Abstract
Objective
Recent evidences suggested that hypertension was associated with changes in gut microbiota composition. As intervention with probiotics might be considered as one of the approaches for modulating gut microbiota, the objective of the present study was to systematically review the meta-analyses of controlled trials (CTs) to elucidate the effects of probiotics on blood pressure.
Methods
We searched PubMed, Web of Science, and Cochrane Library databases until November 2019 to explore all the meta-analyses conducted on the CTs assessing the efficacy of probiotics in the management of blood pressure (BP). Meta-analyses performed on in vitro, animal or observational studies were excluded from the study. References of the included studies were also screened to obtain further eligible publications.
Results
From the 111 records which were identified during the literature search, 5 meta-analyses met the selection criteria. Total sample size was 2703 subjects (1009 subjects with type 2 diabetes mellitus (T2DM)), aged 12–75 years from both sexes. Results of meta-analyses have been shown a moderate effect of probiotics on BP in hypertensive adults with/without T2DM; from 3.10 to 5.04 mmHg for systolic blood pressure (SBP) and from 0.39 to 3.84 mmHg for diastolic blood pressure (DBP) after 3–24 weeks consumption. These effects were greater in adults with BP ≥ 130/85, by dairy products, by Asian fermented products with multiple species and higher dose of probiotics (≥ 1011 colony forming units (CFU)).
Conclusion
It seems probiotic foods and supplements which were contained high dose multiple species of probiotic bacteria could be more effective in BP control.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00525-0) contains supplementary material, which is available to authorized users.
Keywords: Probiotics, Blood pressure, Gut microbiota, Meta-analysis
Introduction
Hypertension (HTN) is one of the most health problems worldwide that strongly associated with the risk of ischemic heart disease, kidney disorders and cerebrovascular events development. The rate of elevated systolic blood pressure (SBP), as leading global health risk, increased substantially from 1990 to 2015, consequently disability-adjusted life-years (DALYs) and deaths associated with elevated SBP also increased [1]. This evidence indicates that antihypertensive medications and dietary regimens which applied for the management of HTN have not been completely successful and development of novel therapeutic modalities like gut microbiota modulation are needed. Human intestine hosts trillions of microorganisms including bacteria, yeasts, parasites, viruses and etc.; this dynamic ecosystem called gut microbiota [2]. The human health maintenance such as immune system modulation, intestinal integrity, and protection against pathogens is acknowledged to symbiotic interaction between microbiota and its host [3]. In recent years, it has been shown that gut microbiome is altered in hypertensive patients [4]. In mechanistic view point, some researchers believe that gut microbiota-derived short chain fatty acids (SCFAs) play an important role in a range of physiological processes, including blood pressure [5]. It has been revealed that certain SCFAs-producing species are lower in individuals with higher blood pressure [6].
Probiotics are live microorganisms which when consumed in appropriate amounts may offer some health benefits for the host [7]. Some evidence suggests that probiotics have advantageous role in the function of immune system [8] as well as protective effects against various chronic disorders [9, 10]. Besides, in recent years a number of trials have been conducted evaluating the beneficial effects of probiotics for controlling HTN with various results. In some meta-analyses performed on these randomized clinical trials (RCTs), researchers observed that there is a significant reduction in blood pressure following probiotic consumption; however, some others did not attain such findings [11–15]. Since, review of these meta-analyses could be provided further information regarding probiotics effects on HTN management we aimed to systematically review the meta-analyses that assessed the effectiveness of probiotics on blood pressure.
Methods
Study design and searches
This review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline for literature searching and reports the study findings [16]. A comprehensive systematic search was performed thorough PubMed, Web of Science, and Cochrane Library databases for all relative systematic reviews with meta-analyses documented up to November 2019. The search terms used for publications retrieval were as follows; “probiotic*”, “ Lactobacillus”, “ Bifidobacterium”, “metabolic syndrome”, “blood pressure”, “hypertension”, “cardiovascular”, “ cardiometabolic”, “ meta-analysis”. The search results were imported in EndNote Reference Manager X7 and duplicates were removed by the software function “remove duplicates” and manually by two independent authors. Moreover, reference lists of the included studies were screened to obtain further eligible publications.
Eligibility criteria and study selection
First of all, two authors (OTM, HSE) screened retrieved publications based on the titles and abstracts. The inclusion criteria consisted of: 1. Meta-analyses that conducted on controlled trials (CTs) about the efficacy of probiotics in the management of blood pressure; 2. Publications that had English language full text.
The exclusion criteria consisted of: 1. Meta-analyses performed on in vitro, animal or observational studies; 2. Meta-analyses on prebiotics or 3. Meta-analyses which included variables rather than variables of interest.
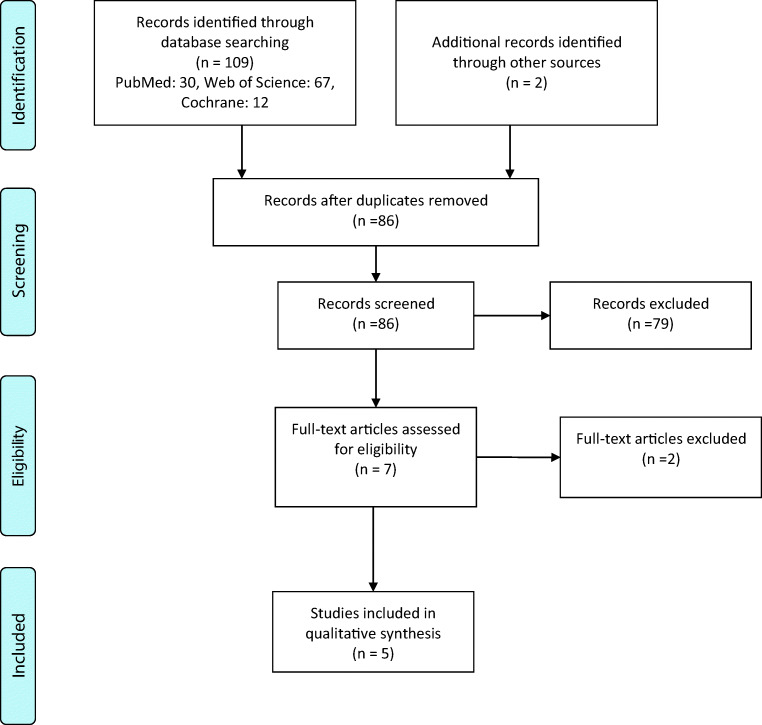
The process of study selection is presented in the PRISMA flow diagram. The full text article of studies that met the inclusion criteria was reviewed.
Data extraction
Data which extracted by two authors was consisted of authors’ name, year of study publication, number and type of included studies in meta-analysis, total sample size, participants’ characteristics, type and dose of probiotic product, and duration of intervention.
Quality assessment
The measurement tool for critical appraisal of systematic reviews entitled ’Assessment of Multiple Systematic Reviews’ (AMSTAR) [17] was used for quality assessment of included studies. AMSTAR is a validated measurement tool for systematic reviews that comprise randomized studies of healthcare interventions. It consisted of 11 questions which answered with ‘yes’, ‘no’ and in some cases ’can’t answer’ or ‘not applicable’, Supplementary file. The AMSTAR score of 8–11 was considered as high quality, 4–7 as medium quality and a score of ≤ 3 as low quality. Blinded quality assessment of each study was performed by two review authors. Any disagreement in scoring process was resolved through discussion until reaching to consensus.
Results
Among total 111 articles which were recorded in the initial search, 5 papers were enrolled in our systematic review after considering inclusion and exclusion criteria, Fig. 1 [11–15]. Type of included studies in the meta-analyses was randomized controlled trials. Characteristics of included meta-analyses are summarized in Table 1. Total sample size was 2703 subjects (1245 normotensive and hypertensive subjects, 1009 subjects with type 2 diabetes (T2DM), and 449 subjects with metabolic syndrome (MetS)), aged 12–75 years old from both genders. The total participants were individuals with obesity, overweight, T2DM, MetS, or dyslipidemia that compared in two groups including normal/hyper blood pressure. Duration of intervention varied from 3 to 24 weeks. Different form of probiotics products were taken in intervention group including foods (fermented milk, yogurt, bread), sachet, pills or capsules which contained single or multiple species of Lactobacillus, Bifidobacterium, Enterococcus, and Streptococcus genera. According to the quality of included studies, three studies had AMSTAR score equal to or greater than 8 which interpreted as high quality and two studies were classified as medium quality (range: 4–7).
Fig. 1.
Flow diagram of the study selection process
Table 1.
Included meta-analysis studies investigating effect of probiotics on blood pressure
| Authors/ Published (year) | Included studies in meta-analysis (n) |
Included studies in meta-analysis (type) |
Total sample size (n) |
Participants’ characteristics | Type and dose of probiotic products | Duration of intervention | Main outcome | Risk of bias assessment | AMSTAR score |
|---|---|---|---|---|---|---|---|---|---|
| Dong J, et al., 2013 [11] | 14 (8 in European countries and 6 in Japan) | DBRCTs, SBRCTs, OLTs |
702 (intervention: UN, Control: UN) |
Normotensive and hypertensive subjects, adults ≥ 18 y, both sexes | Probiotic fermented milk: 100–450 g/day | 4–24 w |
-Sig. SBP: -3.10 mmHg (95% CI: -4.64, -1.56) -Sig. DBP:-1.09 mmHg (95% CI: -2.11, -0.06) |
Yes | 7 |
| Khalesi S, et al., 2014 [12] | 9 (4 in European countries, 4 in Asian countries and 1 in Canada) | DBRCTs, SBRCTs, COTs |
543 (intervention: 282, placebo: 261) |
Normotensive and hypertensive subjects, adults ≥ 18 y, both sexes | Multiple and single species of probiotic dairy products and other sources (109-1012 CFU/day) | 3–9 w |
-Sig. SBP: -3.56 mmHg (95% CI: -6.46, -0.66) -Sig. DBP:-2.38 mmHg (95% CI: -3.84, -0.93) |
Yes | 8 |
| He J, et al., 2017 [13] | 10 (2 in European countries, 7 in Asian countries and 1 in Brazil) | RCTs |
591 (intervention: 297, placebo: 294) |
T2DM, adults ≥ 18 y, both sexes | Probiotic foods (varied amounts) | 4–12 w |
-Sig. SBP:-5·04 mmHg (95% CI: -8.80, -1.20) –Sig. DBP:-0.39 mmHg (95% CI: -0.62, -0·17) |
Yes | 7 |
|
Hendijani F, et al., 2017 [14] |
11 (8 in Asian countries, 1 in Denmark, 1 in Brazil and 1 in USA) | DBRCTs, SBRCTs, COTs |
418 (intervention: 209, placebo: 209) |
T2DM, adults ≥ 18 y, both sexes | Probiotic foods and supplements (varied amounts) | 6–16 w |
-Sig. SBP: -3.28 mmHg (95% CI: -5.38, -1.18) -Sig. DBP:-2.13 mmHg (95% CI: -4.5, -0.24) |
Yes | 9 |
| Dong Y, et al., 2019 [15] | 6 (3 in European countries, 2 in Asian countries and 1 in Australia) | DBRCTs, SBRCTs |
449 (intervention: 242, placebo: 207) |
MetS, adolescents and adults, both sexes | Probiotic foods and supplements (107 to 1010 CFU/day) | 6–24 w | No significant effect on both SBP and DBP | Yes | 8 |
Legend: DBRCTs: double blind randomized controlled trials; SBRCTs: single blind randomized controlled trials; OLTs: open label trials; COTs: cross over trials; UN: Unknown; W: week; SBP: systolic blood pressure; DBP: diastolic blood pressure; ASMRT: A MeaSurement Tool to Assess systematic Reviews; T2DM: type 2 diabetes mellitus; MetS: metabolic syndrome; CFU: colony forming unit.
Dong et al. [11] in their meta-analysis published in 2013 pooled data of 14 RCTs which used probiotic milk fermented with different species of Lactobacillus, Enterococcus, and Streptococcus genera; for example L. casei, L. helveticus, E. faecium, S. thermophilus, and Saccharomyces cerevisiae. They concluded a significant reduction in SBP and diastolic blood pressure (DBP) by -3.10 mmHg (95%CI: -4.64, -1.56) and − 1.09 mmHg (95%CI: -2.11, -0.06), respectively. In subgroup analysis, the greater reduction effect on SBP was estimated in hypertensive individuals compared with normotensive ones; -3.98 mmHg (95%CI: -6.08, -1.88) vs. -2.09 mmHg (95%CI: -4.34, 0.17). In addition, the greater effect was shown in Japanese vs. European populations for both SBP and DBP by -6.12 mmHg (95%CI: -9.17, -3.06) vs. -2.08 mmHg (95%CI: -3.86, -0.30) for SBP, and − 3.45 mmHg (95%CI: -5.77, -1.12) vs. -0.52 mmHg (95%CI: -1.66, 0.62) for DBP. Trials with short term duration (≤ 8 weeks) were shown greater reduction in both SBP and DBP than trials with long term duration by -3.43 mmHg (95%CI: -6.07, -0.79) vs. -3.07 mmHg (95%CI: -5.50, -0.64) for SBP, and − 2.09 mmHg (95%CI: -4.17, -0.01) vs. -0.68 mmHg (95%CI: -2.07, 0.72) for DBP.
Meta-analysis of 9 trials [12] using probiotic dairy products and other sources for 3–9 weeks was shown a statistically significant reduction in SBP and DBP by -3.56 mmHg (95%CI: -6.46, -0.66) and − 3.84 mmHg (95%CI: -2.38, -0.93), respectively. These probiotic products contained Lactobacillus, Bifidobacterium, Enterococcus, and Streptococcus genera which included L. plantarum, L. acidophilus, L. reuteri, L. casei, L. helveticus, B. animalis lactis, B. infantis, E. faecium, S. thermophilus, and Saccharomyces cerevisiae. Subgroup analysis was shown a significant reduction for both of SBP and DBP after long term (≥ 8 weeks) vs. short term duration of intervention (< 8 weeks). These effects for SBP and DBP after long term treatment were − 4.90 mmHg (95%CI: -8.41, -1.40), and − 2.35 mmHg (95%CI: -3.94, -0.75), respectively. A greater reduction was found with multiple vs. single species of probiotics for both SBP and DBP as -5.79 mmHg (95%CI: -8.66, -2.93) and − 2.72 mmHg (95%CI: -4.35, -1.08), respectively. In addition, daily dose of probiotics was shown significant reduction in SBP or DBP when used ≥ 1011 colony-forming units (CFU); -3.78 mmHg (95%CI: -7.30, -0.25) for SBP, -2.86 mmHg (95%CI: -4.96, -0.76) for DBP. Effect of these probiotic products was significant improvement in DBP in subjects with baseline BP ≥ 130/85 mmHg vs. ones with BP < 130/85 mmHg as -2.68 (95%CI: -4.25, -1.10).
Pooling data of 10 RCTs that used different probiotic products for 4–12 weeks in type 2 diabetic patients [13] has been shown a significant reduction in both SBP and DBP as -5.04 (95%CI: -8.8, -1.20) for SBP and − 0.39 (95%CI: -0.62, -0.17) for DBP. The used probiotics was from Lactobacillus, Bifidobacterium, and Streptococcus genera, which included L. acidophilus, L. casei, L. helveticus, L. Sporogenes, L .bulgaricus, B. Longum, B. infantis, B. lactis BB12, B. bifidum, S. thermophilus, and Saccharomyces cerevisiae.
In another meta-analysis of 17 RCTs by varied probiotics in patients with T2DM [14], it was estimated a significant reduction in SBP as -3.28 mmHg (95%CI: -5.38, -1.18), and in DBP as -2.13 mmHg (95%CI: -4.5, -0.24). The prescribed probiotics were from the Lactobacillus and Bifidobacterium genera; including L. plantarum, L. acidophilus, L. casei, L. lactis, L. helveticus, L. Sporogenes, L. thermophilus, L. bulgaricus, B. bifidum, B. Longum, B. infantis, B. lactis BB12, and Saccharomyces cerevisiae.
Pooling data of 6 RCTs which assessed the effects of probiotic foods or supplements on BP in the meta-analysis with 18 RCTs in participants with metabolic syndrome [15] was shown no significant difference in both SBP and DBP between intervention and control groups. These figures were − 0.05 (95%CI: -0.24, 0.14) for SBP and − 0.09 (95%CI: -0.27, 0.10) for DBP. The consumed probiotics were from Lactobacillus and Bifidobacterium genera including L. plantarum, L. acidophilus, L. rhamnosus, L. casei, L. salivarius, B. bifidum, and B. animalis subsp lactis.
Discussion
Our systematic review of meta-analyses was revealed a slight to moderate improvement effect of probiotics on both SBP and DBP after 3–24 week intervention. The beneficial effects of probiotics on BP were shown mostly by dairy products, in adults with BP ≥ 130/85, in Asian fermented products with multiple species and higher dose of probiotics (≥ 1011 CFU).
Since hypertension is known as a major risk factor for cardiovascular diseases, its optimization could be influential on health outcome [18]. A substantial body of evidences based on studies mostly conducted in animal models of hypertension has been supported the link between hypertension and gut microbiota. One of these evidences is Yang et al. study [4] that found significant reduction of BP through gut microbial richness therapy for rats with spontaneously or angiotensin II-induced hypertension and small sample size of humans with essential hypertension. Multiple mechanisms have been claimed for improvement of BP by supplementation of probiotics [19–22] that most of them have been found in animal models. Some of these suggested hypotheses include alteration of autonomic neurotransmission, reduction of lipid peroxidation and oxidative stress, inhibition of angiotensin-converting enzyme (ACE), insulin sensitivity acceleration, improvement of endothelial function, reduction of vascular inflammation, restoring dysbiosis and gut barrier improvement.
Regardless of proposed mechanisms of controlling BP by probiotic products, their beneficial effects were reported in some meta-analyses of RCTs [11–14]. These studies reported a wide variation of BP improvement from 3.10 to 5.04 mmHg for SBP and from 0.39 to 3.84 mmHg for DBP. However, another meta-analysis reported a non-significant slightly reduction in both SBP (0.05 mmHg) and DBP (0.09 mmHg) [15]. The observed discrepancy between results of the meta-analyses [11–15] could be partly attributed to differences in characteristics of studied population, duration of intervention and its cutoff point for subgroup analysis, type of probiotic and fermented products, dose and type of probiotic species. In Dong et al. [15] meta-analysis, the results of probiotics effects on BP in pregnant women and adolescents was pooled with obtained results in adults. However, in other meta-analyses which considered homogeneity of population, it was found a significant beneficial effect of probiotics on BP [11–14]. It should be noticed that even a small reduction in high BP could be implicated a significant effect on health promotion of cardiovascular systems [23]. Although all of included meta-analyses, data of different countries were pooled, just Dong et al [11] performed subgroup analysis to compare effects of probiotics on BP in Asians (Japanese) vs. Caucasians. Small Asians population in this study (211 subjects) compared to European population (494 subjects) makes it difficult to expand observed statistically significant improvement of hypertension to all Asians population worldwide.
Some lactic acid bacteria such as L. johnsonii La1 [24] has shown a hypotensive activity in rats by regulation of the autonomic nervous system. Gomez-Guzman et al. [25] have reported beneficial effects of long-term supplementation of probiotics from L. fermentum, or L. coryniformis plus L. gasseri in rats through reduction of endothelial dysfunction, oxidative stress and vascular inflammation [26]. These formulas could also be restored gut dysbiosis and bacterial abundances that resulted in changing in the cecum microbiota towards increasing counts of Lactobacillus species cluster and decreasing counts of Bacteriodes and Clostridium species [27]. Other studies have demonstrated that the beneficial effect of L. plantarum fermented blueberries on BP can be related to controlling nitric oxide (NO) dependent pathway in the hypertensive rat models [28, 29]. Antihypertensive effects of fermented milk might be related to release of ACE inhibitory peptides that have isolated from milk fermented with L. helveticus and Saccharomyces cerevisiae bacteria [30–32]. Aoyagi et al [33] in their study were highlighted the beneficial effects of fermented milk containing L. casi through increasing prostaglandin I2 biosynthesis and decreasing peripheral vascular resistance that was in agreement with Yap et al. study [34]. In our included meta-analysis, sub-group analysis based on species was not performed. However, the fermented products in our study were from Lactobacillus species that has been proven its beneficial effects on human health in previous studies [35–38]. Therefore, the observed beneficial effects on BP in our study might be related to additive or synergistic effects of multi-species of probiotics.
Our study had some strengths and limitations. The main strength of the current study was the inclusion of all meta-analyses of RCTs that compared the effect of probiotics with a control group. The second strength was high quality of most of the included meta-analyses which has been assessed by AMSTAR checklist. The main limitation was the lack of subgroup analysis to determine effects of each species separately.
In conclusion, it seems modulation of the gut microbiota through high dose multiple species of fermented probiotics products could be effective on BP in adults with/without T2DM.
Electronic supplementary material
(DOCX 13 kb)
Abbreviations
- HTN
Hypertension
- SBP
Systolic blood pressure
- DALYs
Disability-adjusted life-years
- SCFAs
Short chain fatty acids
- RCTs
Randomized clinical trials
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- CTs
Controlled trials
- AMSTAR
Multiple Systematic Reviews’
- T2DM
Type 2 diabetes
- MetS
Metabolic syndrome
- DBP
Diastolic blood pressure
- CFU
Colony forming units
- ACE
Angiotensin-converting enzyme
- NO
Nitric oxide
Authors’ contributions
HSE and OTM designed the study and drafted the manuscript. EA helped to write of final draft of the manuscript, revision and re-analysis. ZHT helped in data extraction. SHR, ARS and BL helped in quality assessment and interpretation of data. All authors read and approved the final manuscript.
Funding information
This study was funded by the Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
Availability of data
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hanieh-Sadat Ejtahed, Email: haniejtahed@yahoo.com.
Edris Ardeshirlarijani, Email: ediardeshir@gmail.com.
Ozra Tabatabaei-Malazy, Email: tabatabaeiml@sina.tums.ac.ir.
Zahra Hoseini-Tavassol, Email: zhtavassol@gmail.com.
Shirin Hasani-Ranjbar, Email: shirinhasanir@yahoo.com.
Ahmad-Reza Soroush, Email: soroush1344@gmail.com.
Bagher Larijani, Email: emrc@tums.ac.ir.
References
- 1.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–82. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley EMM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y) 2013;9(9):560–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pluznick JL. Microbial short-chain fatty acids and blood pressure regulation. Curr Hypertens Rep. 2017;19(4):25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 2017;7(381). 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed]
- 7.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 8.Moro-García MA, Alonso-Arias R, Baltadjieva M, Benítez CF, Barrial MAF, Ruisánchez ED, et al. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age. 2013;35(4):1311–26. doi: 10.1007/s11357-012-9434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong X, Dong J, Wu Z, Li W, Qin L. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65(9):1027. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- 10.Dalmeijer GW, Struijk EA, Van Der Schouw YT, Soedamah-Muthu SS, Verschuren WM, Boer JM, et al. Dairy intake and coronary heart disease or stroke—a population-based cohort study. Int J Cardiol. 2013;167(3):925–9. doi: 10.1016/j.ijcard.2012.03.094. [DOI] [PubMed] [Google Scholar]
- 11.Dong JY, Szeto IM, Makinen K, Gao Q, Wang J, Qin LQ, Zhao Y. Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomized controlled trials. Br J Nutr. 2013;110(7):1188–94. doi: 10.1017/S0007114513001712. [DOI] [PubMed] [Google Scholar]
- 12.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 13.He J, Zhang F, Han Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: a meta-analysis of RCTs. Med (Baltim) 2017;96(51):e9166. doi: 10.1097/MD.0000000000009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37(2):532–41. doi: 10.1016/j.clnu.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Xu M, Chen L, Bhochhibhoya A. Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and meta-analysis of recent clinical trials. Ann Nutr Metab. 2019;74(3):224–41. doi: 10.1159/000499028. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):403–9. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noce A, Marrone G, Daniele FD, Ottaviani E, Jones G, Bernini R, Romani A, Rovella V. Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients. 2019;11:1073. doi: 10.3390/nu11051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upadrasta A, Madempudi RS. Probiotics and blood pressure: current insights. Integr Blood Press Control. 2016;9:33–42. doi: 10.2147/IBPC.S73246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sircana A, Michieli FD, Parente R, Framarin L, Leone N, Berrutti M, Paschetta E, Bongiovanni D, Musso G. Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol Res. 2019;144:390–408. doi: 10.1016/j.phrs.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Schiffrin EJ, Thomas DR, Kumar VB, Brown C, Hager C, Van’t Hof MA, Morley JE, Guigoz Y. Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J Nutr Health Aging. 2007;11(6):475–9. [PubMed] [Google Scholar]
- 23.Daliri EBM, Lee BH, Oh DH. Current perspectives on antihypertensive probiotics. Probiotics Antimicrob Proteins. 2017;9:91–101. doi: 10.1007/s12602-016-9241-y. [DOI] [PubMed] [Google Scholar]
- 24.Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389:109–14. doi: 10.1016/j.neulet.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Guzman M, Toral M, Romero M, Jimenez R, Galindo P, Sanchez M, Zarzuelo MJ, Olivares M, Galvez J, Duarte J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:2326–36. doi: 10.1002/mnfr.201500290. [DOI] [PubMed] [Google Scholar]
- 26.Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla MP, et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and proinflammatory status in obese mice. Clin Sci (Lond) 2014;127:33–45. doi: 10.1042/CS20130339. [DOI] [PubMed] [Google Scholar]
- 27.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR: LA-cp rats. Br J Nutr. 2012;107(4):601–13. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Ahren IL, Prykhodko O, Olsson C, Ahrne S, Molin G. Intake of Blueberry fermented by Lactobacillus plantarum affects the gut microbiota of L-NAME treated rats. Evid Based Complement Altern Med. 2013;2013:809128. doi: 10.1155/2013/809128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahren IL, Xu J, Onning G, Olsson C, Ahrne S, Molin G. Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin Nutr. 2015;34:719–26. doi: 10.1016/j.clnu.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Jauhiainen T, Vapaatalo H, Poussa T, Kyronpalo S, Rasmussen M, Korpela R. Lactobacillus helveticus fermented milk lowers blood pressure in hypertensive subjects in 24-h ambulatory blood pressure measurement. Am J Hypertens. 2005;18:1600–5. doi: 10.1016/j.amjhyper.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Koppe L, Mafra D, Fouque D. Probiotics and chronic kidney disease. Kidney Int. 2015;88:958–66. doi: 10.1038/ki.2015.255. [DOI] [PubMed] [Google Scholar]
- 32.Blood Pressure Lowering Treatment Trialists’ Collaboration. Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, Baigent C, Chalmers J, Li N, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: Meta-analysis of randomised controlled trials. BMJ. 2013;347:f5680. doi: 10.1136/bmj.f5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoyagi Y, Park S, Matsubar S, Honda Y, Amamoto R, Kushiro A, Miyazaki K, Shephard RJ. Habitual intake of fermented milk products containing Lactobacillus casei strain Shirota and a reduced risk of hypertension in older people. Benef Microbes. 2017;8:23–9. doi: 10.3920/BM2016.0135. [DOI] [PubMed] [Google Scholar]
- 34.Yap WB, Ahmad FM, Lim YC, Zainalabidin S. Lactobacillus casei strain C1 attenuates vascular changes in spontaneously hypertensive rats. Korean J Physiol Pharmacol. 2016;20:621–8. doi: 10.4196/kjpp.2016.20.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ardeshirlarijani E, Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar Jalili R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. DARU J Pharm Sci. 2019;27:827. doi: 10.1007/s40199-019-00302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikelsaar M, Zilmer M. Lactobacillus fermentum ME-3- an antimicrobial and antioxidative probiotic. Microb Ecol Health Dis. 2009;21(1):1–27. doi: 10.1080/08910600902815561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang Y, Zhang X, Cai Y, et al. Gut microbiota and metabolic disease: from pathogenesis to new therapeutic strategies. Rev Med Microbiol. 2016;27:141–52. doi: 10.1097/MRM.0000000000000085. [DOI] [Google Scholar]
- 38.Ejtahed HS, Angoorani P, Soroush AR, Atlasi R, Hasani-Ranjbar S, Mortazavian AM, et al. Probiotics supplementation for the obesity management; A systematic review of animal studies and clinical trials. Journal of Functional Foods. 2019;52:228–42. doi: 10.1016/j.jff.2018.10.039. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.