Abstract
People with Parkinson’s disease (PD) exhibit an increase in fixational saccades during the preparatory period prior to target onset in the antisaccade task and this increase is related to an increase in prosaccade errors in the antisaccade task. It was previously shown that bilateral, but not unilateral, subthalamic nucleus deep brain stimulation (STN DBS) in people with PD further increases the prosaccade error rate on the antisaccade task. We investigated whether bilateral STN DBS also increases the number of fixational saccades in the preparatory period of the antisaccade task and if this increase in the number of fixational saccades is related to prosaccade errors. We found that: (1) there were a greater number of fixational saccades during the preparatory period of the antisaccade task during bilateral STN DBS compared to no STN DBS (p < 0.001), unilateral STN DBS (p < 0.001), and healthy controls (p = 0.02), and (2) the increase in the number of fixational saccades increased the probability of a prosaccade error for the antisaccade task during bilateral STN DBS (p = 0.005). This association between number of fixational saccades and probability of a prosaccade error was similar across no STN DBS, unilateral stimulation, and healthy controls. In addition, we found that the proportion of express prosaccade errors and prosaccade error latency were similar across stimulation conditions. We propose that bilateral STN DBS disrupts the integrated activity of cortico-basal ganglia-collicular processes underlying antisaccade preparation and that this disruption manifests as an increase in both fixational saccades and prosaccade error rate.
Keywords: Deep brain stimulation, Preparatory set, Parkinson’s disease, Fixational saccades, Antisaccade
Introduction
A primary symptom of Parkinson’s disease (PD) is impaired execution of voluntary movement (Marsden 1982). Voluntary movement requires the preparation of neural circuits to coordinate the facilitation of a behaviorally appropriate motor program as well as the inhibition of automatic and reflexive competing motor programs (Haggard 2008; Watanabe et al. 2013). The neural dynamics underlying these preparatory processes are dysfunctional in PD (Cunnington et al. 1997; Praamstra et al. 1996a, b).
It has been hypothesized that fixational saccades, which are microsaccades generated during ocular fixation, serve as an index for preparatory set (Betta and Turatto 2006; Hermens et al. 2010; Watanabe et al. 2013), which is defined as the intention to make a specific response and the readiness to respond (Evarts et al. 1984; Hebb 1972). Healthy individuals show a reduction in fixational saccades generated during the preparatory period preceding voluntary eye and limb movements compared to the number of fixational saccades they make when no subsequent movement is required (Betta and Turatto 2006; Watanabe et al. 2013). For example, when given prior instructions to either make a manual response to a stimulus or ignore a stimulus and make no response, subjects reduce the number of fixational saccades when prior instructions are to make the manual response compared to when the prior instructions are to refrain from responding (Betta and Turatto 2006).
In contrast to healthy controls, people with PD exhibit an increase in fixational saccades during the preparatory period prior to target onset in the antisaccade task and this increase has been shown to be related to an increase in prosaccade errors on the antisaccade task (McInnis 2014). This association between increased prosaccade error and increased fixational saccades has also been shown in other neurological populations, such as schizophrenia (Barton et al. 2008) and attention-deficit hyperactivity disorder (Munoz et al. 2003). Altogether, previous studies have shown that failure to reduce the number of fixational saccades in the antisaccade task is related to an increase in prosaccade error in healthy controls (Barton et al. 2008; Watanabe et al. 2013) and this association is exaggerated in some neurological populations, such as PD (McInnis 2014).
Although the precise mechanism of generation of fixational saccades is unknown, eye positions during fixation and saccade execution are both dependent on the coordination of neural activity between the two superior colliculi (Krauzlis et al. 2017). Neural activity of the rostral superior colliculus is related to central gaze fixation and fixational saccades (Coe and Munoz 2017; Krauzlis et al. 2017; Martinez-Conde et al. 2009). Descending cortical and basal ganglia projections strongly influence superior collicular activity. Considering the STN is a critical node of the oculomotor circuit of the basal ganglia (Coe and Munoz 2017; Hikosaka et al. 2000) and that it has been shown to be involved with the preparation of voluntary saccades (Fawcett et al. 2007; Yugeta et al. 2013), it is highly likely that stimulating the STN could alter saccade preparatory processes, including the generation of fixational saccades.
The current study investigated the effects of bilateral deep brain stimulation of the subthalamic nucleus (STN DBS) on preparatory set for the antisaccade task using fixational saccades prior to target onset as the measure of task preparation. We have previously shown that bilateral STN DBS increases prosaccade error rate on the antisaccade task compared to unilateral STN DBS and no stimulation (off STN DBS) in people with PD (Goelz et al. 2017). Further, previous studies have shown an association between the number of fixational saccades prior to target onset and number of prosaccade errors (Barton et al. 2008; McInnis 2014; Munoz et al. 2003; Watanabe et al. 2013). Therefore, the current study investigated whether bilateral STN DBS also increases the number of fixational saccades prior to target onset in the antisaccade task and whether the number of fixational saccades is related to prosaccade error rate.
An alternative explanation for an increase in prosaccade error rate is that inhibitory oculomotor circuits themselves are impaired. This inhibitory impairment can lead to impulsivity and this can affect goal-directed behavior (Bari and Robbins 2013). Prosaccade errors may be triggered despite proper preparatory processes (i.e., although preparatory processes are implemented correctly, impaired inhibitory circuits are unable to suppress saccade to target) (Barton et al. 2008). Prosaccade errors due to impaired inhibitory circuits are characterized by shortened prosaccade error latencies that fall in the express range (90–140 ms), whereas prosaccade errors due to impaired failure to implement correct task set are characterized by saccade latencies in the regular saccade range (> 140 ms) (Coe and Munoz 2017). It is currently unknown neither how STN DBS affects the proportion of express prosaccade errors nor how it affects the prosaccade error latency. If the increase in prosaccade error rate during bilateral STN DBS (Goelz et al. 2017) is due to stimulation interference with oculomotor inhibitory circuits, one would expect to find a greater proportion of express prosaccade errors and a reduction in prosaccade error latency during bilateral STN DBS.
Methods
Participants
Ten patients (7 male, 3 female; mean age 58 years ± SD 6.8) with advanced PD and bilaterally implanted STN stimulators (Medtronic Inc., Minneapolis, MN, USA) were tested along with 10 healthy, age- and sex-matched controls (57 years ± 8.2). All patients and controls recruited for this study were right hand dominant. This was confirmed by the Edinburgh Handedness Inventory (Oldfield 1971). Patients were examined by a movement disorders neurologist and included in the study if they (1) had PD as outlined by the Parkinson’s disease Society Brain bank diagnostic criteria (Hughes et al. 1992), (2) had undergone bilateral STN DBS surgery, (3) scored 25 or greater on the Mini-Mental State Examination (Folstein et al. 1975), and (4) exhibited no eyelid opening apraxia or other clinically evident eye movement abnormalities. With the exception of PD, both patients and controls had no known neurological disorders. Participants gave informed consent to all experimental procedures, which were approved by the Institutional Review Board at the University of Illinois at Chicago. Patient demographics, clinical rating scores, and stimulator parameters are shown in Table 1.
Table 1.
Patient characteristics, MDS-UPDRS motor scores, and stimulator settings
| ID | Sex | Age (years) |
Date of surgery |
Disease duration at time of surgery (years) |
Years since surgery at time of testing |
MDS-UPDRS motor score |
Left stimulator |
Right stimulator |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left electrode |
Right electrode |
OFF | UNI |
BOTH | Voltage (v) |
Frequency (Hz) |
Pulse width (μS) |
Voltage (v) |
Frequency (Hz) |
Pulse width (μs) |
||||||
| Left | Right | |||||||||||||||
| 1 | F | 50 | 7/10/2001 | 1/11/2002 | 8 | 11 | 34 | 40 | 36 | 40 | 2.2 | 185 | 120 | 2.0 | 185 | 120 |
| 2 | M | 59 | 6/6/2006 | 6/6/2006 | 13 | 6 | 74 | 48 | 51 | 50 | 4.1 | 185 | 80 | 4.5 | 185 | 100 |
| 3 | M | 50 | 4/14/2006 | 4/14/2006 | 24 | 7 | 55 | 47 | 51 | 31 | 2.9 | 185 | 60 | 2.6 | 185 | 60 |
| 4 | M | 58 | 4/14/2009 | 4/14/2009 | 7 | 4 | 53 | 43 | 49 | 37 | 2.6 | 185 | 90 | 4.0 | 185 | 60 |
| 5 | M | 58 | 12/20/2011 | 12/20/2011 | 14 | 1 | 97 | 45 | 73 | 12 | 4.1 | 140 | 120 | 3.5 | 140 | 210 |
| 6 | M | 63 | 3/19/2010 | 3/19/2010 | 8 | 3 | 38 | 26 | 34 | 13 | 2.3 | 130 | 60 | 2.2 | 130 | 450 |
| 7 | M | 59 | 6/28/2002 | 6/1/2001 | 11 | 11 | 56 | 74 | 36 | 31 | 3.1 | 160 | 60 | 3.1 | 160 | 60 |
| 8 | M | 73 | 10/8/2007 | 10/8/2007 | 4 | 6 | 53 | 27 | 40 | 25 | 5.5 | 185 | 120 | 4.0 | 185 | 120 |
| 9 | F | 61 | 3/28/2006 | 3/28/2006 | 5 | 7 | X | 42 | 29 | 30 | 2.9 | 185 | 60 | 3.7 | 125 | 80 |
| 10 | F | 53 | 6/8/2004 | 10/18/2002 | 6 | 9 | 65 | 52 | 61 | 43 | 2.5 | 130 | 60 | 2.6 | 180 | 60 |
Procedure
Patients completed a battery of eye movement tasks on four separate, consecutive days. In this paper, we only present data from the antisaccade task. Only one stimulator condition was tested each day. The order of stimulation conditions was randomized for the first five patients and counterbalanced for the last five patients to average out practice, order, and fatigue effects. The patients were tested after a 12-h overnight withdrawal from PD medication under 1 of 4 stimulator conditions: off stimulation (OFF), left unilateral stimulation (LEFT), right unilateral stimulation (RIGHT), or both stimulators on (BOTH). The LEFT, RIGHT, and BOTH on conditions were tested with stimulators set to their respective clinical settings. Each testing session began 3 h after the appropriate stimulators were turned off for the condition of that day. One of the ten patients was unable to perform testing for the OFF stimulation condition due to extreme discomfort from excessive rigidity. Healthy controls performed one block of the antisaccade task.
The antisaccade task was performed in a completely darkened room. Participants sat upright in a height-adjustable chair with arm rests. In order to control for head movement, participants placed their chin on a stationary chin rest and head movements were recorded using a 3-D motion capture system (Optotrak 3020, Northern Digital, Inc.). Eye movements were collected using an eye-tracking system consisting of two miniature cameras mounted on a comfortable padded headband (Eyelink II, SR Research Ltd, 2002) which has a spatial resolution (RMS) of 0.01° and velocity noise < 0.5°/s. Pupil position was sampled at a frequency of 500 Hz. A 5 degrees of freedom robot arm (CRS Robotics Corp., A255) presented green LED visual targets (3 mm green LED, 70 mcd) in a plane located 47 cm in front of the subject. Eye and head movement data were synchronized and stored using The Motion Monitor software (Innovative Sports Training, Inc. 2011).
Fixation prior to antisaccade and instructional set
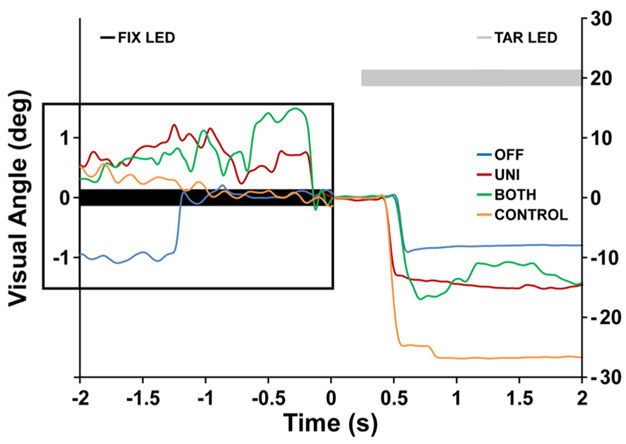
Each trial began with the subject fixating a central LED (0° visual angle) for 3000 ms. Next, the central fixation cue disappeared, and after a 200 ms gap a target was presented at 20° of visual angle for 1800 ms to the left or right of center. The instructional set was “look to the mirror image location in the opposite direction as quickly and accurately as possible”. Inter-trial intervals were constant (2000 ms) and no stimulus was presented during the inter-trial intervals. Target location (left or right) was randomized for each trial such that each target location was presented 25 times. The fixation prior to the antisaccade task was completed in a single block of 50 trials. Figure 1 shows eye movement traces of representative trials for each stimulation condition (OFF, UNI, and BOTH), as well as for a healthy control (CONTROL) during a single antisaccade trial. The filled black line at 0° visual angle represents the presentation of the fixation LED for 2 s. This is followed by a gap of 0.2 s when no visual stimulus is presented. The filled gray line at 20° visual angle represents the presentation of the target LED in the right visual field. Subjects are shown fixating during the fixation period and then making an antisaccade to the opposite side (left) toward the mirror image location of the target during OFF, UNI, and BOTH STN DBS.
Fig. 1.
The eye movement traces of representative trials for each stimulation condition (OFF, UNI, and BOTH), as well as for a healthy control (CONTROL) during a single antisaccade trial. The double y-axis is visual angle in degrees. The distance between tick marks on the y-axis on the left corresponds to 1° of visual angle, while the y-axis on the right corresponds to 10° of visual angle. 0° visual angle corresponds to location of the fixation LED (filled black rectangle). Positive values on the y-axis correspond to the right of the visual field and negative values correspond to the left of the visual field. The x-axis is time in seconds. The inset illustrates the eye movement traces during the fixation period of the trial. The fixation LED (filled black rectangle) remains illuminated from – 2 to 0 s. The number of fixational saccades was computed between – 2 and 0 s. A gap, when no stimulus was illuminated, is shown from 0 to 0.2 s. The filled gray rectangle at 20° visual angle (to the right of the visual field) denotes the target LED. The target LED remained illuminated from 0.2 to 2 s. After the onset of the target LED, i.e., at 0.2 s, participants are shown making antisaccades to the opposite side toward the mirror image location of the target LED, i.e., – 20° visual angle to the left
Data processing
Of the 3000 ms fixation period, the first 1000 ms was ignored to allow for gaze to stabilize on the fixation LED and only the last 2000 ms was used to obtain fixational saccadic measures. The data were analyzed using a custom Matlab script (The MathWorks, Natick, MA, USA). A 100 Hz low-pass 2nd order Butterworth filter was applied to the position data of the right and left eye. Eye velocity was calculated from the filtered eye position data. There were no differences between left and right eye velocities for each of the STN DBS conditions for the first four subjects analyzed; therefore, the average of the left and right eye velocities was used to determine saccade metrics. Fixational saccades had to meet a minimum peak velocity criterion of 30°/s and a minimum distance between successive peaks of 60 ms in order to be considered a fixational saccade. The number of these peaks corresponded to the number of fixational saccades for a given trial. All horizontal saccades made during attempted fixation, regardless of size, were defined as fixational saccades (Otero-Millan et al. 2013). Fixational saccades included square wave jerks, microsaccades, and any other kind of intrusional saccades as there is evidence to suggest that these saccades are generated by a common oculomotor mechanism (Otero-Millan et al. 2011). After analyzing all 1883 antisaccade trials for all STN DBS conditions and healthy controls, we found that using a fixed peak velocity threshold of 30°/s lacked specificity in 305 trials and sensitivity in 131 trials. To obtain the greatest specificity and sensitivity, these trials were visually analyzed and peak thresholds adjusted by rater input on a trial-by-trial basis so that peaks that exceeded the 30°/s but that were not a fixational saccade were rejected and peaks that were below the 30°/s but were a fixational saccade were accepted by the algorithm. To determine prosaccade error latency, a constant threshold of 30°/s was used to determine onset based on the velocity profile of the saccade. The algorithm searched backward from the peak velocity to determine the first point in time when the threshold was crossed. This point was determined as the onset of a saccade. Saccade latencies between 90 and 140 ms were characterized as express saccades (Barton et al. 2008; Coe and Munoz 2017; Fischer and Ramsperger 1984). Trials were rejected and omitted from analyses if the subject blinked or failed to perform the task.
LEFT unilateral STN DBS and RIGHT unilateral STN DBS were collapsed into a UNI STN DBS condition as initial analyses revealed no systematic lateralization patterns between LEFT and RIGHT unilateral STN DBS (see Goelz et al. 2017, supplementary material).
Statistical analysis
Generalized linear mixed models were used to assess the effect of STN DBS condition on number of fixational saccades prior to antisaccades (PROC GLIMMIX, SAS 9.4). The random effect was subject. The fixed effect was STN DBS condition (OFF, UNI, and BOTH). Log likelihood ratio tests were performed to assess model fit. In the event of a significant effect of condition, comparisons between STN DBS conditions were evaluated using post hoc paired sample t tests. In addition, we present independent sample t tests comparing each STN DBS condition to age- and sex-matched healthy controls.
Generalized linear mixed models were also used to assess whether the number of fixational saccades during the preparatory phase of the antisaccade task was related to the probability of committing a prosaccade error (PROC GLIMMIX, SAS 9.4). We also assessed whether the association between number of fixational saccades and the probability of committing a prosaccade error varied as a function of STN DBS condition. The random effect was subject. There were three fixed effects: (1) the grand mean centered number of fixational saccades, (2) STN DBS condition (OFF, UNI, and BOTH), and (3) the interaction between number of fixational saccades and STN DBS condition. The change in – 2 log likelihood was used to assess the model with the best fit. In the event of a significant effect of condition, comparisons between STN DBS conditions were evaluated using post hoc paired sample t tests. In addition, we present the association between number of fixational saccades and estimated probability of error for CONTROLS and independent sample t tests comparing each STN DBS condition to age- and sex-matched healthy controls. Results from the most parsimonious model are reported in the results section.
Generalized linear mixed models were also used to assess the effect of STN DBS condition on the proportion of express prosaccade errors (PROC GLIMMIX, SAS 9.4). The random effect was subject. The fixed effect was STN DBS condition (OFF, UNI, and BOTH). Log likelihood ratio tests were performed to assess model fit. In the event of a significant effect of condition, comparisons between STN DBS conditions were evaluated using post hoc paired sample t tests. In addition, we present independent sample t tests comparing each STN DBS condition to age- and sex-matched healthy controls.
A linear mixed model was used to assess the effect of STN DBS condition on prosaccade error latency (PROC MIXED, SAS 9.4). The random effect was subject. The fixed effect was STN DBS condition (OFF, UNI, and BOTH). Log likelihood ratio tests were performed to assess model fit. In the event of a significant effect of condition, comparisons between STN DBS conditions were evaluated using post hoc paired sample t tests. In addition, we present independent sample t tests comparing each STN DBS condition to age- and sex-matched healthy controls.
All the above statistical tests were two-tailed, critical alpha was 0.05, and p values were corrected using the Bonferroni method.
Results
The effect of STN DBS on antisaccade latency, gain, and prosaccade error has been previously published in Goelz et al. (2017).
Effects of STN DBS condition on fixational saccades prior to antisaccades
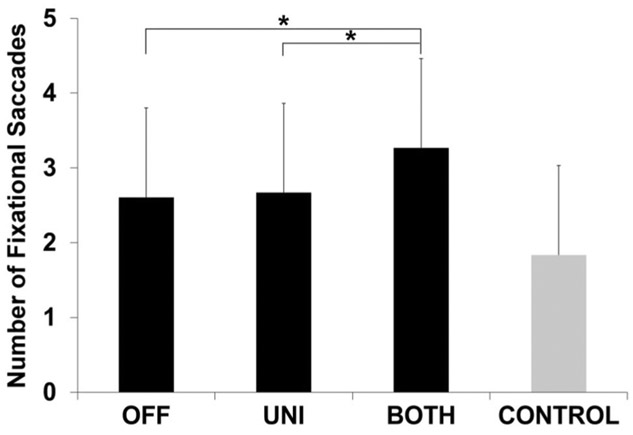
There was a main effect of STN DBS condition on mean number of saccades prior to making an antisaccade (F2,1469 = 13.87, p < 0.001) as shown in Fig. 2. Post hoc pairwise comparisons revealed that the BOTH condition significantly increased number of saccades compared to OFF (estimated mean difference in number of saccades, 0.66; p < 0.001) and UNI (0.60; p < 0.001). In addition, while the number of fixational saccades during BOTH STN DBS was well above CONTROL, this difference was not statistically significant after Bonferroni correction (1.2; p = 0.132; uncorrected p = 0.022) (Fig. 2).
Fig. 2.
Fixational saccades prior to antisaccades. This plot shows the estimated mean and standard error for number of fixational saccades prior to antisaccade for PD patients during OFF, UNI, and BOTH stimulation conditions (black bars) and healthy, age- and sex-matched controls (gray bar). Asterisk indicates significant differences between the conditions
Association between number of fixational saccades prior to antisaccades and prosaccade errors
The most parsimonious model with the best fit that estimated the association between number of fixational saccades and prosaccade errors was one that included the number of fixational saccades and STN DBS condition. The interaction term was not significant and was dropped from the final model.
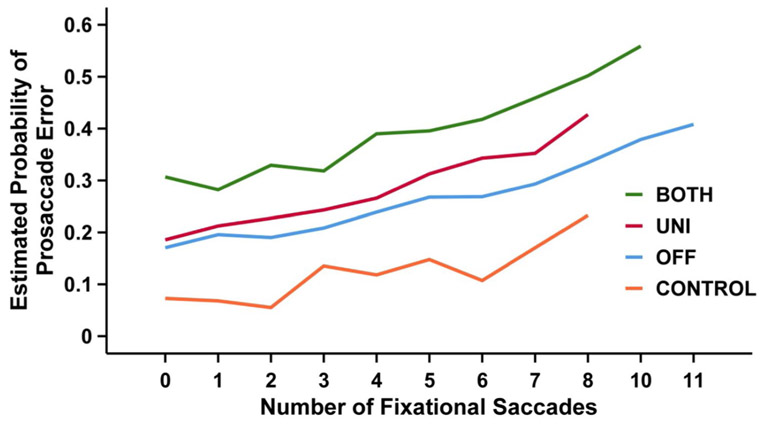
The association between number of fixational saccades and probability of committing a prosaccade error was significant (F1,1163 = 8.12, p = 0.005) as shown in Fig. 3. To elaborate, the number of fixational saccades during the preparatory phase was significantly associated with the log odds (or the probability) of committing a prosaccade error (log odds, 0.105; 95% CI 0.03, 0.18; p = 0.005). Averaging across STN DBS conditions, an increase in one fixational saccade was associated with approximately an 11% increase in the log odds of committing a prosaccade error.
Fig. 3.
Association between number of fixational saccades and the probability of a prosaccade error. This plot shows the mean estimated probability of committing a prosaccade error (y-axis) for a given number of fixational saccades (x-axis) for the OFF (blue line), UNI (red line), and BOTH (green line) stimulation conditions in patients with PD and age- and sex-matched healthy controls (orange line)
The main effect for STN DBS condition was also significant (F2,1163 = 5.87, p = 0.003). This can be seen as a difference in intercepts in Fig. 3. Post hoc paired sample analyses revealed that the log odds of committing a prosaccade error during BOTH was significantly greater than UNI (estimated difference in log odds, 0.5; 95% CI 0.08, 0.9; p = 0.01) and OFF (0.7; 95% CI 0.17, 1.2; p = 0.005). In addition, independent sample analyses also revealed that the log odds of committing a prosaccade error during BOTH (1.8; 95% CI 0.8, 2.8; p < 0.001), UNI (1.3; 95% CI 0.3, 2.3; p = 0.003), and OFF (1.1; 95% CI 0.06, 2.2; p = 0.03) were significantly greater than CONTROL.
Proportion of express prosaccade errors
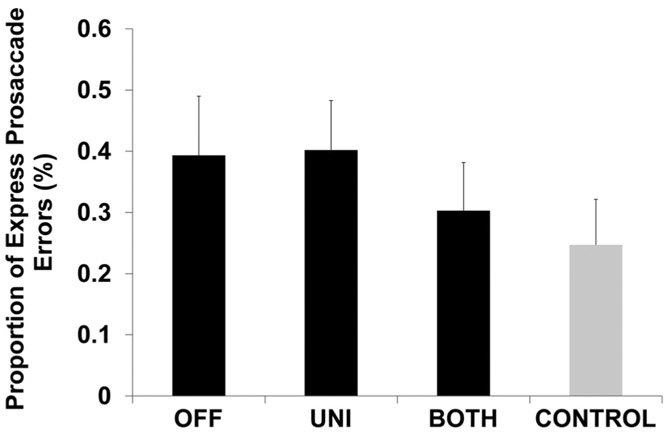
There was no effect of STN DBS condition on the proportion of express prosaccade errors (F2,377 = 1.38, p = 0.25, Fig. 4). In addition, there were no differences between any of the STN DBS conditions and CONTROL (OFF vs. CONTROL, p = 1; UNI vs. CONTROL, p = 0.48; BOTH vs. CONTROL, p = 1; Fig. 4).
Fig. 4.
Proportion of express prosaccade errors. This plot shows the estimated proportion of express prosaccade errors and standard error for participants with PD during OFF, UNI, and BOTH stimulation conditions (black bars) and healthy, age- and sex-matched controls (gray bar). There were no significant differences between conditions
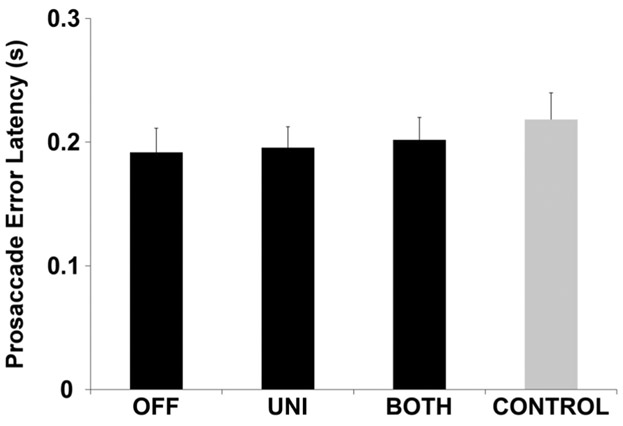
Prosaccade error latency
Mean prosaccade error latency was in the regular range (> 140 ms) for all STN DBS conditions. There was no effect of STN DBS condition on prosaccade error latency (F2,377 = 0.24, p = 0.79, Fig. 5). In addition, there were no differences between any of the STN DBS conditions and CONTROL (OFF vs. CONTROL, p = 1; UNI vs. CONTROL, p = 1; BOTH vs. CONTROL, p = 1; Fig. 5).
Fig. 5.
Prosaccade error latency. This plot shows the estimated mean prosaccade error latency and standard error for participants with PD during OFF, UNI, and BOTH stimulation conditions (black bars) and healthy, age- and sex-matched controls (gray bar). There were no significant differences between conditions
Discussion
The current study adds to a line of previous work showing a differential effect of bilateral and unilateral STN DBS on voluntary motor control (Alberts et al. 2008; David et al. 2018; Goelz et al. 2017). We have previously reported that bilateral STN DBS, but not unilateral STN DBS, increases prosaccade error rate on the antisaccade task, a task with high volitional control demands requiring the coordination of facilitatory and inhibitory saccade processes (Goelz et al. 2017). The findings of the current study extend our understanding of this error induced by bilateral STN DBS. As shown in Fig. 2, there are an increased number of fixational saccades prior to the anti-saccade task during bilateral STN DBS compared to no STN DBS, unilateral STN DBS, and healthy controls. In addition, we show that an increase in the number of fixational saccades is associated with an increase in the probability of committing a prosaccade error (Fig. 3). While this association is the same between conditions, bilateral STN DBS is associated with a higher probability of a prosaccade error for any given number of fixational saccades (Fig. 3). Our findings are in line with previous work which has shown an association between an increase in the number of fixational saccades and an increase in prosaccade error (Barton et al. 2008; McInnis 2014; Munoz et al. 2003; Watanabe et al. 2013). This suggests that STN DBS disrupts preparatory set for the antisaccade task, resulting in a deficit in implementing the correct saccade motor program.
An alternative interpretation for these data is that the increase in the number of prosaccade errors could indicate a compromised motor inhibitory system (Barton et al. 2008; Coe and Munoz 2017). A compromised motor inhibitory system would result in the early release of reflexive, or express saccades (90–140 ms) to target, whereas impaired implementation of inhibition (Barton et al. 2008) or altered frontal lobe connectivity (Coe and Munoz 2017) would result in prosaccade errors in a regular saccade latency range (> 140 ms). Our findings show that stimulation condition had no effect on the proportion of express prosaccade errors (Fig. 4) or on the prosaccade error latency (Fig. 5) during the antisaccade task. More importantly, the mean prosaccade error latency was in the regular range. Taken together, these findings do not support the argument that disruption of inhibitory processes underlies the increase in prosaccade error rate and indicate that the increased prosaccade errors were likely due to a disruption in processes involved with preparation and execution of the proper task set.
Mechanisms underlying the increased prosaccade error rate during bilateral STN DBS
It has been posited that people with PD exhibit impaired generation and inhibition of saccades due to progressive changes in neural drive to the superior colliculus (Terao et al. 2011, 2013). These changes include altered cortical and sub-cortical drive to the superior colliculus. Cortical changes include a reduced voluntary drive from saccade-related cortical areas such as the frontal eye field and the supplementary eye field to the superior colliculus (Terao et al. 2011, 2013). Sub-cortical changes include an increased output of the basal ganglia resulting in excessive inhibition of superior colliculus saccade-generating cells and fluctuating STN bursting patterns resulting in phasic disinhibition of the superior colliculus (Terao et al. 2011, 2013). It is likely that high-frequency stimulation of the STN alters cortical and sub-cortical drive to the superior colliculus, thereby improving some saccade metrics, such as antisaccade gain, while impairing other metrics, such as prosaccade error rate on the antisaccade task (Goelz et al. 2017).
Preparatory set impairment due to cortical drive disruption
The modulation of voluntary drive in preparation for saccade generation includes both excitatory and inhibitory cortical saccade processes (Terao et al. 1998). For example, the FEF ipsilateral to the target is involved with the generation of the antisaccade in the opposite direction while it also inhibits activity in the contralateral FEF possibly through callosal fibers (Terao et al. 1998). It is also known that the STN receives input from the supplementary eye field (SEF), frontal eye field (FEF) (Nambu et al. 2002), and the dorsolateral prefrontal cortex (DLPFC) (Benarroch 2008; Morris et al. 2017), which are primary cortical areas associated with preparatory set of the antisaccade task (DeSouza et al. 2003; Everling and Munoz 2000; Schlag-Rey et al. 1997; Sweeney et al. 1996). High-frequency STN DBS could disrupt preparatory set neural cortical activity antidromically via the direct projections to the STN from the FEF, SEF, and DLPFC or orthodromically via basal ganglia-thalamo-cortical projections. In support of this idea, previous studies have shown that STN DBS alters activity in frontal (Hilker et al. 2004; Mayer et al. 2016) and prefrontal areas such as the DLPFC (Campbell et al. 2008; Kalbe et al. 2009; Limousin et al. 1997). It is argued that STN DBS either replaces or superimposes an invariant high-frequency signal (> 130 Hz) upon the pathological signal in the STN and throughout the striato-thalamo-cortical network (Grill et al. 2004). It is possible that the invariant high-frequency STN DBS disrupts the modulatory capacity of these cortical areas involved with antisaccade preparatory set, thus reducing the integrity of the cortical voluntary drive to the superior colliculus.
Preparatory set impairment vs. inhibitory impairment: evidence against inhibitory impairment
Alternatively, and possibly in conjunction with preparatory set disruption, bilateral STN DBS may reduce the inhibitory output from the basal ganglia to levels below that of normal inhibitory output thereby leading to excessive disinhibition of the superior colliculus. The deficit in inhibitory input to the superior colliculus could occur via reduced inhibitory influence from altered STN-substantia nigra pars reticulata-superior colliculus projections. It has been shown that STN DBS reduces STN firing rate in patients with PD (Toleikis et al. 2012; Welter et al. 2004). It has also been shown that STN DBS can modify both the excitatory signals from the STN to the substantia nigra pars reticulata as well as the inhibitory signals from the globus pallidus externus to the substantia nigra pars reticulata via antidromic stimulation (Chiken and Nambu 2014; Maurice et al. 2003). Thus, it is possible that bilateral STN DBS reduces the excessive inhibitory output of the substantia nigra pars reticulata (Benazzouz et al. 1995), thereby allowing the superior colliculus to more easily and quickly activate brain stem saccade generators which could facilitate saccade generation and compromise saccade inhibition.
Antisaccade initiation is dependent on a threshold level of preparatory pre-saccadic activity that is required to initiate a saccade (Carpenter and Williams 1995; LeVasseur et al. 2001; Munoz et al. 2002; Noorani and Carpenter 2013). During the antisaccade task, neural preparatory activity associated with an antisaccade motor program must reach threshold before the neural preparatory activity for a prosaccade motor program reaches threshold. Successful antisaccade generation requires inhibition of activity underlying the prosaccade program while simultaneously increasing the activity involved with the antisaccade program (Noorani and Carpenter 2013). Failure to inhibit the prosaccade program, or an improper balance of excitation and inhibition of these pathways, could result in prosaccade errors. The prosaccade error latency is directly related to the time it takes the prosaccade program to reach threshold (Noorani and Carpenter 2013) and can provide insight into the type of disruption likely to contribute to the prosaccade errors observed during bilateral STN DBS. It has been previously shown that impaired implementation of inhibition (Barton et al., 2008) or altered frontal lobe connectivity (Coe and Munoz 2017) could result in prosaccade errors in a regular saccade latency range (> 140 ms), whereas impaired inhibitory circuits could result in the early release of reflexive, or express saccades (90–140 ms) to target (Coe and Munoz 2017). Our findings show that bilateral STN DBS, relative to off and unilateral STN DBS had no effect on the proportion of express prosaccade errors. In fact, bilateral STN DBS reduced the proportion of express prosaccade errors (Fig. 4). It is possible that we did not have enough power to show this difference. However, even if we did it would only further support the idea that bilateral STN DBS does not impair inhibition. This is because a significant reduction in proportion of express prosaccade errors during bilateral STN DBS would be opposite to what one would expect if bilateral STN DBS impaired inhibition. In addition, bilateral STN DBS relative to off and unilateral STN DBS also had no effect on the prosaccade error latency (Fig. 5). These two findings suggest that impaired inhibition due to pathological disinhibition of the superior colliculus was not the primary driving source of increased prosaccade error during bilateral STN DBS. We do not discount the fact that previous research has shown that bilateral STN DBS significantly reduced prosaccade latency on the prosaccade task (Fawcett et al. 2010; Goelz et al. 2017) indicating a reduction in basal ganglia inhibitory drive to the superior colliculus. Bilateral STN DBS may in fact reduce the pathological inhibitory output from the basal ganglia to the superior colliculus, but this is not necessarily the primary driving factor underlying the increased prosaccade error rate during bilateral STN DBS compared to off and unilateral STN DBS.
Potential microlesion effect
One possible limitation is because we did not collect pre-surgical data we cannot rule out an underlying surgical lesion effect. For example, the non-significant increase in express prosaccade errors in the OFF STN DBS condition compared to the controls (Fig. 4) may be due to an interaction between PD disease symptomatology along with the lesion effect. Despite the above consideration, the absence of lesion information in our study does not affect the within-group findings (i.e., OFF vs. UNI vs. BOTH) reported.
Differential effect of unilateral vs. bilateral STN DBS
The finding that bilateral, but not unilateral, STN DBS induced an increased number of fixational saccades and an increased number of prosaccade errors suggests that bilateral, but not unilateral, STN DBS disrupts antisaccade preparatory set. In contrast, it is known that unilateral STN DBS improves skeletomotor function, such as movement speed (Vaillancourt et al. 2004) and amplitude (Stegemoller et al. 2013), and that bilateral STN DBS provides greater improvements in motor function than unilateral STN DBS (Bastian et al. 2003; Kumar et al. 1999). This differential effect of unilateral and bilateral STN DBS between tasks supports the idea that STN DBS has differential effects on the prefrontal and motor basal ganglia loops (Alexander et al. 1986; Alhourani et al. 2015). It is probable that STN DBS reduces pathological neural activity of the basal ganglia nuclei (Anidi et al. 2018; Grill et al. 2004; Vitek 2002) in a manner that facilitates the activity of the primary motor cortex (Alhourani et al. 2015), while simultaneously disrupting activity of prefrontal cortical areas such as the DLPFC (Campbell et al. 2008; Kalbe et al. 2009; Limousin et al. 1997; Mayer et al. 2016).
The fact that bilateral, and not unilateral, STN DBS resulted in disrupted preparatory set compared to OFF STN DBS (Figs. 2, 3) could indicate that during unilateral STN DBS the non-stimulated side can compensate or maintain behavioral control (Alberts et al. 2008). Previous modeling studies have shown that, in general, unilateral lesions may have little to no negative effect on behavior, whereas bilateral lesions produce measurable deficits in behavior (Schapiro et al. 2013). It is thought that behavioral control is maintained under a unilateral lesion condition because the non-lesioned side can compensate for the lesioned side (Schapiro et al. 2013). Thus, cortical- or basal ganglia-collicular disruption during unilateral STN DBS may not necessarily result in deleterious behavioral output because the non-stimulated side can compensate for the disrupted side.
Conclusion
In the current study, we showed that there was an increased number of fixational saccades during the antisaccade task during bilateral, but not unilateral, STN DBS and that this increase was related to increased prosaccade errors suggesting that bilateral STN DBS interferes with preparatory set during the antisaccade task. These findings add to a line of previous work indicating a deleterious effect of bilateral STN DBS on tasks requiring greater levels of voluntary control, such as those required for dual-tasking and reaching memorized sequential targets (Alberts et al. 2008; David et al. 2018).
Bilateral STN DBS is effective at improving measures of motor function such as movement speed and amplitude (Stegemoller et al. 2013; Vaillancourt et al. 2004), but it is detrimental to certain voluntary tasks that rely heavily on prefrontal networks (Alberts et al. 2008; David et al. 2018). We propose that high-frequency STN DBS may improve neural activity of basal ganglia-primary motor pathways (Anidi et al. 2018; Grill et al. 2004; Vitek 2002) while simultaneously interfering with prefrontal cortical pathways associated with higher-order activity underlying complex voluntary movement (Alberts et al. 2008; David et al. 2018; Goelz et al. 2017). It should be noted that the neural circuitry for preparatory set remains unclear. The circuitry can involve both prefrontal cortical areas, as well as sub-cortical basal ganglia areas. We have not ruled out the possibility that bilateral STN DBS could disrupt local basal ganglia processes that might disrupt preparatory activity. We have only ruled out the possibility that inhibitory processes are not driving the increased prosaccade error rate during bilateral STN DBS. Future work should investigate the effect of STN DBS on neural areas associated with preparatory set during voluntary tasks to probe the neural changes associated with the behavioral impairment reported.
Acknowledgements
The authors thank the participants and our professional colleagues, Jay Pillai and Christiane Alford, for their important contributions to the successful implementation of this project.
Sources of funding This study was supported by the National Institutes of Health (R56NS040902 and R01NS09295001A1). The sponsors were not involved in the design, conduct, collection, management, analysis, and/or interpretation of the study results and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIH.
Conflict of interest LCG and FJD received Grant support from NIH. LCG, MLC, and FJD have no conflict of interest to report. DMC is a Full Professor at Northwestern University and receives a salary, has additional NIH funding (5R01NS074343, 5R01HD075777, 1R01DK110699, 5T15HD074546), and receives honoraria and/or consults for the following: University of Florida, Ohio University Athens, Temple University, Iowa State University, University of Alabama, Birmingham, Oregon Health Sciences Institute, University of Westminster, University of Waterloo, University of Colorado, Denver, Several NIH Study Sections, ACRM, ASNR, University of New Hampshire, University of Minnesota, Movement Disorders Society. LVM has foundation research support from Michael J. Fox Foundation; commercial research support from Medtronic, Inc., US World-Meds LLC, Pfizer Inc, Boston Scientific, Avanir Pharmaceuticals, Inc., and Adamas Pharmaceuticals, Inc.; is on the scientific advisory board of St. Jude Medical, AbbVie, Inc., and Britannia Pharmaceuticals Ltd.; and consults for St. Jude Medical, AbbVie, Inc., Medtronic, Inc., and Boston Scientific.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL (2008) Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain 131:3348–3360. 10.1093/brain/awn238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. 10.1146/annurev.ne.09.030186.002041 [DOI] [PubMed] [Google Scholar]
- Alhourani A et al. (2015) Network effects of deep brain stimulation. J Neurophysiol 114:2105–2117. 10.1152/jn.00275.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anidi C, O’Day JJ, Anderson RW, Afzal MF, Syrkin-Nikolau J, Velisar A, Bronte-Stewart HM (2018) Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease. Neurobiol Dis 120:107–117. 10.1016/j.nbd.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Barton JJ, Pandita M, Thakkar K, Goff DC, Manoach DS (2008) The relation between antisaccade errors, fixation stability and prosaccade errors in schizophrenia. Exp Brain Res 186:273–282. 10.1007/s00221-007-1235-2 [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW (2003) Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson’s disease. Mov Disord 18:1000–1007. 10.1002/mds.10493 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2008) Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology 70:1991–1995. 10.1212/01.wnl.0000313022.39329.65 [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Piallat B, Pollak P, Benabid AL (1995) Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett 189:77–80 [DOI] [PubMed] [Google Scholar]
- Betta E, Turatto M (2006) Are you ready? I can tell by looking at your microsaccades Neuroreport 17:1001–1004. 10.1097/01.wnr.0000223392.82198.6d [DOI] [PubMed] [Google Scholar]
- Campbell MC et al. (2008) Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia 46:3162–3169. 10.1016/j.neuropsychologia.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML (1995) Neural computation of log likelihood in control of saccadic eye movements. Nature 377:59–62. 10.1038/377059a0 [DOI] [PubMed] [Google Scholar]
- Chiken S, Nambu A (2014) Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci 8:33 10.3389/fnsys.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe BC, Munoz DP (2017) Mechanisms of saccade suppression revealed in the anti-saccade task. Philos Trans R Soc Lond B Biol Sci. 10.1098/rstb.2016.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Johnson KA, Bradshaw JL (1997) Movement-related potentials in Parkinson’s disease. Motor imagery and movement preparation. Brain 120(Pt 8):1339–1353 [DOI] [PubMed] [Google Scholar]
- David FJ, Goelz LC, Tangonan RZ, Metman LV, Corcos DM (2018) Bilateral deep brain stimulation of the subthalamic nucleus increases pointing error during memory-guided sequential reaching. Exp Brain Res 236:1053–1065. 10.1007/s00221-018-5197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S (2003) Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol 89:1016–1023. 10.1152/jn.00562.2002 [DOI] [PubMed] [Google Scholar]
- Evarts E, Shinoda Y, Wise S (1984) Neurophysiological approaches to higher brain functions. Wiley, New York [Google Scholar]
- Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20:387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett AP, Cunic D, Hamani C, Hodaie M, Lozano AM, Chen R, Hutchison WD (2007) Saccade-related potentials recorded from human subthalamic nucleus. Clin Neurophysiol 118:155–163. 10.1016/j.clinph.2006.09.016 [DOI] [PubMed] [Google Scholar]
- Fawcett AP, Gonzalez EG, Moro E, Steinbach MJ, Lozano AM, Hutchison WD (2010) Subthalamic nucleus deep brain stimulation improves saccades in Parkinson’s disease. Neuromodulation 13:17–25. 10.1111/j.1525-1403.2009.00246.x [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E (1984) Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res 57:191–195 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- Goelz LC, David FJ, Sweeney JA, Vaillancourt DE, Poizner H, Metman LV, Corcos DM (2017) The effects of unilateral versus bilateral subthalamic nucleus deep brain stimulation on prosaccades and antisaccades in Parkinson’s disease. Exp Brain Res 235:615–626. 10.1007/s00221-016-4830-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S (2004) Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15:1137–1140 [DOI] [PubMed] [Google Scholar]
- Haggard P (2008) Human volition: towards a neuroscience of will. Nat Rev Neurosci 9:934–946. 10.1038/nrn2497 [DOI] [PubMed] [Google Scholar]
- Hebb D (1972) Textbook of psychology. Saunders, Philadelphia [Google Scholar]
- Hermens F, Zanker JM, Walker R (2010) Microsaccades and preparatory set: a comparison between delayed and immediate, exogenous and endogenous pro- and anti-saccades. Exp Brain Res 201:489–498. 10.1007/s00221-009-2061-5 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978 [DOI] [PubMed] [Google Scholar]
- Hilker R et al. (2004) Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab 24:7–16. 10.1097/01.WCB.0000092831.44769.09 [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E et al. (2009) Frontal FDG-PET activity correlates with cognitive outcome after STN-DBS in Parkinson disease. Neurology 72:42–49. 10.1212/01.wnl.0000338536.31388.f0 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Goffart L, Hafed ZM (2017) Neuronal control of fixation and fixational eye movements. Philos Trans R Soc Lond B Biol Sci. 10.1098/rstb.2016.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Lozano AM, Sime E, Halket E, Lang AE (1999) Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology 53:561–566 [DOI] [PubMed] [Google Scholar]
- LeVasseur AL, Flanagan JR, Riopelle RJ, Munoz DP (2001) Control of volitional and reflexive saccades in Tourette’s syndrome. Brain 124:2045–2058. 10.1093/brain/124.10.2045 [DOI] [PubMed] [Google Scholar]
- Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R (1997) Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol 42:283–291. 10.1002/ana.410420303 [DOI] [PubMed] [Google Scholar]
- Marsden CD (1982) The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology 32:514–539 [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Hubel DH (2009) Microsaccades: a neurophysiological analysis. Trends Neurosci 32:463–475. 10.1016/j.tins.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Maurice N, Thierry AM, Glowinski J, Deniau JM (2003) Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci 23:9929–9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Neimat J, Folley BS, Bourne SK, Konrad PE, Charles D, Park S (2016) Deep brain stimulation of the subthalamic nucleus alters frontal activity during spatial working memory maintenance of patients with Parkinson’s disease. Neurocase 22:369–378. 10.1080/13554794.2016.1197951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis H (2014) Microsaccades in Parkinson’s disease. Master’s Thesis, Queen’s University [Google Scholar]
- Morris LS, Baek K, Voon V (2017) Distinct cortico-striatal connections with subthalamic nucleus underlie facets of compulsivity. Cortex 88:143–150. 10.1016/j.cortex.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Le Vasseur AL, Flanagan JR (2002) Control of volitional and reflexive saccades in Tourette’s syndrome. Prog Brain Res 140:467–481 [DOI] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD (2003) Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J Neurophysiol 90:503–514. 10.1152/jn.00192.2003 [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43:111–117 [DOI] [PubMed] [Google Scholar]
- Noorani I, Carpenter RH (2013) Antisaccades as decisions: LATER model predicts latency distributions and error responses. Eur J Neurosci 37:330–338. 10.1111/ejn.12025 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Serra A, Leigh RJ, Martinez-Conde S (2011) Triggering mechanisms in microsaccade and saccade generation: a novel proposal. Ann N Y Acad Sci 1233:107–116. 10.1111/j.1749-6632.2011.06177.x [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Schneider R, Leigh RJ, Macknik SL, Martinez-Conde S (2013) Saccades during attempted fixation in parkinsonian disorders and recessive ataxia: from microsaccades to squarewave jerks. PLoS ONE 8:e58535 10.1371/journal.pone.0058535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P, Cools AR, Stegeman DF, Horstink MW (1996a) Movement-related potential measures of different modes of movement selection in Parkinson’s disease. J Neurol Sci 140:67–74 [DOI] [PubMed] [Google Scholar]
- Praamstra P, Meyer AS, Cools AR, Horstink MW, Stegeman DF (1996b) Movement preparation in Parkinson’s disease. Time course and distribution of movement-related potentials in a movement precueing task. Brain 119(Pt 5):1689–1704 [DOI] [PubMed] [Google Scholar]
- Schapiro AC, McClelland JL, Welbourne SR, Rogers TT, Lambon Ralph MA (2013) Why bilateral damage is worse than unilateral damage to the brain. J Cogn Neurosci 25:2107–2123. 10.1162/jocn_a_00441 [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J (1997) Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390:398–401. 10.1038/37114 [DOI] [PubMed] [Google Scholar]
- Stegemoller EL, Zadikoff C, Rosenow JM, Mackinnon CD (2013) Deep brain stimulation improves movement amplitude but not hastening of repetitive finger movements. Neurosci Lett 552:135–139. 10.1016/j.neulet.2013.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR (1996) Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 75:454–468 [DOI] [PubMed] [Google Scholar]
- Terao Y et al. (1998) Erroneous prosaccades provoked by TMS during antisaccade task In: Hashimoto I, Kakigi R (eds) Proceedings of the 6th International Evoked Potentials Symposium, Okazaki, Japan, 1998. Elsevier, Netherlands, pp 984–989 [Google Scholar]
- Terao Y et al. (2011) Initiation and inhibitory control of saccades with the progression of Parkinson’s disease—changes in three major drives converging on the superior colliculus. Neuropsychologia 49:1794–1806. 10.1016/j.neuropsychologia.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Fukuda H, Ugawa Y, Hikosaka O (2013) New perspectives on the pathophysiology of Parkinson’s disease as assessed by saccade performance: a clinical review. Clin Neurophysiol 124:1491–1506. 10.1016/jxlinph.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleikis JR, Metman LV, Pilitsis JG, Barborica A, Toleikis SC, Bakay RA (2012) Effect of intraoperative subthalamic nucleus DBS on human single-unit activity in the ipsilateral and contralateral subthalamic nucleus. J Neurosurg 116:1134–1143. 10.3171/2011.12.jns102176 [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM (2004) Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain 127:491–504. 10.1093/brain/awh057 [DOI] [PubMed] [Google Scholar]
- Vitek JL (2002) Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 17(Suppl 3):S69–S72 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Matsuo Y, Zha L, Munoz DP, Kobayashi Y (2013) Fixational saccades reflect volitional action preparation. J Neurophysiol 110:522–535. 10.1152/jn.01096.2012 [DOI] [PubMed] [Google Scholar]
- Welter ML et al. (2004) Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol 61:89–96. 10.1001/archneur.61.1.89 [DOI] [PubMed] [Google Scholar]
- Yugeta A et al. (2013) Modulation of Beta oscillations in the subthalamic nucleus with prosaccades and antisaccades in Parkinson’s disease. J Neurosci 33:6895–6904. 10.1523/jneurosci.2564-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]