in metabolite identification.
2.11. Microbiome-Metabolome Association Analysis
Pearson statistical method was used to calculate the correlation coefficients (rho) and P values of the contents of differential metabolites and relative abundances of differential bacteria. The absolute value of rho bigger than 0.6 and P value smaller than 0.05 were considered significant. Scatterplot and heat map of correlation analysis were drawn according to the results of Pearson statistical method.
2.12. Statistical Analyses
Data are shown as mean ± Standard Error of Mean (SEM). First, the D'Agostino-Pearson omnibus normality test (Prism 7.0) and Kolmogorov–Smirnov test (Prism 7.0) were applied to examine whether the data were in Gaussian distribution. If the data were in Gaussian distribution with equal variance, it would be analyzed by the unpaired t-test (Prism 7.0). If the data were in Gaussian distribution but with unequal variance, it would be analyzed by the unpaired t-test with Welch's correction (Prism 7.0). If the data were not in Gaussian distribution, it would be analyzed by the nonparametric test (Mann–Whitney U test, Prism 7.0). Differences with P < 0.05 were considered significant.
3. Results
3.1. GABA Supplementation Has No Effect on the Growth Performance and Organ Indices in Weanling Piglets
No significant difference was observed in the body weight gain, average daily gain, average daily feed intake, and feed conversion ratio between the CON1 and GABA1 groups (Fig. S1A). The growth performances of ETEC-infected piglets were not markedly influenced by GABA supplementation (Fig. S1B). In the first stage, organ indices had no difference between the CON1 and GABA1 groups (Table S2). From the perspective of the whole experiment, the organ indices of the GABA group were all higher than those of the CON group but without significant differences (Table S2).
3.2. GABA Supplementation Has Little Effect on the Serum Amino Acid Profile and Biochemical Indices
Compared with the CON1 group, the aspartic acid level in serum of the GABA1 group was significantly decreased (P < 0.05), while the histidine level in serum increased (P < 0.05) (Table 1). However, there was no difference in amino acid levels of serum between the CON2 and GABA2 groups (Table 1). As for the serum biochemical parameters, the calcium level in the serum of the GABA1 group was higher than that of the CON1 group (P < 0.05) (Table 2). For the ETEC-infected piglets, the albumin level in the serum was decreased in the GABA2 group compared with the CON2 group (P < 0.05) (Table 2).
Table 1.
Serum amino acid profiles of piglets in stages 1 and 2.
| CON1 | GABA1 | P value (1) | CON2 | GABA2 | P value (2) | |
|---|---|---|---|---|---|---|
| Taurine | 53 ± 6.62 | 47.09 ± 3.93 | 0.464 | 77.05 ± 12.9 | 84.51 ± 9.47 | 0.652 |
| Aspartic acid | 12.9 ± 12.9 | 7.11 ± 1.22∗ | 0.016 | 11.46 ± 2.91 | 11.11 ± 1.54 | 0.918 |
| Threonine | 27.82 ± 7.32 | 22.05 ± 5.6 | 0.547 | 28.68 ± 5.29 | 33.65 ± 7.71 | 0.608 |
| Serine | 48.89 ± 4.44 | 44.34 ± 2.2 | 0.388 | 46.72 ± 5.11 | 43.74 ± 7.44 | 0.749 |
| Glutamic acid | 119.99 ± 8.85 | 106.94 ± 12.61 | 0.419 | 130.03 ± 10.46 | 115.37 ± 10.49 | 0.346 |
| Sarcosine | 1.47 ± 0.43 | 1.77 ± 0.25 | 0.567 | 1.71 ± 0.45 | 1.75 ± 0.54 | 0.957 |
| α-Aminoadipic acid | 26.12 ± 3.03 | 26.13 ± 2.76 | 0.998 | 21.3 ± 4.17 | 22.79 ± 2.82 | 0.775 |
| Glycine | 368.99 ± 41.85 | 326.62 ± 28.04 | 0.423 | 234.47 ± 40.5 | 210.24 ± 66.01 | 0.762 |
| Alanine | 179.52 ± 18.95 | 143.21 ± 17.19 | 0.187 | 132.82 ± 19.87 | 124.6 ± 17.84 | 0.765 |
| Citrulline | 18.83 ± 0.78 | 22.88 ± 2.08 | 0.115 | 16.3 ± 1.4 | 14.79 ± 0.69 | 0.364 |
| 2-Aminobutanoic acid | 4.12 ± 1.3 | 3.86 ± 0.73 | 0.866 | 10.94 ± 4.99 | 12.97 ± 3.13 | 0.738 |
| Valine | 57.92 ± 9.09 | 57.29 ± 5.27 | 0.954 | 72.64 ± 17.19 | 75.41 ± 7.8 | 0.888 |
| Cysteine | 11.97 ± 2.04 | 7.52 ± 1.16 | 0.095 | 12.47 ± 2.45 | 15.2 ± 3 | 0.498 |
| Methionine | 7.68 ± 0.87 | 7.57 ± 0.59 | 0.916 | 6.91 ± 0.78 | 7.29 ± 0.37 | 0.672 |
| Cystathionine | 7.75 ± 0.98 | 8.29 ± 1.09 | 0.720 | 6.01 ± 0.87 | 8.68 ± 1.24 | 0.112 |
| Isoleucine | 31.79 ± 3.12 | 33.52 ± 3.65 | 0.727 | 44.11 ± 9.79 | 54.03 ± 5.82 | 0.408 |
| Leucine | 59.29 ± 3.2 | 63.65 ± 2.31 | 0.298 | 61.87 ± 8.29 | 67.57 ± 3.78 | 0.551 |
| Tyrosine | 31.27 ± 3.21 | 36.63 ± 3.21 | 0.265 | 29.22 ± 2.03 | 26.57 ± 1.53 | 0.322 |
| Phenylalanine | 36.57 ± 2.39 | 38.35 ± 1.56 | 0.548 | 51.35 ± 8.64 | 56.15 ± 4.54 | 0.636 |
| β-Alanine | 7.82 ± 0.72 | 6.27 ± 0.5 | 0.112 | 6.38 ± 1.41 | 6.09 ± 1.04 | 0.874 |
| 3-Aminoisobutyric acid | 0.26 ± 0.07 | 0.32 ± 0.09 | 0.597 | 0.23 ± 0.08 | 0.22 ± 0.06 | 0.915 |
| γ-Aminobutyric acid | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.761 | 0.07 ± 0.02 | 0.04 ± 0.02 | 0.380 |
| Ethanolamine | 2.39 ± 0.25 | 2.22 ± 0.12 | 0.553 | 2.5 ± 0.19 | 2.68 ± 0.2 | 0.521 |
| Hydroxylysine | 1.21 ± 0.59 | 2.98 ± 0.77 | 0.099 | 1.16 ± 0.34 | 3.3 ± 1.61 | 0.245 |
| Ornithine | 31.15 ± 2.43 | 30.07 ± 1.74 | 0.726 | 32.15 ± 3.84 | 27.19 ± 1.59 | 0.273 |
| Lysine | 1.74 ± 11.02 | 93.03 ± 8.84 | 0.762 | 70.334 ± 7.35 | 78.54 ± 5.77 | 0.402 |
| 1-Methyl-L-histidine | 6.01 ± 2.31 | 6.02 ± 1.74 | 1.000 | 4.77 ± 1.41 | 4.18 ± 1.41 | 0.773 |
| Histidine | 24.48 ± 1.99 | 32.26 ± 2.45∗ | 0.034 | 22.91 ± 2.95 | 23.62 ± 1.33 | 0.832 |
| 3-Methyl-L-histidine | 4.3 ± 0.38 | 5.59 ± 0.46 | 0.059 | 5.68 ± 1.23 | 5.83 ± 0.48 | 0.915 |
| Carnosine | 10.51 ± 1.38 | 12.03 ± 0.96 | 0.389 | 6.01 ± 1.3 | 4.31 ± 0.89 | 0.307 |
| Arginine | 84.21 ± 7.29 | 87.61 ± 7.76 | 0.756 | 105.45 ± 17.44 | 106.34 ± 5.2 | 0.962 |
| Proline | 103.14 ± 5.08 | 95.3 ± 4.36 | 0.269 | 94.64 ± 12.63 | 84.46 ± 9.65 | 0.537 |
Means with ∗ are significantly different from the control group (P < 0.05).
Table 2.
Serum biochemistry parameters of piglets in stages 1 and 2.
| CON1 | GABA1 | P value (1) | CON2 | GABA2 | P value (2) | |
|---|---|---|---|---|---|---|
| Total protein | 46.933 ± 0.750 | 45.8 ± 1.035 | 0.398 | 48.433 ± 1.410 | 47.267 ± 1.506 | 0.584 |
| Albumin | 37.75 ± 1.628 | 39.7167 ± 1.148 | 0.349 | 34.833 ± 1.114 | 31.200 ± 0.733∗ | 0.024 |
| Alanine aminotransferase | 31.85 ± 3.609 | 30.0833 ± 1.537 | 0.667 | 40.017 ± 8.316 | 43.217 ± 3.088 | 0.730 |
| Aspartate aminotransferase | 55.5 ± 9.549 | 53 ± 7.024 | 0.838 | 56.167 ± 10.058 | 51.500 ± 6.479 | 0.706 |
| Alkaline phosphatase | 309.833 ± 23.494 | 380.667 ± 49.876 | 0.239 | 199.333 ± 32.278 | 170.500 ± 20.343 | 0.470 |
| Lactate dehydrogenase | 555.167 ± 70.002 | 532.5 ± 25.160 | 0.770 | 612.667 ± 72.638 | 524.333 ± 46.760 | 0.335 |
| Blood urea nitrogen | 1.8333 ± 0.233 | 2.1333 ± 0.265 | 0.416 | 2.817 ± 1.009 | 2.617 ± 0.547 | 0.866 |
| Glucose | 5.6 ± 0.259 | 4.9667 ± 0.362 | 0.189 | 3.833 ± 0.549 | 4.467 ± 0.506 | 0.146 |
| Ca | 2.7017 ± 0.051 | 2.915 ± 0.070∗ | 0.036 | 2.632 ± 0.171 | 2.493 ± 0.082 | 0.490 |
| P | 2.208 ± 0.099 | 2.025 ± 0.191 | 0.420 | 2.868 ± 0.150 | 2.480 ± 0.107 | 0.064 |
| Triglyceride | 0.422 ± 0.030 | 0.405 ± 0.015 | 0.637 | 0.638 ± 0.178 | 0.845 ± 0.151 | 0.398 |
| Cholesterol | 1.821 ± 0.102 | 1.675 ± 0.119 | 0.373 | 2.127 ± 0.111 | 2.563 ± 0.202 | 0.096 |
| High-density lipoprotein cholesterol | 0.702 ± 0.054 | 0.580 ± 0.029 | 0.084 | 0.562 ± 0.099 | 0.520 ± 0.068 | 0.767 |
| Low-density lipoprotein cholesterol | 0.897 ± 0.099 | 0.832 ± 0.086 | 0.630 | 1.245 ± 0.112 | 1.648 ± 0.195 | 0.110 |
| D-Lactic acid | 8.967 ± 0.789 | 7.417 ± 0.320 | 0.114 | 9.763 ± 0.820 | 8.06 ± 0.683 | 0.143 |
| Blood ammonia | 172.567 ± 8.083 | 193.35 ± 6.764 | 0.078 | 299.367 ± 16.071 | 263.017 ± 5.825 | 0.075 |
| Immunoglobulin M | 0.067 ± 0.014 | 0.080 ± 0.012 | 0.481 | 0.172 ± 0.112 | 0.167 ± 0.090 | 0.973 |
| Diamine oxidase | 1.2 ± 0.157 | 1.433 ± 0.203 | 0.257 | 1.767 ± 0.275 | 1.500 ± 0.197 | 0.451 |
Means with ∗ are significantly different from the control group (P < 0.05).
3.3. GABA Supplementation Promotes Intestinal SIgA Production
To examine the effect of GABA supplementation on intestinal SIgA secretion, the jejunum, ileum, and colon were applied to the immunohistochemistry analysis. As the results show, GABA1 significantly improved the jejunal (P < 0.01) and ileal (P < 0.05) SIgA levels compared to CON1 (Figures 1(a) and 1(b)). However, GABA supplementation had no effect on the jejunal and ileal SIgA levels in ETEC-infected piglets (Figures 1(a) and 1(b)). With regard to the colonic SIgA level, neither healthy nor ETEC-infected piglets were affected by GABA supplementation (Figure 1(c)). Results from ELISA analysis further verified that GABA supplementation increased the jejunal SIgA secretion in both healthy piglets and infected piglets (P < 0.01) (Figure 1(d)).
Figure 1.
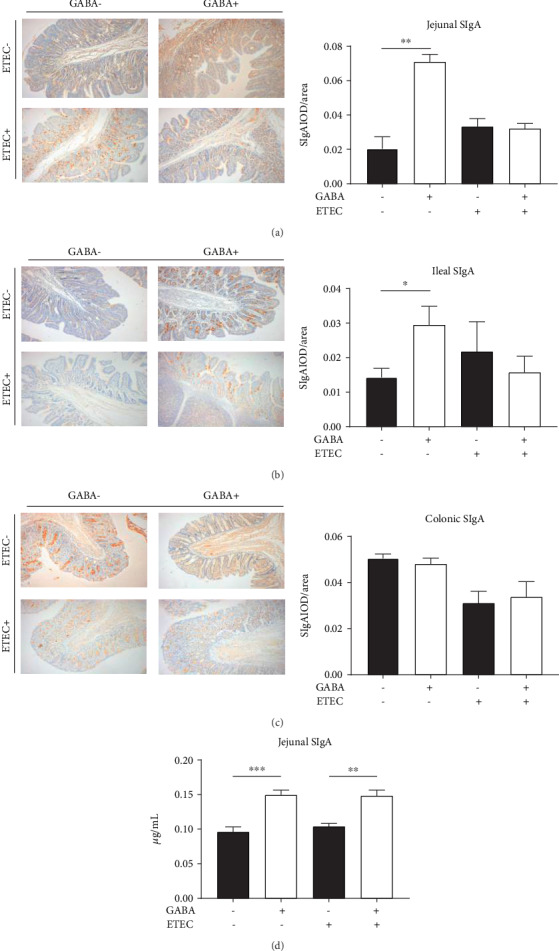
Dietary GABA promoted the production of SIgA in the intestine of piglets. Immunohistochemistry analysis of the IgA levels in the (a) jejunum, (b) ileum, and (c) colon of piglets in stage 1 and stage 2. (d) ELISA test of the jejunal SIgA level of piglets in stage 1 and stage 2. Data (n = 6) are presented as mean ± SEM and analyzed by the unpaired t-test with Welch's correction or Mann–Whitney U test. Differences were denoted as follows: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3.4. GABA Supplementation Improves the Expression of SIgA-Related Cytokines, Especially in ETEC-Infected Piglets
To explore the underlying mechanisms of the regulating effect of GABA supplementation on jejunal SIgA production, the protein levels and mRNA expression of cytokines in the jejunum of piglets were examined. The results of ELISA analysis showed that the jejunal concentration of IL-4 in the GABA1 group was much higher than that in the CON1 group (P < 0.0001) (Figure 2(a)). Compared with the CON2 group, GABA2 improved the jejunal concentrations of IL-4 (P < 0.0001), IFN-γ (P < 0.01), IL-1β (P < 0.01), and IL-17 (P < 0.001) (Figure 2(b)). Compared with the CON1 group, the mRNA expression of jejunal IFN-γ (P < 0.05) and IL-13 (P < 0.01) was significantly increased in the GABA1 group, while no significant alterations in the mRNA expression of any jejunal cytokines were observed in the GABA2 group (Figures 2(c) and 2(d)).
Figure 2.
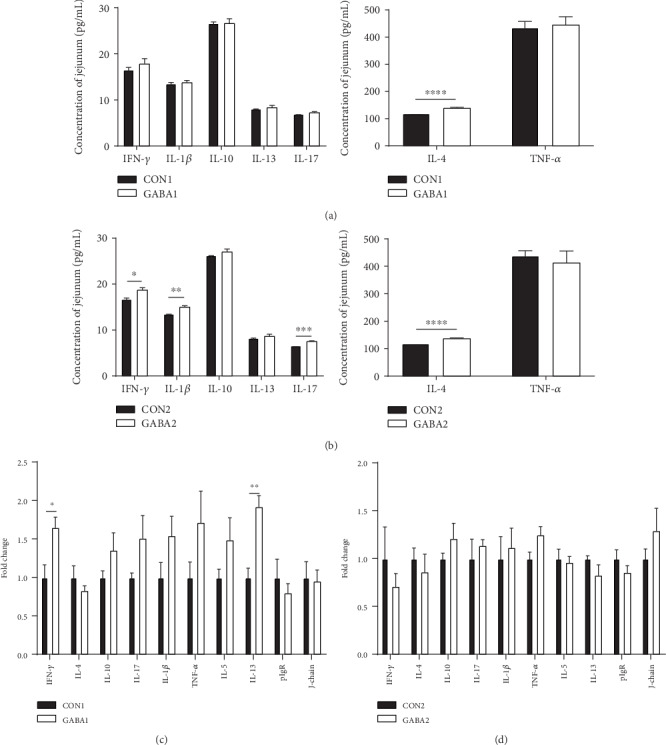
GABA promoted RNA expression and production of cytokines. (a) Jejunal concentrations of cytokines in normal piglets; (b) jejunal concentrations of cytokines in ETEC-infected piglets; (c) mRNA expression levels of cytokines in normal piglets; (d) mRNA expression levels of cytokines in ETEC-infected piglets. Data (n = 6) are presented as mean ± SEM and analyzed by the unpaired t-test with Welch's correction or Mann–Whitney U test. Differences were denoted as follows: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
3.5. GABA Supplementation Alters the Relative Abundances of Gut Bacteria
To examine if GABA supplementation increased intestinal SIgA production through modulating gut microbiota, the fecal microbiota of piglets was analyzed by bacterial 16S rRNA sequencing (V3-V4 regions). An average of 85,463 raw reads was generated for each sample. After removing the low-quality sequences, 80,121 clean tags were clustered into OTUs for the following analysis, based on the 97% similarity level. As shown in Table S3, no matter in the healthy piglets or the ETEC-infected piglets, GABA supplementation had no effect on the diversity indices (Shannon and Simpson) or the richness indices (Chao1, ACE, and observed species). In addition, PCoA revealed that the gut microbiota composition of piglets basically was little affected by GABA treatment (Figs. S2F and S2L). The relative abundances of the top ten strains in the phylum, class, order, family, and genus level were not significantly altered by GABA supplementation (Figs. S2A to S2E). The same goes for the gut microbiota of ETEC-infected piglets (Figs. S2G to S2K). However, when the scope was not limited to the top ten strains anymore, there were some low-abundance strains that were markedly altered by GABA supplementation. In normal piglets, the relative abundances of Phascolarctobacterium (P < 0.05) and Butyricicoccus (P < 0.05) were decreased by GABA, while the relative abundance of Enterococcus (P < 0.05) was increased (Figure 3(a)). In ETEC-infected piglets, GABA supplementation increased the relative abundance of Bacteroides (P < 0.05) and an unidentified Ruminococcaceae (P < 0.05) (Figure 3(b)). These results suggested that GABA supplementation affected the relative abundances of certain low-abundance bacteria in weanling piglets.
Figure 3.
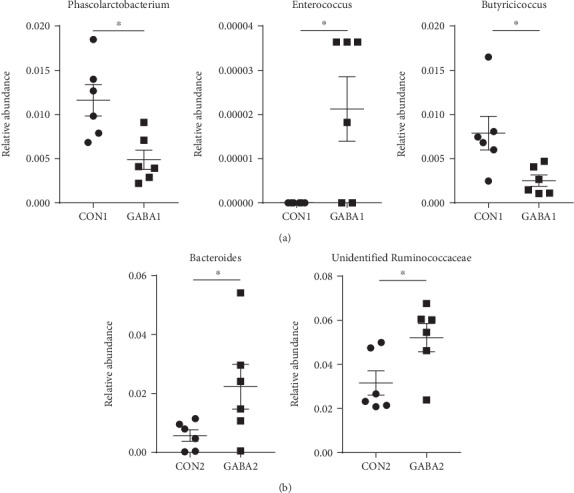
GABA altered the relative abundance of gut microbiota. (a) Relative abundance of Phascolarctobacterium, Enterococcus, and Butyricicoccus in normal piglets. (b) Relative abundance of Bacteroides and unidentified Ruminococcaceae in ETEC-infected piglets. Data are presented as mean ± SEM and analyzed by the unpaired t-test with Welch's correction or Mann–Whitney U test. Differences were denoted as follows: ∗P < 0.05.
3.6. GABA Supplementation Modulates Metabolism of Gut Microbiota, Especially in Normal Weanling Piglets
The fecal metabolites were analyzed to examine whether GABA supplementation altered the metabolism of intestinal microbiota in weanling piglets. Firstly, a PLS-DA analysis was applied to have a better view of the different metabolic patterns in normal piglets. As shown in the PLS-DA score plot, the CON1 group and GABA1 group were distributed separately (Figure 4(a)). Quality of the resulting discriminant models suggested that the model was available and had good fitness and prediction (Figure 4(b)). Significant variables responsible for group separation were selected using the variable importance in the projection (VIP) statistic of the first principal component of the PLS-DA model (threshold > 1), together with the P value of Student's t-test (threshold < 0.05). As listed in Table 3, 10 metabolites were markedly upregulated in the GABA1 group. These metabolites were identified as potential markers: 2-hydroxybutanoic acid (P < 0.05), 1,3-diaminopropane (P < 0.05), IS (P < 0.05), O-phosphorylethanolamine (P < 0.05), methyl-beta-D-galactopyranoside (P < 0.05), 3-hydroxybutyric acid (P < 0.05), fructose 2 (P < 0.01), 10-hydroxy-2-decenoic acid (P < 0.05), norleucine 1 (P < 0.01), and hydroxylamine (P < 0.01) (Figure 4(c)). The results of KEGG enrichment analysis were presented as a scatterplot which showed that GABA markedly altered the sphingolipid signaling pathway, sphingolipid metabolism, nitrogen metabolism, cationic antimicrobial peptide (CAMP) resistance, glycerophospholipid metabolism, and microbial metabolism in diverse environments (Figure 4(d)).
Figure 4.
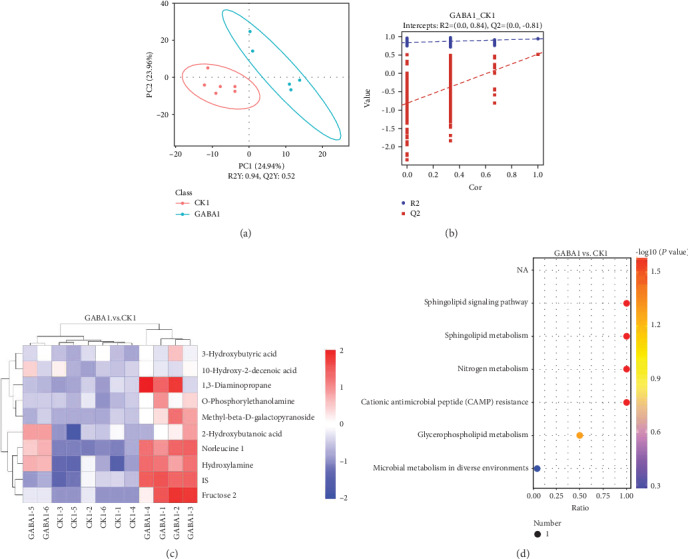
GABA highly changes the metabolism of gut microbiota in healthy piglets. (a) GABA1 vs. CON1 PLS-DA score plot; (b) GABA1 vs. CON1 PLS-DA valid plot; (c) heat map of significant variables; (d) scatterplot of KEGG enrichment analysis. The ratio in the scatterplot means the ratio of the number of altered metabolites to the number of all metabolites in this pathway. The CK group in this figure means the CON group.
Table 3.
Significant variables responsible for group separation of the CON1 group and GABA1 group.
| Metabolites | RT | VIP | P value | Fold change |
|---|---|---|---|---|
| 2-Hydroxybutanoic acid | 8.39152,0 | 1.0943912 | 0.0219111 | 2.08685768 |
| 1,3-Diaminopropane | 15.2455,0 | 1.5156162 | 0.0389701 | 3.578994496 |
| IS | 16.6102,0 | 1.0352327 | 0.0446863 | 2.116515269 |
| O-Phosphorylethanolamine | 16.7466,0 | 1.1989025 | 0.0272898 | 2.718830077 |
| Methyl-beta-D-galactopyranoside | 17.4716,0 | 2.662989 | 0.0125204 | 8.738756131 |
| 3-Hydroxybutyric acid | 8.87752,0 | 1.0999058 | 0.0356118 | 2.488895428 |
| Fructose 2 | 17.7379,0 | 8.2363709 | 0.0037375 | 15.0103342 |
| 10-Hydroxy-2-decenoic acid | 19.8381,0 | 1.1643369 | 0.0498178 | 2.53080591 |
| Norleucine 1 | 11.1259,0 | 5.3345447 | 0.0008329 | 21.17542765 |
| Hydroxylamine | 8.2099,0 | 1.7659162 | 0.0021158 | 3.131884356 |
RT: retention time (min); VIP: variable importance in the projection of the PLS-DA first principal component; P value: t-test significance; fold change: GABA1 group to CON1 group.
In the PLS-DA score plot, the GABA2 group and CON2 group were not separated from each other (Figure 5(a)). Only 4 metabolites were significantly altered: 1,2,4-benzenetriol (P < 0.01) and methyl-beta-D-galactopyranoside (P < 0.05) were upregulated, and oleic acid (P < 0.05) and DL-dihydrosphingosine 1 (P < 0.05) were downregulated (Figure 5(c)). The results of VIP statistic and fold changes are listed in Table 4. The scatterplot suggested that GABA significantly affected the longevity regulating pathway-worm in ETEC-infected piglets (Figure 5(d)).
Figure 5.
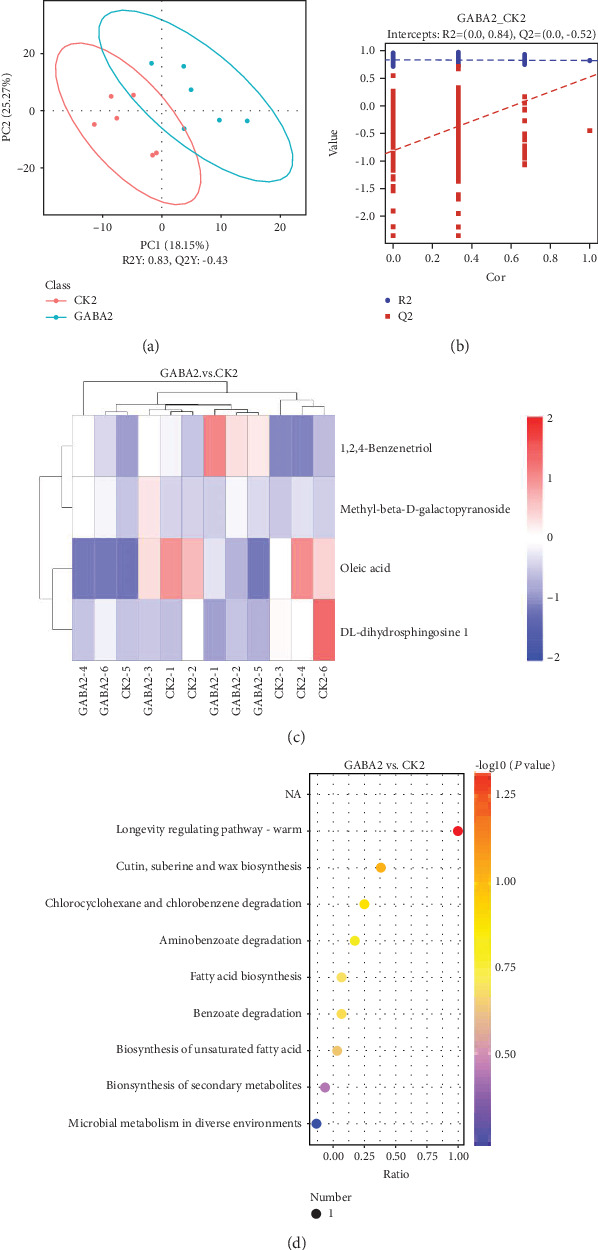
GABA regulated the metabolism of gut microbiota in ETEC-infected piglets. (a) GABA2 vs. CON2 PLS-DA score plot; (b) GABA2 vs. CON2 PLS-DA valid plot; (c) heat map of significant variables; (d) scatterplot of KEGG enrichment analysis. The ratio in the scatterplot means the ratio of the number of altered metabolites to the number of all metabolites in this pathway. The CK group in this figure means the CON group.
Table 4.
Significant variables responsible of the CON2 group and GABA2 group.
| Metabolites | RT | VIP | P value | Fold change |
|---|---|---|---|---|
| 1,2,4-Benzenetriol | 14.717,0 | 1.331900147 | 0.005181206 | 2.074787321 |
| Methyl-beta-D-galactopyranoside | 17.4716,0 | 2.865360744 | 0.030532857 | 7.741120774 |
| Oleic acid | 21.1446,0 | 1.239038753 | 0.038381941 | 0.520647409 |
| DL-dihydrosphingosine 1 | 23.2836,0 | 2.333778351 | 0.03935667 | 0.271034926 |
RT: retention time (min); VIP: variable importance in the projection of the PLS-DA first principal component; P value: t-test significance; fold change: GABA1 group to CON1 group.
We identified the specific metabolites related with enriched KEGG pathways and found that, in healthy piglets, all enriched KEGG pathways were related with O-phosphorylethanolamine or hydroxylamine (Table 5). In ETEC-infected piglets, two metabolites, oleic acid and 1,2,4-benzenetriol (Table 6), might mediate the effect of GABA supplementation on gut microbial metabolism in ETEC infection.
Table 5.
Enriched KEGG pathway and related differential metabolites in normal piglets.
| Map ID | Map title | Metabolite |
|---|---|---|
| map00600 | Sphingolipid metabolism | O-Phosphorylethanolamine |
| map00910 | Nitrogen metabolism | Hydroxylamine |
| map01503 | Cationic antimicrobial peptide (CAMP) resistance | O-Phosphorylethanolamine |
| map04071 | Sphingolipid signaling pathway | O-Phosphorylethanolamine |
| map00564 | Glycerophospholipid metabolism | O-Phosphorylethanolamine |
| map01120 | Microbial metabolism in diverse environments | Hydroxylamine |
Map ID: ID of the enriched KEGG pathway; map title: title of the enriched KEGG pathway; metabolite: specific differential metabolites related with the corresponding enriched KEGG pathway.
Table 6.
Enriched KEGG pathway and related differential metabolites in ETEC-infected piglets.
| Map ID | Map title | Metabolite |
|---|---|---|
| map04212 | Longevity regulating pathway-worm | Oleic acid |
| map00073 | Cutin, suberine, and wax biosynthesis | Oleic acid |
| map00361 | Chlorocyclohexane and chlorobenzene degradation | 1,2,4-Benzenetriol |
| map00627 | Aminobenzoate degradation | 1,2,4-Benzenetriol |
| map00061 | Fatty acid biosynthesis | Oleic acid |
| map00362 | Benzoate degradation | 1,2,4-Benzenetriol |
| map01040 | Biosynthesis of unsaturated fatty acids | Oleic acid |
| map01060 | Biosynthesis of plant secondary metabolites | Oleic acid |
| map01120 | Microbial metabolism in diverse environments | 1,2,4-Benzenetriol |
Map ID: ID of the enriched KEGG pathway; map title: title of the enriched KEGG pathway; metabolite: specific differential metabolites related with the corresponding enriched KEGG pathway.
3.7. There Are Significant Correlations between Differential Metabolites and Altered Low-Abundance Bacteria
We performed a microbiome-metabolome association analyses to examine the possible correlation between the altered low-abundance bacteria and the changed microbial metabolites. The results showed that hydroxylamine was positively correlated with Enterococcus (∣rho | >0.6) (Figure 6(b)) but negatively correlated with Phascolarctobacterium (∣rho | >0.6) (Figure 6(c)) and Butyricicoccus (∣rho | >0.6) in healthy piglets (Figure 6(d)). The absolute values of the correlation coefficient between other observed differential metabolites, O-phosphorylethanolamine, and Enterococcus and Phascolarctobacterium were lower than 0.6 (Figure 6(a)). In addition, we also found 1,2,4-benzenetriol in the colon was positively correlated with Bacteroides (Figures 7(a) and 7(b)), and oleic acid was negatively correlated with an unidentified Ruminococcaceae (Figures 7(a) and 7(c)).
Figure 6.
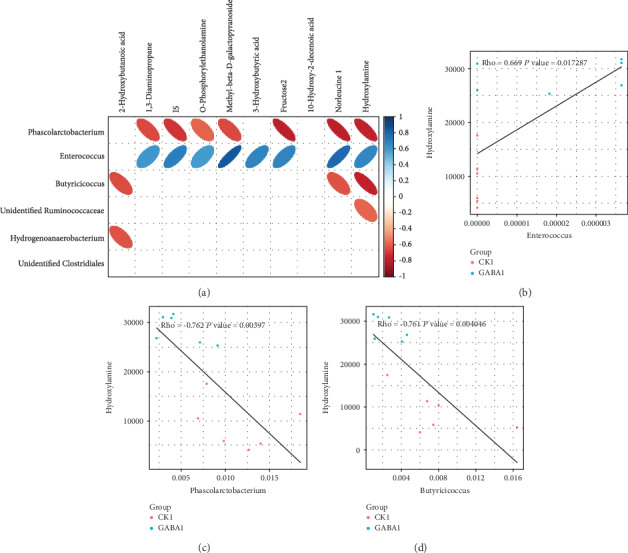
The relationship between gut microbiota and metabolites in healthy piglets. (a) Correlation analysis heat map of differential bacteria and differential metabolites in normal piglets; (b) correlation analysis scatterplot of the content of hydroxylamine and the abundance of Enterococcus in normal piglets; (c) correlation analysis scatterplot of the content of hydroxylamine and the abundance of Phascolarctobacterium in normal piglets. The CK group in this figure means the CON group.
Figure 7.
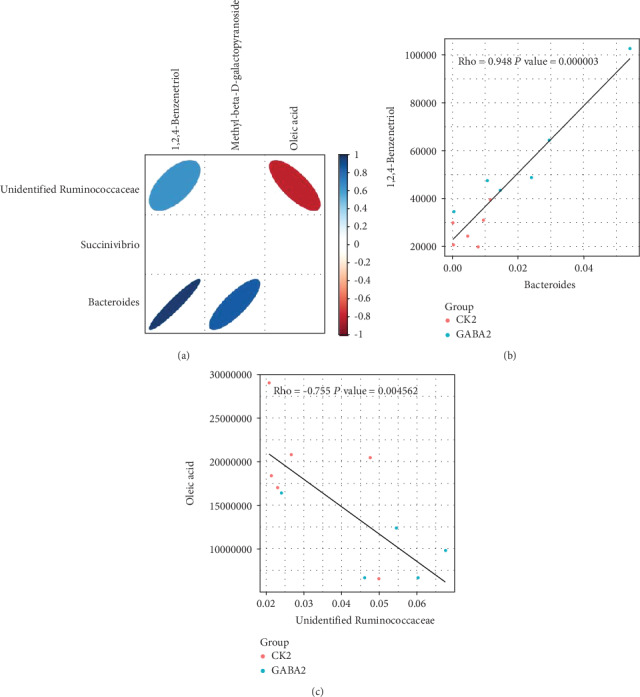
The relationship between gut microbiota and metabolites in ETEC-infected piglets. (a) Correlation analysis heat map of differential bacteria and differential metabolites in ETEC-infected piglets; (b) correlation analysis scatterplot of the content of 1,2,4-benzenetriol and the abundance of Bacteroides in ETEC-infected piglets; (c) correlation analysis scatterplot of the content of oleic acid and the abundance of unidentified Ruminococcaceae in ETEC-infected piglets. The CK group in this figure means the CON group.
4. Discussion
As a multifunctional neurotransmitter, GABA has received a lot of attention for its essential role in conducting neural signals, improving sleep quality, and alleviating hot stress [17, 26, 27]. Dietary GABA supplementation improves the growth performance, serum parameters, feed intake, and immune function of various animals, such as broilers, lambs, and cows [27–29]. However, consistent with our results, there is no direct evidence which revealed that GABA supplementation contributed to the growth of weaning piglets in previous studies [19, 21]. But previous studies and our results both demonstrated that GABA could modify intestine immunity and metabolism condition [21, 22]. In the present study, 40 mg·kg−1 of GABA supplementation decreased the aspartic acid level but increased the histidine level in the serum of normal piglets, while there are no significant changes in serum amino acid contents of ETEC-infected piglets. Aspartic acid can be metabolized to glutamate, which is the precursor of GABA, through transamination [30]. Glutamate could also degrade to histidine [31]. GABA supplementation does not affect the serum glutamate concentration maybe due to the inhibition of absorption of aspartic acid and the promotion of glutamate degradation to histidine in piglets. Surprisingly, GABA supplementation increases the calcium concentration of serum in normal piglets but decreases the albumin content of serum in ETEC-infected piglets. Serum albumin represents the main determinant of plasma oncotic pressure [32], and serum calcium is closely related to calcium and phosphorus metabolism. Therefore, GABA may affect the plasma oncotic pressure and calcium and phosphorus metabolism.
SIgA is a 400 kDa molecule composed of the secretory components, J-chain and dimeric IgA [33]. As a major component of the intestinal immune barrier, SIgA plays an important role in maintaining intestinal health by clearing pathogenic microorganisms and interacting with intestinal commensal microorganisms [5, 34, 35]. Furthermore, the host may discriminate symbionts from pathogens by recognizing the coating of commensal bacteria by SIgA [36]. Thus, enough SIgA secretion in the gut is essential for the intestinal homeostasis. However, piglets, especially weaning piglets, are unable to get maternal immunoglobulins and usually cannot secret enough SIgA because of their underdeveloped intestinal immune system [11]. Interestingly, in the current study, results of the ELISA and immunohistochemistry analyses confirmed that GABA supplementation increases the jejunal and ileal SIgA levels in normal piglets. Various Th2 cytokines, such as transforming growth factor- (TGF-) β1, interleukin- (IL-) 4, IL-5, IL-6, IL-10, and IL-13, can promote the immature B cells differentiated into IgA-secreting plasma cells [13]. To further verify our results about jejunal SIgA secretion and explore the underlying effects of GABA supplementation on cytokine production, we examined the SIgA-secreting cytokines in mRNA and protein levels. We found that jejunal concentrations of IFN-γ, IL-1β, IL-4, and IL-17 were upregulated by GABA treatment in ETEC-infected piglets. IL-1β mediates the host inflammatory response to prevent infection [37]. IFN-γ not only can activate macrophages to enhance phagocytosis of pathogenic bacteria [38] but also is implicated in the induction of pIgR synthesis and dimeric IgA binding [39]. Differentiation of B cells into plasma cells secreting IgA occurs upon interactions with T-cells in the lamina propria in an environment rich in IL-4 and other Th2 cytokines [39]. Moreover, IL-17 is an IgA-inducing cytokine that can increase pIgR expression and therefore the rate of SIgA secreted into the lumen [40]. In total, our study provided evidences that GABA enhances intestinal immunity by promoting SIgA secretion, and this might be the result of elevated levels of Th2 cytokines which further promotes the maturation of IgA-secreting plasma cells.
An extensive body of the literature has confirmed the interaction between SIgA and gut microbiota [13, 35, 41–43]. Interestingly, we detected several noteworthy strains altered by GABA supplementation. For example, GABA supplementation increased the relative abundance of Enterococcus, which has been widely proposed to be a probiotics that could be applied in porcine, murine, chicken, and even human models [44–47]. Feeding Enterococcus faecium significantly increases the intestinal SIgA level and promotes the proliferation of IgA+ cells in chicken and murine models [48–50]. Moreover, feeding dehydrated Enterococcus faecium increases the concentration of IL-4 in jejunal mucosa and decreases intestinal colonization of Escherichia coli in broilers [51]. Therefore, our results suggested GABA supplementation might enhance the SIgA secretion of intestine through increasing the relative abundance of Enterococcus.
The metabolite produced by gut microbes also exerts crucial roles in the health maintenance and modulation of physiologic function [52–56]. In the present study, we identified two metabolites, O-phosphorylethanolamine and hydroxylamine, which are significantly enriched by GABA supplementation in normal piglets. Interestingly, the content of hydroxylamine is positively correlated with the abundance of Enterococcus and associated with two enriched KEGG pathways: nitrogen metabolism and microbial metabolism in diverse environments. These results provided stronger implication that Enterococcus might play a key role in mediating the effect of GABA on intestinal SIgA secretion. On the other hand, the sphingolipid signaling pathway and sphingolipid metabolism are also enriched by GABA supplementation in normal piglets. Brown et al. demonstrated that sphingolipid produced by Bacteroides species can promote symbiosis with the host [57]. Meanwhile, the elevated abundance of Bacteroides in ETEC-infected piglets suggested that GABA supplementation might also affect SIgA secretion through Bacteroides and sphingolipid. However, the changes in microbial metabolism of ETEC-infected piglets are not as significant and organized as those in normal piglets. This could be attributed to the interference of ETEC infection, and further studies will be needed to clarify this issue.
In conclusion, although GABA supplementation had little effect on the growth performance, organ indices, serum amino acid profile, and serum biochemistry, it enhances intestinal immunity by promoting jejunal SIgA secretion. In addition, we observed interesting alterations in gut microbiota and microbial metabolism, which implies the potential mechanisms underlying the promotion of GABA supplementation on SIgA secretion. Our study provides insight into functional patterns of dietary supplementation on gut microbiome and host immune response and stresses the possibility of GABA utilization on intestinal health improvement.
Acknowledgments
This study was funded from the National Key R&D Program (2017YFD0500503 and 2018YFD0500600), the National Natural Science Foundation of China (31922079, 31872365, and 31790411), the Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), the Earmarked Fund for China Agriculture Research System (CARS-35), and the project funded by China Postdoctoral Science Foundation (BX20180096).
Contributor Information
Jing Wang, Email: jingwang90@g.ucla.edu.
Yulong Yin, Email: yinyulong@isa.ac.cn.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
There are no conflicts between the authors to declare.
Authors' Contributions
Y. Yin and J. Wang devised the experiment. Y. Zhao, J. Wang, and Y. Huang conducted the experiment. Y. Zhao analyzed the data and prepared all tables, figures, and manuscript. H. Wang, M. Qi, S. Liao, and P. Bin revised the manuscript. J. Wang and Y. Yin approved the final version of manuscript.
Supplementary Materials
Supplementary Table 1: primers used in this study. Supplementary Table 2: organ indices of piglets in stages 1 and 2. Supplementary Table 3: gut microbial alpha diversity indices of piglets in stages 1 and 2. Figure S1: dietary GABA did not affect the growth performance of piglets. Growth performance of piglets in (A) stage 1 and (B) stage 2. Data are presented as mean ± SEM, analyzed by the unpaired t-test with Welch's correction or Mann–Whitney U test. Figure S2: dietary GABA had little effect on the composition of gut microbiota. (A–E) The relative abundances of main strains in the phylum, class, order, family, and genus level in normal piglets; (F) PCoA of gut microbiota in normal piglets; (G, H) the relative abundances of main strains in the phylum, class, order, family, and genus level in ETEC-infected piglets; (F) PCoA of gut microbiota in ETEC-infected piglets.
References
- 1.Tang Y., Li J., Liao S., et al. The effect of dietary protein intake on immune status in pigs of different genotypes. Food and Agricultural Immunology. 2018;29(1):776–784. doi: 10.1080/09540105.2018.1455812. [DOI] [Google Scholar]
- 2.Pohl C. S., Medland J. E., Mackey E., et al. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterology & Motility. 2017;29(11, article e13118) doi: 10.1111/nmo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsuda K., Kohmoto M., Kawashima K., Tsunemitsu H. Frequency of enteropathogen detection in suckling and weaned pigs with diarrhea in Japan. Journal of Veterinary Diagnostic Investigation. 2006;18(4):350–354. doi: 10.1177/104063870601800405. [DOI] [PubMed] [Google Scholar]
- 4.Guan G., Ding S., Yin Y., Duraipandiyan V., al-Dhabi N. A., Liu G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Science China Life Sciences. 2019;62(8):1019–1027. doi: 10.1007/s11427-018-9494-6. [DOI] [PubMed] [Google Scholar]
- 5.Chairatana P., Nolan E. M. Defensins, lectins, mucins, and secretory immunoglobulin A: microbe-binding biomolecules that contribute to mucosal immunity in the human gut. Critical Reviews in Biochemistry and Molecular Biology. 2017;52(1):45–56. doi: 10.1080/10409238.2016.1243654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Frontiers in Immunology. 2013;4:p. 185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macpherson A. J., Geuking M. B., McCoy K. Homeland security: IgA immunity at the frontiers of the body. Trends in Immunology. 2012;33(4):160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Mantis N. J., Rol N., Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunology. 2011;4(6):603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pabst O. New concepts in the generation and functions of IgA. Nature Reviews Immunology. 2012;12(12):821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 10.Herich R. Is the role of IgA in local immunity completely known? Food and Agricultural Immunology. 2016;28(2):223–237. doi: 10.1080/09540105.2016.1258547. [DOI] [Google Scholar]
- 11.Ushida K., Kameue C., Tsukahara T., Fukuta K., Nakanishi N. Decreasing traits of fecal immunoglobulin A in neonatal and weaning piglets. The Journal of Veterinary Medical Science. 2008;70(8):849–852. doi: 10.1292/jvms.70.849. [DOI] [PubMed] [Google Scholar]
- 12.Ren W., Wang K., Yin J., et al. Glutamine-induced secretion of intestinal secretory immunoglobulin A: a mechanistic perspective. Frontiers in Immunology. 2016;7:p. 503. doi: 10.3389/fimmu.2016.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M., Xiao H., Liu G., et al. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Molecular Nutrition & Food Research. 2016;60(7):1637–1648. doi: 10.1002/mnfr.201600026. [DOI] [PubMed] [Google Scholar]
- 14.Song B., Zheng C., Zha C., et al. Dietary leucine supplementation improves intestinal health of mice through intestinal SIgA secretion. Journal of Applied Microbiology. 2020;128(2):574–583. doi: 10.1111/jam.14464. [DOI] [PubMed] [Google Scholar]
- 15.Ren W., Yin J., Duan J., et al. Mouse intestinal innate immune responses altered by enterotoxigenic Escherichia coli (ETEC) infection. Microbes and Infection. 2014;16(11):954–961. doi: 10.1016/j.micinf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Liu G., Ren W., Fang J., et al. L-Glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids. 2017;49(12):1945–1954. doi: 10.1007/s00726-017-2410-9. [DOI] [PubMed] [Google Scholar]
- 17.Bak L. K., Schousboe A., Waagepetersen H. S. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y. The immunological function of GABAergic system. Frontiers in Bioscience. 2017;22(7):1162–1172. doi: 10.2741/4539. [DOI] [PubMed] [Google Scholar]
- 19.Li Y. H., Li F., Liu M., et al. Effect of γ-aminobutyric acid on growth performance, behavior and plasma hormones in weaned pigs. Canadian Journal of Animal Science. 2015;95(2):165–171. doi: 10.4141/cjas2013-148. [DOI] [Google Scholar]
- 20.Xie W. Y., Hou X. Y., Yan F. B., Sun G. R., Han R. L., Kang X. T. Effect of γ-aminobutyric acid on growth performance and immune function in chicks under beak trimming stress. Animal Science Journal. 2013;84(2):121–129. doi: 10.1111/j.1740-0929.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen S., Tan B., Xia Y., et al. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food & Function. 2019;10(1):366–378. doi: 10.1039/c8fo02161a. [DOI] [PubMed] [Google Scholar]
- 22.Ren W., Liao Y., Ding X., et al. Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunology. 2019;12(2):531–544. doi: 10.1038/s41385-018-0111-7. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y., Chen S., Zhao Y., et al. GABA attenuates ETEC-induced intestinal epithelial cell apoptosis involving GABAAR signaling and the AMPK-autophagy pathway. Food & Function. 2019;10(11):7509–7522. doi: 10.1039/C9FO01863H. [DOI] [PubMed] [Google Scholar]
- 24.Ren W., Yin J., Xiao H., et al. Intestinal microbiota-derived GABA mediates interleukin-17 expression during enterotoxigenic Escherichia coli infection. Frontiers in Immunology. 2017;7:p. 685. doi: 10.3389/fimmu.2016.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren W., Liu G., Yin J., et al. Draft genome sequence of enterotoxigenic Escherichia coli strain W25K. Genome Announcements. 2014;2(3) doi: 10.1128/genomea.00593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamatsu A., Yamashita Y., Maru I., Yang J., Tatsuzaki J., Kim M. The improvement of sleep by oral intake of GABA and Apocynum venetum leaf extract. Journal of Nutritional Science and Vitaminology. 2015;61(2):182–187. doi: 10.3177/jnsv.61.182. [DOI] [PubMed] [Google Scholar]
- 27.Hu H., Bai X., Shah A. A., et al. Dietary supplementation with glutamine and γ-aminobutyric acid improves growth performance and serum parameters in 22- to 35-day-old broilers exposed to hot environment. Journal of Animal Physiology and Animal Nutrition. 2016;100(2):361–370. doi: 10.1111/jpn.12346. [DOI] [PubMed] [Google Scholar]
- 28.Wang D. M., Chacher B., Liu H. Y., Wang J. K., Lin J., Liu J. X. Effects of γ-aminobutyric acid on feed intake, growth performance and expression of related genes in growing lambs. Animal. 2015;9(3):445–448. doi: 10.1017/S1751731114002651. [DOI] [PubMed] [Google Scholar]
- 29.Wang D. M., Wang C., Liu H. Y., Liu J. X., Ferguson J. D. Effects of rumen-protected γ-aminobutyric acid on feed intake, lactation performance, and antioxidative status in early lactating dairy cows. Journal of Dairy Science. 2013;96(5):3222–3227. doi: 10.3168/jds.2012-6285. [DOI] [PubMed] [Google Scholar]
- 30.Birsoy K., Wang T., Chen W. W., Freinkman E., Abu-Remaileh M., Sabatini D. M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanarek N., Keys H. R., Cantor J. R., et al. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature. 2018;559(7715):632–636. doi: 10.1038/s41586-018-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanali G., di Masi A., Trezza V., Marino M., Fasano M., Ascenzi P. Human serum albumin: from bench to bedside. Molecular Aspects of Medicine. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Woof J. M., Russell M. W. Structure and function relationships in IgA. Mucosal Immunology. 2011;4(6):590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 34.Stokes C. R., Soothill J. F., Turner M. W. Immune exclusion is a function of IgA. Nature. 1975;255(5511):745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 35.Lindner C., Wahl B., Föhse L., et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. The Journal of Experimental Medicine. 2012;209(2):365–377. doi: 10.1084/jem.20111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sansonetti P. J. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunology. 2011;4(1):8–14. doi: 10.1038/mi.2010.77. [DOI] [PubMed] [Google Scholar]
- 37.Han S., Yu H., Yang F., Qiao S., He P. Effect of dietary supplementation with hyperimmunized hen egg yolk powder on diarrhoea incidence and intestinal health of weaned pigs. Food and Agricultural Immunology. 2019;30(1):333–348. doi: 10.1080/09540105.2019.1581732. [DOI] [Google Scholar]
- 38.Ren D., Wang D., Liu H., Shen M., Yu H. Two strains of probioticLactobacillusenhance immune response and promote naive T cell polarization to Th1. Food and Agricultural Immunology. 2019;30(1):281–295. doi: 10.1080/09540105.2019.1579785. [DOI] [Google Scholar]
- 39.Nelson R., Katayama S., Mine Y., Duarte J., Matar C. Immunomodulating effects of egg yolk low lipid peptic digests in a murine model. Food and Agricultural Immunology. 2007;18(1):1–15. doi: 10.1080/09540100601178623. [DOI] [Google Scholar]
- 40.Levkut M., Husáková E., Bobíková K., et al. Inorganic or organic zinc and MUC-2, IgA, IL-17, TGF-β4 gene expression and sIgA secretion in broiler chickens. Food and Agricultural Immunology. 2017;28(5):801–811. doi: 10.1080/09540105.2017.1313202. [DOI] [Google Scholar]
- 41.Catanzaro J. R., Strauss J. D., Bielecka A., et al. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-49923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macpherson A. J., Yilmaz B., Limenitakis J. P., Ganal-Vonarburg S. C. IgA function in relation to the intestinal microbiota. Annual Review of Immunology. 2018;36:359–381. doi: 10.1146/annurev-immunol-042617-053238. [DOI] [PubMed] [Google Scholar]
- 43.Auteri M., Zizzo M. G., Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacological Research. 2015;93:11–21. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Beirão B. C. B., Ingberman M., Fávaro C., et al. Effect of an Enterococcus faecium probiotic on specific IgA following live Salmonella Enteritidis vaccination of layer chickens. Avian Pathology. 2018;47(3):325–333. doi: 10.1080/03079457.2018.1450487. [DOI] [PubMed] [Google Scholar]
- 45.Benyacoub J., Pérez P. F., Rochat F., et al. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. The Journal of Nutrition. 2005;135(5):1171–1176. doi: 10.1093/jn/135.5.1171. [DOI] [PubMed] [Google Scholar]
- 46.Kreuzer S., Machnowska P., Aßmus J., et al. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Veterinary Research. 2012;43(1):p. 58. doi: 10.1186/1297-9716-43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surono I. S., Koestomo F. P., Novitasari N., Zakaria F. R., Yulianasari K. Novel probiotic Enterococcus faecium IS-27526 supplementation increased total salivary sIgA level and bodyweight of pre-school children: a pilot study. Anaerobe. 2011;17(6):496–500. doi: 10.1016/j.anaerobe.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Bobíková K., Revajová V., Karaffová V., Levkutová M., Levkut M. IgA gene expression and quantification of cecal IgA+, IgM+, and CD4+ cells in chickens treated with EFAL41 and infected with Salmonella Enteritidis. Acta Histochemica. 2015;117(7):629–634. doi: 10.1016/j.acthis.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Hendrickx A. P. A., van de Kamer D., Willems R. J. L. Primary murine mucosal response during cephalosporin-induced intestinal colonization by Enterococcus faecium. MicrobiologyOpen. 2018;7(5, article e00602) doi: 10.1002/mbo3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Husáková E., Bobíková K., Stašová D. Total IgA in spleen, bursa and intestine of chickens pretreated withE. faeciumAL41 and challenged withSalmonellaEnteritidis PT4. Food and Agricultural Immunology. 2014;26(3):366–370. doi: 10.1080/09540105.2014.918587. [DOI] [Google Scholar]
- 51.Cao G. T., Zeng X. F., Chen A. G., et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Science. 2013;92(11):2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- 52.Fandriks L. Roles of the gut in the metabolic syndrome: an overview. Journal of Internal Medicine. 2017;281(4):319–336. doi: 10.1111/joim.12584. [DOI] [PubMed] [Google Scholar]
- 53.Jonsson A. L., Backhed F. Role of gut microbiota in atherosclerosis. Nature Reviews. Cardiology. 2017;14(2):79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 54.Lin L., Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunology. 2017;18(1):p. 2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Postler T. S., Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metabolism. 2017;26(1):110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonnenburg J. L., Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown E. M., Ke X., Hitchcock D., et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host & Microbe. 2019;25(5):668–680.e7. doi: 10.1016/j.chom.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: primers used in this study. Supplementary Table 2: organ indices of piglets in stages 1 and 2. Supplementary Table 3: gut microbial alpha diversity indices of piglets in stages 1 and 2. Figure S1: dietary GABA did not affect the growth performance of piglets. Growth performance of piglets in (A) stage 1 and (B) stage 2. Data are presented as mean ± SEM, analyzed by the unpaired t-test with Welch's correction or Mann–Whitney U test. Figure S2: dietary GABA had little effect on the composition of gut microbiota. (A–E) The relative abundances of main strains in the phylum, class, order, family, and genus level in normal piglets; (F) PCoA of gut microbiota in normal piglets; (G, H) the relative abundances of main strains in the phylum, class, order, family, and genus level in ETEC-infected piglets; (F) PCoA of gut microbiota in ETEC-infected piglets.
Data Availability Statement
The data used to support the findings of this study are included within the article.


