Abstract
Objective
Inflammation is the primary mechanism of lung ischemia-reperfusion injury (LIRI) and neurologic factors can regulate inflammatory immune responses. Netrin-1 is an axonal guidance molecule, but whether Netrin-1 plays a role in LIRI remains unclear.
Methods
A mouse model of LIRI was established. Immunohistochemistry was used to detect expression of Netrin-1 and to enumerate macrophages and T cells in lung tissue. The proportion of regulatory T cells (Tregs) was assessed by flow cytometry. Levels of apoptosis were assessed by terminal deoxynucleotidyl transferase dUTP nick end staining.
Results
Numbers of macrophages and T cells in the lung tissues of mice with LIRI were elevated, while expression of netrin-1 was significantly decreased. Flow cytometry showed that the proportion of Tregs in mice with LIRI was significantly decreased. The proportion of Tregs among lymphocytes was positively correlated with netrin-1 expression. In vitro experiments showed that netrin-1 promoted an increase in Treg proportion through the A2b receptor. Animal experiments showed that netrin-1 could inhibit apoptosis and reduce T cell and macrophage infiltration by increasing the proportion of Tregs, ultimately reducing LIRI. Treg depletion using an anti-CD25 monoclonal antibody blocked the effects of netrin-1.
Conclusion
Netrin-1 reduced LIRI by increasing the proportion of Tregs.
Keywords: Netrin-1, ischemia-reperfusion injury, regulatory T cell, inflammation, lung, A2b receptor
Introduction
Multiple factors can lead to lung ischemia-reperfusion injury (LIRI) including pulmonary embolism, lung transplantation, cardiac arrest and severe trauma. LIRI has a high mortality rate, so prevention and treatment are both clinically important.1,2 Inflammation is the primary mechanism of LIRI. During reperfusion of ischemic lung tissues, inflammatory cell infiltrates release pro-inflammatory molecules such as tumor necrosis factor-α and interleukin (IL)-6, causing further damage to lung tissues.3,4 In addition, inflammatory molecules released from ischemic sites can enter the peripheral blood, causing systemic organ and tissue damage.5 Therefore, regulation of inflammatory responses is important for the prevention and treatment of LIRI.
Regulatory T cells (Tregs) are immune regulatory cells that play an important role in maintaining immune homeostasis.6 Tregs inhibit the proliferation and activation of effector T cells by directly contacting them or secreting immunosuppressive molecules.7 Human leukocyte antigen (HLA)-G and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) play important roles in immunosuppression by Tregs. Several studies have found that Tregs can protect the brain, heart, liver and kidney against ischemia-reperfusion injuries.8–10 Tregs reduced the infiltration of macrophages and lymphocytes into renal tissue following ischemia-reperfusion by secreting IL-10 and transforming growth factor (TGF)-β. The number of Tregs was negatively correlated with the degree of ischemia-reperfusion injury.11 Therefore, increasing the number of Tregs may have a therapeutic effect on LIRI.
In recent years, many neurologic factors have been found to regulate immune responses. Netrin-1 is an axonal guidance molecule that plays an important role in nerve growth and axon formation.12 Many studies have found that netrin-1 can reduce myocardial ischemia-reperfusion injury and inhibit cardiomyocyte apoptosis, although the specific mechanisms remain unclear.13,14 Regulation of inflammatory responses may be one mechanism through which netrin-1 exerts these effects. Netrin-1 can inhibit the migration and aggregation of white blood cells and reduce the release of pro-inflammatory cytokines.15–17 The goals of this study were to investigate whether netrin-1 can be used to treat LIRI and to explore the role of Tregs in its mechanism of action.
Materials and methods
Establishment of a mouse model of LIRI
The mouse model of LIRI was established as previously reported.18 Briefly, following anesthesia, the mouse trachea was incised and mechanical ventilation was performed using a small animal ventilator. The second and third ribs were cut along the left sternum, the thoracic cavity was opened, and the left lung was exposed. The left hilum was clamped for half an hour, and then the arterial clamp was loosened to allow reperfusion of lung tissue. The study was approved by the Ethics Committee of Wenzhou Central Hospital (No: 20170361) and was performed according to the Guide for the Care and Use of Laboratory Animals of Wenzhou Central Hospital.
Histological staining
Mice were divided into a control group (sham operation) and a LIRI group (LIRI model). After 3 hours of reperfusion, lung tissues of mice in both groups were collected, fixed with 10% formalin, and then paraffin sections were prepared. Some sections were stained with hematoxylin and eosin (H&E) to observe the overall morphology of lung tissue. Some sections were used for immunohistochemistry to assess expression of netrin-1 as well as numbers of CD68+ macrophages and CD3+ T cells in lung tissue. All antibodies were purchased from Abcam (Cambridge, UK). Netrin-1 expression was quantified using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA). The numbers of macrophages and T cells in each high power field were counted using a light microscope.
Flow cytometry
After 3 hours of reperfusion, peripheral blood was collected and lymphocytes were separated using lymphocyte separation medium (TBD, Tianjin, China). Anti-CD4 and -CD25 antibodies were added for 15 minutes, then the lymphocytes were washed three times with phosphate-buffered saline (PBS). One hour following membrane rupture, an anti-Foxp3 antibody was added and incubated for 15 minutes. After three washes with PBS, the proportion of CD4+CD25+Foxp3+ Tregs was assessed by flow cytometry. Tregs were sorted by fluorescence-activated cell sorting and cultured in RPMI-I640 containing 10% fetal bovine serum (Hyclone, Logan, UT, USA). After 48 hours, levels of IL-10 and TGF-β in the supernatant were assessed by ELISA (Huijia, Xiamen, China) and expression of HLA-G and CTLA-4 on Tregs was assessed by flow cytometry. All antibodies were purchased from BD Biosciences (San Diego, CA, USA).
In vitro experiments
Peripheral blood of mice in the LIRI group was collected. Lymphocytes were separated using lymphocyte separation medium and cultured in RPMI-1640 containing 10% fetal bovine serum. The cells were divided into four groups: (i) a control group (an equivalent volume of PBS was added to the culture medium), (i) a netrin-1 group (10 ng/mL of netrin-1 was added to the culture medium), (iii) an A2b blockade group (10 ng/mL of netrin-1 and an A2b neutralizing antibody were added to the culture medium), and a deleted in colorectal cancer (DCC) blockade group (10 ng/mL of netrin-1 and a DCC neutralizing antibody were added to the culture medium). After 48 hours of continuous culture, the proportion of Tregs among lymphocytes was assessed by flow cytometry.
Animal experiments
Mice were divided into three groups: (i) a LIRI group (LIRI model mice), (ii) a netrin-1 group (receiving intraperitoneal injections of netrin-1 4 hours prior to LIRI model establishment), and (iii) a PC61 group (receiving intraperitoneal injections of netrin-1 and the anti-CD25 monoclonal antibody PC61 4 hours prior to LIRI model establishment).19,20 After 24 hours of reperfusion, peripheral blood was collected and the number of Tregs in peripheral blood was assessed by flow cytometry. Lung tissues were collected and overall pathological changes were assessed by H&E staining. The numbers of CD3+ T cells and CD68+ macrophages were assessed by immunohistochemistry. Levels of apoptosis were assessed using terminal deoxynucleotidyl transferase dUTP nick end (TUNEL) staining.
Statistical analysis
Statistical analyses were carried out using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Data were presented as means ± standard deviation. Differences between multiple groups were assessed using one-way analysis of variance and the Bonferroni post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Expression of netrin-1 was significantly decreased in lung tissues of LIRI mice
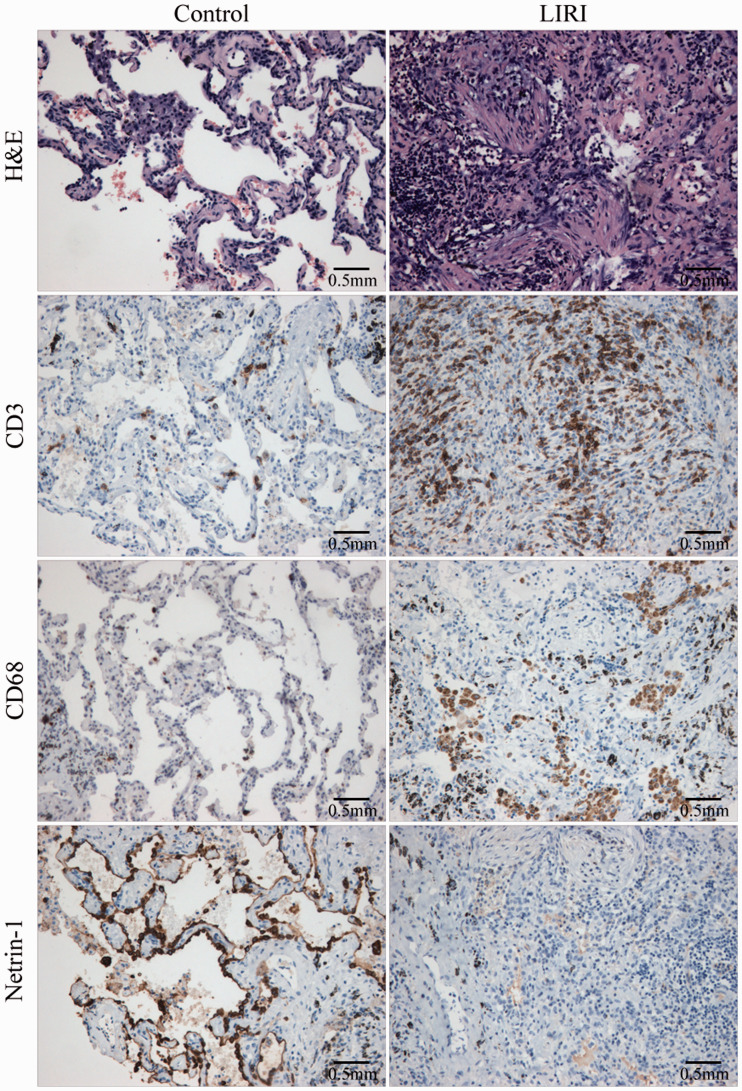
H&E staining showed that lung tissue structures in the control group were basically normal with a small number of infiltrating inflammatory cells detected. In the LIRI group, lung tissue was necrotic and infiltrated with many inflammatory cells. Immunohistochemistry showed that the numbers of macrophages and T cells in lung tissues of LIRI mice were significantly increased compared with the control group. By contrast, the expression of netrin-1 was significantly decreased in LIRI mice (Figure 1).
Figure 1.
Histological staining. Compared with the control group, the numbers of macrophages and T cells in lung tissues of LIRI mice were significantly increased, while expression of netrin-1 was significantly decreased.
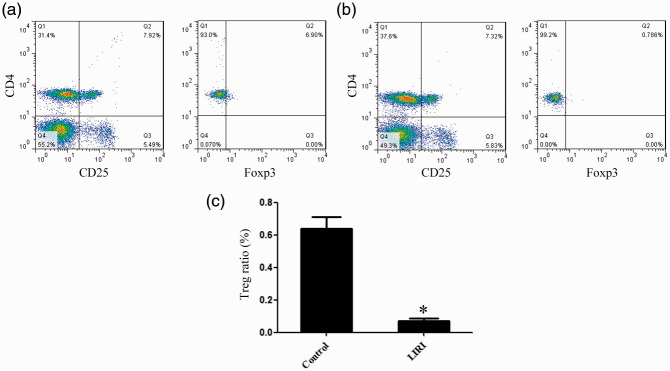
The proportion of Tregs was significantly decreased in LIRI mice
Compared with the control group, the proportion of Tregs among lymphocytes in the LIRI group was significantly decreased. Treg proportion was positively correlated with netrin-1 expression (Figure 2).
Figure 2.
Changes in Treg proportion associated with LIRI. Flow cytometry was used to assess the proportion of Tregs in control (a) and LIRI mice (b). The proportion of Tregs in LIRI mice was significantly decreased (c).
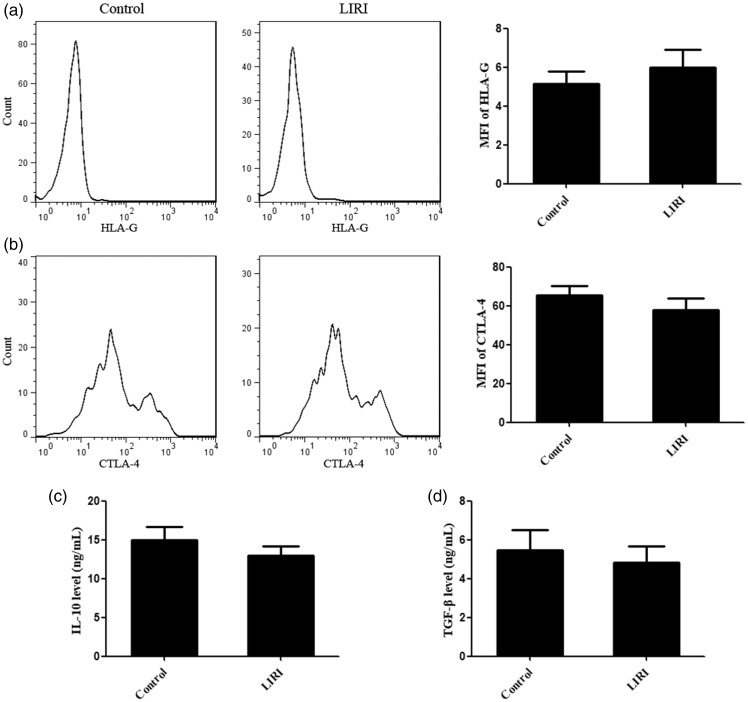
We further studied the functions of Tregs in LIRI. We found that there was no significant difference in IL-10 and TGF-β concentrations in the supernatants of Tregs purified from control and LIRI mice. There was also no significant difference in the expression of HLA-G and CTLA-4 on the Tregs of control and LIRI mice. These results suggested that there was no significant difference in Treg function in these two groups of mice (Figure 3).
Figure 3.
Treg function in LIRI and control mice. (a, b) There was no significant difference in the expression of HLA-G and CTLA-4 on Tregs derived from control and LIRI mice. (c, d) There was no significant difference in levels of IL-10 and TGF-β in the supernatants of Tregs purified from control and LIRI mice. MFI, mean fluorescence intensity.
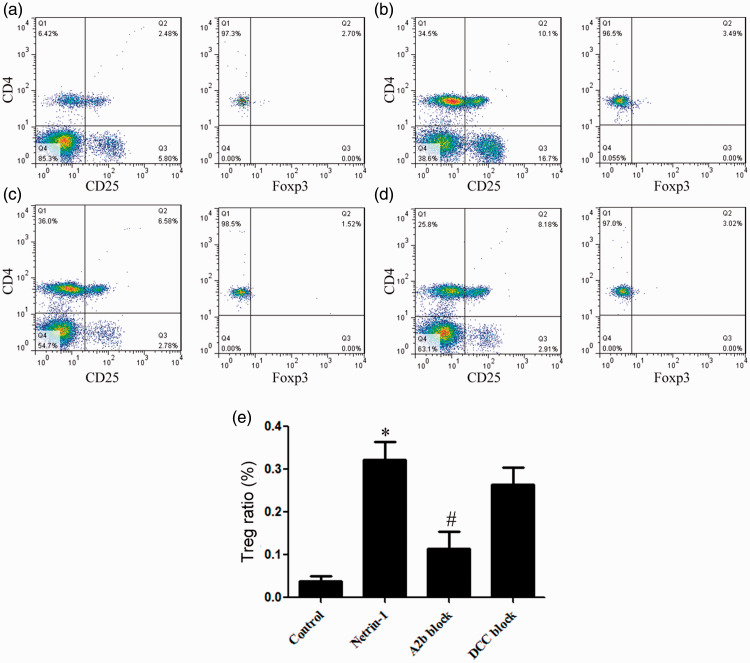
Netrin-1 promoted an increase in Treg proportion in vitro
Compared with the control group, the proportion of Tregs in netrin-1 treated lymphocytes from LIRI mice was significantly increased. A2b receptor blockade significantly inhibited the function of netrin-1, while DCC blockade had no significant effect on its function (Figure 4).
Figure 4.
Netrin-1 treatment promoted an increase in Treg proportion. Flow cytometry was used to assess the proportion of Tregs in control (a), netrin-1-treated (b), A2b blockade (c) and DCC blockade (d) mice. (e) Compared with the control group, the Treg proportion in netrin-1-treated mice was significantly increased. A2b receptor blockade significantly inhibited the function of netrin-1, while DCC blockade had no significant effect on its function. *vs. control, P<0.05; # vs. netrin-1, P < 0.05.
Netrin-1 protected the lung against ischemia-reperfusion injury
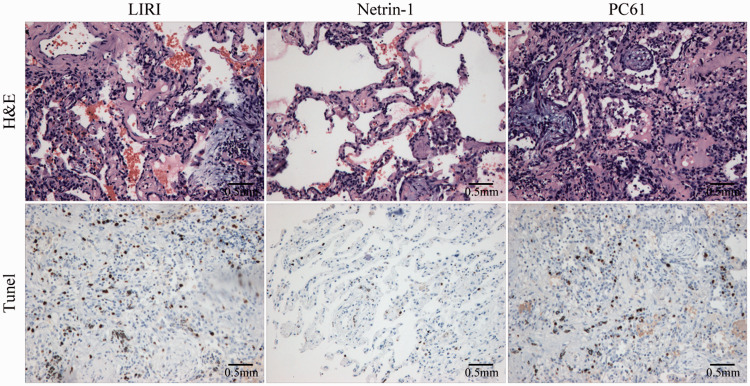
H&E staining demonstrated partial necrosis of lung tissue and infiltration by a large number of inflammatory cells in LIRI mice. Compared with the LIRI group, netrin-1 treatment resulted in decreased lung tissue necrosis and inflammatory cell infiltration. Depletion of Tregs using the anti-CD25 antibody PC61 completely blocked the effects of netrin-1. TUNEL staining showed that 16.4% of cells were apoptotic in LIRI mice, while treatment with netrin-1 decreased the percentage of apoptotic cells by 76.3%. PC61 completely blocked the effects of netrin-1 on apoptosis (Figure 5).
Figure 5.
Netrin-1 protected the lung against ischemia-reperfusion injury. Netrin-1 inhibited apoptosis induced by ischemia-reperfusion. Compared with the LIRI group, the percentage of apoptotic cells decreased by 76.3%. Treatment with the anti-CD25 antibody, PC61 completely blocked the effect of netrin-1. (100×).
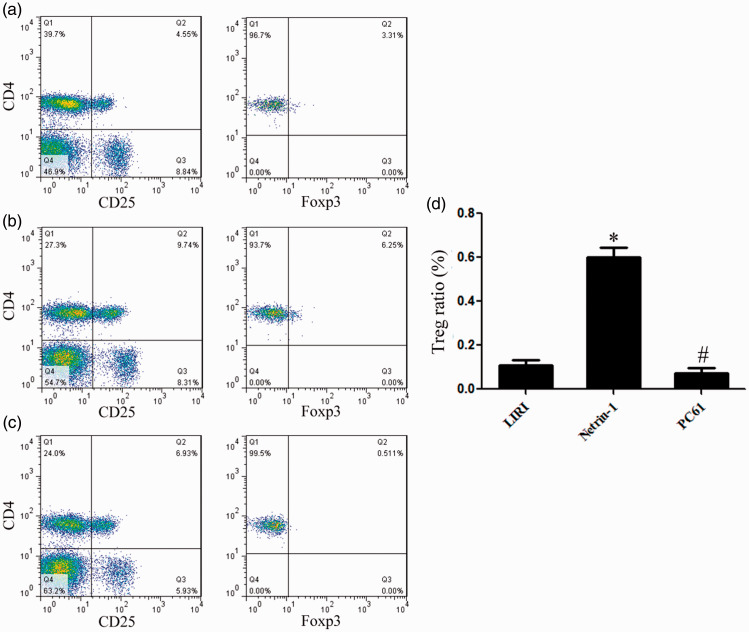
Netrin-1 increased the Treg proportion in vivo
Compared with the LIRI group, the proportion of Tregs in netrin-1-treated mice was 6.07-fold higher. Compared with netrin-1-treated mice, the proportion of Tregs in PC61-treated mice decreased by 84.64% (Figure 6).
Figure 6.
Netrin-1 increased the Treg proportion in vivo. Flow cytometry was used to assess the proportion of Tregs in the LIRI (a), netrin-1 (b) and netrin-1 + PC61 (c) groups. (d) Compared with the LIRI group, the proportion of Tregs in the netrin-1 group was 6.07-fold higher. Compared with the netrin-1 group, the proportion of Tregs in the PC61 group decreased by 84.64%. *vs. LIRI, P<0.05; # vs. netrin-1, P<0.05.
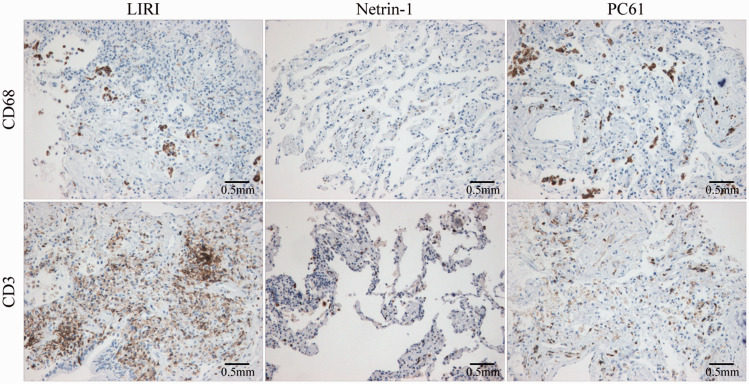
Netrin-1 inhibited inflammatory responses in lung tissue following ischemia-reperfusion
Immunohistochemistry showed that netrin-1 inhibited the infiltration of macrophages and T cells into lung tissue. The numbers of macrophages and T cells present in the lung tissues of PC61-treated mice were significantly higher than those of mice treated with netrin-1 alone (Figure 7).
Figure 7.
Netrin-1 inhibited inflammatory responses in lung tissue following ischemia-reperfusion. Netrin-1 also inhibited infiltration of macrophages and T cells into lung tissue. The numbers of macrophages and T cells in lung tissue of PC61-treated mice were significantly higher compared with mice treated with netrin-1 alone. (100×).
Discussion
In recent years, the increasing number of patients undergoing lung transplantation and cardiac arrest has significantly increased the incidence of LIRI. The mortality rate of patients with LIRI is very high, but there is no effective treatment at present.21 Thus, it is very important to identify new prevention and treatment methods. Inflammation is the primary mechanism of ischemia-reperfusion injury in lung tissue and other organs. Regulating inflammation can effectively alleviate LIRI.22 Tregs are the most important immune regulatory cells in peripheral blood, and suppress immune responses through two mechanisms: (i) Tregs express immunosuppressive molecules (HLA-G and CTLA-4) that can inhibit inflammatory cells through direct cell contact, and (ii) Tregs can secrete large amounts of IL-10 and TGF-β to inhibit inflammation and promote the proliferation the activities of other types of regulatory immune cells.23,24 Our study found that the immunoregulatory functions of Tregs in control and LIRI mice were similar, suggesting that LIRI had no significant effect on Treg function.
Many clinical experiments have shown that Tregs can have therapeutic effects on organ transplant rejection and autoimmune diseases.25,26 For ischemia-reperfusion injury, Treg transplantation can significantly reduce infiltration of macrophages and lymphocytes into ischemic tissue and can inhibit apoptosis.27,28 Our results showed that the proportion of Tregs was significantly decreased in LIRI mice, suggesting that the inflammatory response was activated in these mice. Excessive inflammation can cause further damage to lung tissues. Many inflammatory factors can also enter the peripheral blood and damage other important tissues and organs. Therefore, increasing the proportion of Tregs and maintaining immune homeostasis may be an effective strategy to treat LIRI.
There is a strong connection between the nervous system and inflammatory responses. In recent years, many studies have found that neurological factors can regulate inflammation. Brain-derived neurotrophic factor can regulate the inflammatory microenvironment, promote IL-10 expression, and induce macrophage polarization from M1 to M2.29 Netrin-1 can also regulate inflammatory responses. Some studies have found that netrin-1 can inhibit the proliferation of macrophages and reduce the damage caused by inflammatory cells in ischemic tissue.30,31 Our study points to a new mechanism through which netrin-1 can regulate inflammation. Netrin-1 increases the proportion of Tregs and inhibits inflammation induced during ischemia-reperfusion injury. Immunohistochemistry showed that netrin-1 treatment could significantly inhibit LIRI-associated apoptosis and reduce lymphocyte and macrophage infiltration into ischemic lung tissue. A2b receptors may play a key role in this process. A2b receptor is the primary adenosine receptor expressed in endothelial cells, dendritic cells and lymphocytes, and plays a key role in pulmonary inflammation.32,33
In conclusion, netrin-1 reduced LIRI by increasing the proportion of Tregs. Our data suggest a new strategy for the treatment and prevention of LIRI.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Science and Technology Projects of Wenzhou Science and Technology Bureau (Y2018053).
ORCID iD
Xiaobo Wang https://orcid.org/0000-0002-4633-5928
References
- 1.Zhao YR, Liu Y, Wang D, et al. Effects of sulfur dioxide on alveolar macrophage apoptosis in acute lung injury induced by limb ischemia/reperfusion in rats. Beijing Da Xue Bao Yi Xue Ban 2019; 51: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S, Tian J, Zhang F, et al. The protective effects of heat shock protein 22 in lung ischemia-reperfusion injury mice. Biochem Biophys Res Commun 2019; 512: 698–704. DOI: S0006-291X(19)30425-5 [pii] 10.1016/j.bbrc.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Martinu T, Chruscinski A, et al. A B cell-dependent pathway drives chronic lung allograft rejection after ischemia-reperfusion injury in mice. Am J Transplant 2019; 19: 3377–3389. DOI: 10.1111/ajt.15550. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima D, Watanabe Y, Ohsumi A, et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant 2019; 38: 1214–1223. DOI: S1053-2498(19)31599-2 [pii] 10.1016/j.healun.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Gong J, Ju YN, Wang XT, et al. Ac2-26 ameliorates lung ischemia-reperfusion injury via the eNOS pathway. Biomed Pharmacother 2019; 117: 109194. DOI: S0753-3322(19)31710-X [pii] 10.1016/j.biopha.2019.109194. [DOI] [PubMed] [Google Scholar]
- 6.Rubin AJ, Parker KR, Satpathy AT, et al. Coupled single-cell CRISPR screening and epigenomic profiling reveals causal gene regulatory networks. Cell 2019; 176: 361–376.e317. DOI: S0092-8674(18)31514-9 [pii] 10.1016/j.cell.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamali S, Sarafnejad A, Ahmadpoor P, et al. Sirolimus vs mycophenolate moftile in tacrolimus based therapy following induction with antithymocyte globulin promotes regulatory T cell expansion and inhibits RORgammat and T-bet expression in kidney transplantation. Hum Immunol 2019; 80: 739–747. DOI: S0198-8859(18)30800-0 [pii] 10.1016/j.humimm.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Katsumata H, Ikemiyagi M, Hirai T, et al. Impact of activated invariant natural killer T cells on the expansion of regulatory T cell precursors in murine thymocytes in vitro. Immunol Lett 2019; 206: 41–48. DOI: S0165-2478(18)30432-2 [pii] 10.1016/j.imlet.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Hirata Y, Kakiuchi M, Robson SC, et al. CD150(high) CD4 T cells and CD150(high) regulatory T cells regulate hematopoietic stem cell quiescence via CD73. Haematologica 2019; 104: 1136–1142. DOI: 10.3324/haematol.2018.198283 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Du J, Cheng X, et al. Effect of netrin-1 anti-inflammatory factor on acute lung injury in sepsis rats. Med Sci Monit 2019; 25: 7928–7935. DOI: 10.12659/MSM.917279917279 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Carrasco M, Soto-Santillan P, Mendoza-Pinto C, et al. The role of circulating regulatory T cell levels on subclinical atherosclerosis and cardiovascular risk factors in women with systemic lupus erythematosus. Mediators Inflamm 2018; 2018: 3271572. DOI: 10.1155/2018/3271572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrani S, Haider KH, Ahmed RP, et al. Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury. Stem Cells Dev 2012; 21: 1769–1778. DOI: 10.1089/scd.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui MZ. Potential therapeutics for myocardial ischemia-reperfusion injury. Focus on “Induction of cardioprotection by small netrin-1-derived peptides”. Am J Physiol Cell Physiol 2015; 309: C97–C99. DOI: 10.1152/ajpcell.00150.2015 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao X, Xing H, Mao A, et al. Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages. Inflammation 2014; 37: 573–580. DOI: 10.1007/s10753-013-9771-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Xu J, Gong J, et al. Expression of netrin-1 and its receptors, deleted in colorectal cancer and uncoordinated locomotion-5 homolog B, in rat brain following focal cerebral ischemia reperfusion injury. Neural Regen Res 2013; 8: 64–69. DOI: 10.3969/j.issn.1673-5374.2013.01.008 NRR-8-64 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Z, Jin J, Bai W, et al. Netrin-1 prevents the attachment of monocytes to endothelial cells via an anti-inflammatory effect. Mol Immunol 2018; 103: 166–172. DOI: S0161-5890(18)30690-4 [pii] 10.1016/j.molimm.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Ranganathan P, Mohamed R, Jayakumar C, et al. Guidance cue netrin-1 and the regulation of inflammation in acute and chronic kidney disease. Mediators Inflamm 2014; 2014: 525891. DOI: 10.1155/2014/525891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd-o JM, Hristopoulos ML, Kibler K, et al. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am J Physiol Lung Cell Mol Physiol 2008; 294: L714–L723. DOI: 10.1152/ajplung.00185.2007 00185.2007 [pii]. [DOI] [PubMed] [Google Scholar]
- 19.Tang CL, Yang J, Cheng LY, et al. Anti-CD25 monoclonal antibody enhances the protective efficacy of Schistosoma japonicum GST vaccine via inhibition of CD4(+)CD25(+)Foxp3(+) regulatory T cells. Parasitol Res 2017; 116: 2727–2732. DOI: 10.1007/s00436-017-5581-0 10.1007/s00436-017-5581-0 [pii]. [DOI] [PubMed] [Google Scholar]
- 20.Arce Vargas F, Furness AJS, Solomon I, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 2017; 46: 577–586. DOI: S1074-7613(17)30123-1 [pii] 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone ML, Zhao Y, Robert Smith J, et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res 2017; 18: 212. DOI: 10.1186/s12931-017-0704-9 10.1186/s12931-017-0704-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan CC, Peng CK, Tang SE, et al. Carbonic anhydrase inhibitor attenuates ischemia-reperfusion induced acute lung injury. PLoS One 2017; 12: e0179822 DOI: 10.1371/journal.pone.0179822 PONE-D-16-40648 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Li Z, Ling W, et al. Exosomes derived from dendritic cells attenuate liver injury by modulating the balance of Treg and Th17 cells after ischemia reperfusion. Cell Physiol Biochem 2018; 46: 740–756. DOI: 10.1159/000488733 000488733 [pii]. [DOI] [PubMed] [Google Scholar]
- 24.Grosjean F. Ischemia-reperfusion injury and Treg: old knowledge, new prospects. G Ital Nefrol 2009; 26: 560. [PubMed] [Google Scholar]
- 25.Lakkis FG. Immunology: Treg cells in transplantation – a double-edged sword? Nat Rev Nephrol 2014; 10: 185–186. DOI: 10.1038/nrneph.2014.35 [pii]. [DOI] [PubMed] [Google Scholar]
- 26.Kielsen K, Ryder LP, Lennox-Hvenekilde D, et al. Reconstitution of Th17, Tc17 and Treg cells after paediatric haematopoietic stem cell transplantation: impact of interleukin-7. Immunobiology 2018; 223: 220–226. DOI: S0171-2985(17)30164-X [pii] 10.1016/j.imbio.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Panduro M, Benoist C, Mathis D. Treg cells limit IFN-gamma production to control macrophage accrual and phenotype during skeletal muscle regeneration. Proc Natl Acad Sci U S A 2018; 115: E2585–E2593. DOI: 10.1073/pnas.1800618115 1800618115 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Q, Liu J, Wu G, et al. Co-expression of LAG3 and TIM3 identifies a potent Treg population that suppresses macrophage functions in colorectal cancer patients. Clin Exp Pharmacol Physiol 2018; 45: 1002–1009. DOI: 10.1111/1440-1681.12992. [DOI] [PubMed] [Google Scholar]
- 29.Boakye PA, Rancic V, Whitlock KH, et al. Receptor dependence of BDNF actions in superficial dorsal horn: relation to central sensitization and actions of macrophage colony stimulating factor 1. J Neurophysiol 2019; 121: 2308–2322. DOI: 10.1152/jn.00839.2018. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Schlegel M, Brown EJ, et al. Netrin-1 alters adipose tissue macrophage fate and function in obesity. Immunometabolism 2019; 1: e190010 DOI: e190010 [pii] 10.20900/immunometab20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor L, Brodermann MH, McCaffary D, et al. Netrin-1 reduces monocyte and macrophage chemotaxis towards the complement component C5a. PLoS One 2016; 11: e0160685. DOI: 10.1371/journal.pone.0160685 PONE-D-16-13727 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J, Seel CJ, Temirak A, et al. A2B adenosine receptor antagonists with picomolar potency. J Med Chem 2019; 62: 4032–4055. DOI: 10.1021/acs.jmedchem.9b00071. [DOI] [PubMed] [Google Scholar]
- 33.Vecchio EA, White PJ, May LT. The adenosine A2B G protein-coupled receptor: recent advances and therapeutic implications. Pharmacol Ther 2019; 198: 20–33. DOI: S0163-7258(19)30009-9 [pii] 10.1016/j.pharmthera.2019.01.003. [DOI] [PubMed] [Google Scholar]