Abstract
Background
The main goal of diabetes therapy is to control blood glucose levels.
Objectives
In this study, the effect of Matricaria chamomilla L. oil as an herbal agent, on therapeutic properties of poly L-lactic acid-based (PLLA) scaffold loaded with differentiated stem cells, is examined in the diabetic rabbit.
Methods
Adipose mesenchymal stem cells (AMSCs) were isolated from male New Zealand White rabbits and after seeding on the PLLA scaffold differentiated in the pancreatic region. In vivo differentiation of AMSCs toward pancreatic progenitor cells was evaluated by quantitative analysis of gene expressions and immunohistochemistry. Then, one normal and five diabetic groups including blank diabetic, scaffold, oil + scaffold, and differentiated cell + scaffold or oil + scaffold were assessed after 21 days of treatment. After the assessment, the diabetic groups were evaluated by clinical parameters and pancreatic histological sections.
Results
It was found that AMSCs were differentiated to insulin-producing cells (IPCs) in the pancreatic environment which then used for implantation. Blood glucose in the oil + scaffold, cell + scaffold, and oil + cell + scaffold groups showed a significant decrease after 21 days. In the above mentioned three groups, insulin secretion was increased significantly. Chamomile oil also caused a significant decrease in High-density lipoprotein (HDL), Low-density lipoprotein (LDL), and total cholesterol levels. According to histological sections results, in cell + scaffold and oil + cell + scaffold groups, β cells were significantly increased compared to blank diabetic group.
Conclusions
Together these data demonstrated chamomile oil along with in vivo-differentiated stem cell is a promising new treatment for diabetes.
Keywords: Chamomile Oil, Diabetes, PLLA Scaffold, Rabbit, Stem Cells
Introduction
The frequency of diabetes disease has been increased for the past 3 decades and its outbreak is growing most rapidly in low and middle-income countries. Diabetes type 2 is the major type of diabetes worldwide in the prevalence of obesity and overweight people [32]. Many clinical trials and research projects have been studied about several treatment strategies for the safety and successful treatment of this chronic metabolic disease with high morbidity and mortality. Recently, stem cell-based therapy with its advantages and also predicted challenges has shown considerable promise for both types of diabetes [18, 26, 42]. Stem cell therapies can provide an alternative approach for tissues and organs repair and regeneration [36]. These cells can replicate, transform and differentiate into different cell types [10]. Mesenchymal stem cells (MSCs) are capable candidates for cell-based treatments [13]. Bone marrow and adipose tissue have provided two suitable sources of MSCs [5]. AMSCs have convenient accessibility and broad differentiation potential into different cell lines [28]. Due to their capacity to generate multiple cell lineages, MSCs can be affected by certain conditions in vivo or in vitro. This can influence its conversion to cells with specific functions, like heart muscle cells or insulin-producing cells in the pancreas [3]. Results of new in vivo research using real-time quantitative polymerase chain reaction (qRT-PCR), Western blotting and flow cytometric analysis showed that MSC-derived exosomes recover diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-beta1/Smad2 signaling pathway [23]. Regenerative medicine depends heavily on three-dimensional cultivation techniques on scaffolds [8], which are supporting structures similar to the cell-matrix [29]. PLLA and Gelatinous scaffolds are considered as two of the principal biocompatible and dissoluble polymers used in tissue engineering [25]. The three-dimensional microenvironments supplied by these scaffolds like other ones provide a stable structure for the transplanted cells [14], playing a role similar to the secretion of extracellular matrix [24, 40]. MSCs - PLLA scaffold system can be used as cell therapy for treating chronic diseases such as diabetes.
Traditional medicine is another option to treat some diseases. Diabetes therapy is one of the major areas of traditional medicine. Matricaria chamomilla L. is a safe plant and is used in different commercially available forms such as tea, infusion, liquid, and capsules in human nutrition. Chamomile (Matricaria chamomilla L.) also has some useful properties like pain therapy, anti-anxiety, oral and dermal wound treatment [12, 33, 41]. It was also reported chamomile improves diabetes and its complications by decreasing blood sugar levels, increasing glycogen stores in the liver and red blood cells contained sorbitol [20]. It seems that the combination of this agent with cell therapy methods and tissue engineering can be useful to repair and improve diabetes disease. The purpose of this investigation was to evaluate the use of stem cells derived from adipose tissue in the prevention and control of diabetes using biological scaffold soaked in chamomile oil.
Materials and methods
Animals
In this investigation, 40 male New Zealand White rabbits (2.0–2.5 kg; Razi Institute, Iran) at postnatal week ten were used. The Animal Ethics Committee of Shiraz University and the National Institute of Health Animal Care Guidelines approved all experimental processes of this project with the code of 2272329. During the evaluation process, all rabbits were given food and tap water ad libitum and preserved in a temperature and humidity controlled room on a 12-h light/12-h dark cycle.
Isolation and characterization of AMSCs
Isolation and culture of AMSCs were the same as in our previous work [15]. The cell surface antigen profile expression of AMSCs was characterized using flow cytometry as described previously [15, 17]. Differentiation ability of extracted AMSCs to three categories was carried out as previously described [4, 15].
Scaffold fabrication and Fourier transform infrared (FTIR) spectroscopy
Oil extraction from M. chamomilla L. was performed the same as our previous report [15]. Scaffold fabrication and FTIR spectroscopy of PLLA scaffold and PLLA coating with oil was according to the previous report and surface morphology of fabricated scaffold was investigated by scanning electron microscopy (SEM) as previously described [15].
AMSCs seeding into the PLLA and in vivo-differentiation of AMSC toward pancreatic progenitor cells
These steps were performed the same as our previous report [15]. AMSCs with the scaffold was inserted for 21 days in the caudal of a stomach, between the pancreas and spleen. Ultrasound image was used for implantation of scaffold assessment. Differentiation confirmation of AMSCs to IPCs was performed by quantitative analysis of gene expressions and immunohistochemistry [15].
Diabetic rabbit model preparation
Streptozotocin (STZ) was produced by Sigma (St Louis, MO, United States). Rabbits were injected intraperitoneally with 80 mg/kg body weight. STZ dissolved in 0.1 M citrate buffer, pH 4.5. Rabbits were weaned after 21 days and maintained on the normal diet in individual cages throughout the experimental period.
Experimental group’s classification
36 male rabbits were randomly classified into 6 groups; a standard and 5 diabetic groups. Diabetic groups contained diabetic (blank), scaffold, oil + scaffold, cell + scaffold, and cell + oil + scaffold. All experimental groups were assessed after 21 days of treatment and then evaluated by clinical parameters.
Clinical parameters assessment and Haematoxylin and Eosin (H&E) staining of islets of langerhans
Serum glucose, LDL, HDL, and total cholesterol were measured by the colorimetric method (Auto analyzer, Hitachi 912, Japan) and insulin by chemiluminescence (Liaison, Italy) kit as per the manufacturer’s instructions. After rabbit scarification, islets of Langerhans were evaluated with the H&E staining of pancreatic histological sections [16].
Statistical analyses
This study was basic research of stem cell and animal study in regenerative medicine. Statistical analyses were performed using SPSS 16.0. Statistical significance was measured by using one -way analyses of variance and Tukey’s multiple comparison tests. A confidence level of 95% (P ≤ 0.05) was well-thought-out statistically noteworthy. All data are offered as the mean value ± SD in 40 rabbits.
Results
Culture characteristics
Two days after primary cultivation, spindle-shaped adherent cells were detected. Cells showed fibroblast-like homogenous morphology and expanded rapidly in 5–6 days. As the same as our previous report [15], AMSCs were positive for the MSCs markers (94% and 95% for CD44 and Cd45 respectively) and negative for hematopoietic markers according to flow cytometric results. These cells had also differentiation ability to 3 lines of cells contained adipogenic, osteogenic and chondrogenic line after 21 days of differentiation.
Morphological structures of PLLA scaffold
The SEM images demonstrate the electrospun PLLA and highly porous fiber and an ECM-like structure of scaffolds [15].
In vivo differentiation of AMSC on PLLA ± Oil to IPCs
Ultrasound image showed that scaffold coated oil loaded by AMSCs near the pancreatic region in caudal of the stomach, between spleen and pancreas as a mass with hetero echogenicity [15]. Quantitative analysis of gene expressions and immunohistochemistry results confirmed the differentiation [15].
Confirmation of diabetic model after STZ injection
As shown in Table 1, blood glucose was increased after STZ injection, after 72 h and 10 days. These results indicate that STZ treatment induces a diabetic state in this rabbit model.
Table 1.
Blood glucose levels before and after STZ injection
| Time of Measuring | Average Glucose Level (mg/cc) |
|---|---|
| Before STZ injection | 93 |
| 72 h after STZ injection | 248 |
| 10 days after STZ injection | 303 |
Clinical parameters after different scaffold implantations
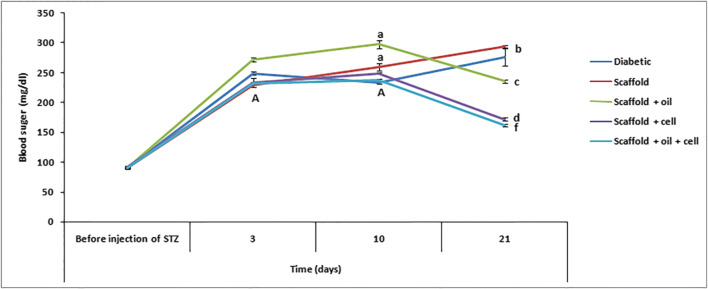
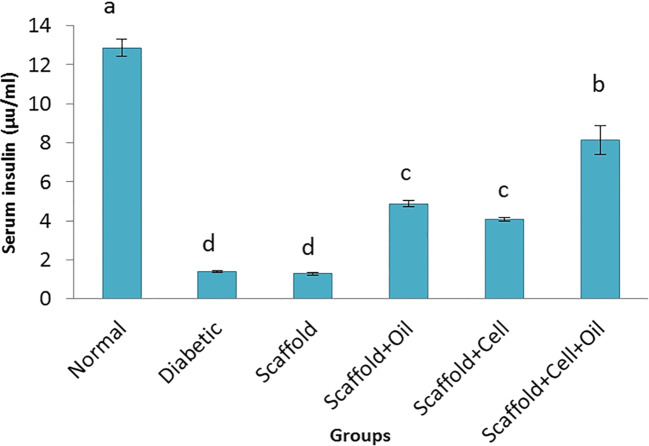
Blood glucose in oil + cells + scaffold, oil scaffold, and cell + scaffold groups showed significantly (P ≤ 0.05) lower levels of blood glucose compared to STZ and scaffold groups (Fig. 1). As shown in Fig. 2, treatment with oil or cell + scaffold and cell + oil + scaffold increased insulin secretion significantly (P ≤ 0.05), while serum insulin level increase in cell + oil + scaffold group was more than in and oil or cell + scaffold groups. Diabetic and scaffold had the lowest level of insulin secretion compared to all experimental groups.
Fig. 1.
Blood glucose levels at 0, 3, 10, and 21 days after treatment of diabetic rabbit model in experimental groups. The b, c, d & f show the significant difference between the groups with each other at the same time point. The A and a: the significant difference in the same group at a different time point (P ≤ 0.05)
Fig. 2.
Serum insulin levels after treatment of diabetic rabbit model in experimental groups. a-d are averages of insulin levels which are significantly different from each other (P ≤ 0.05)
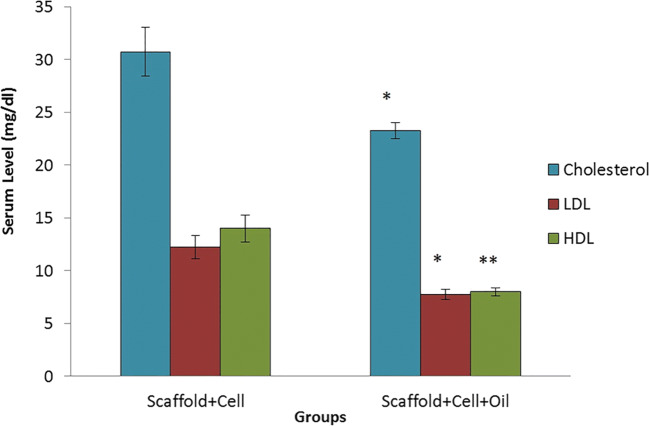
Oil + cell + scaffold rabbit group showed significantly (P ≤ 0.05) lower levels of HDL, LDL, and total cholesterol compared to cells with a scaffold (Fig. 3).
Fig. 3.
Cholesterol, LDL, and HDL levels at 21 days after treatment of diabetic rabbit model in experimental groups (P ≤ 0.05)
Evaluation of pancreatic histological sections
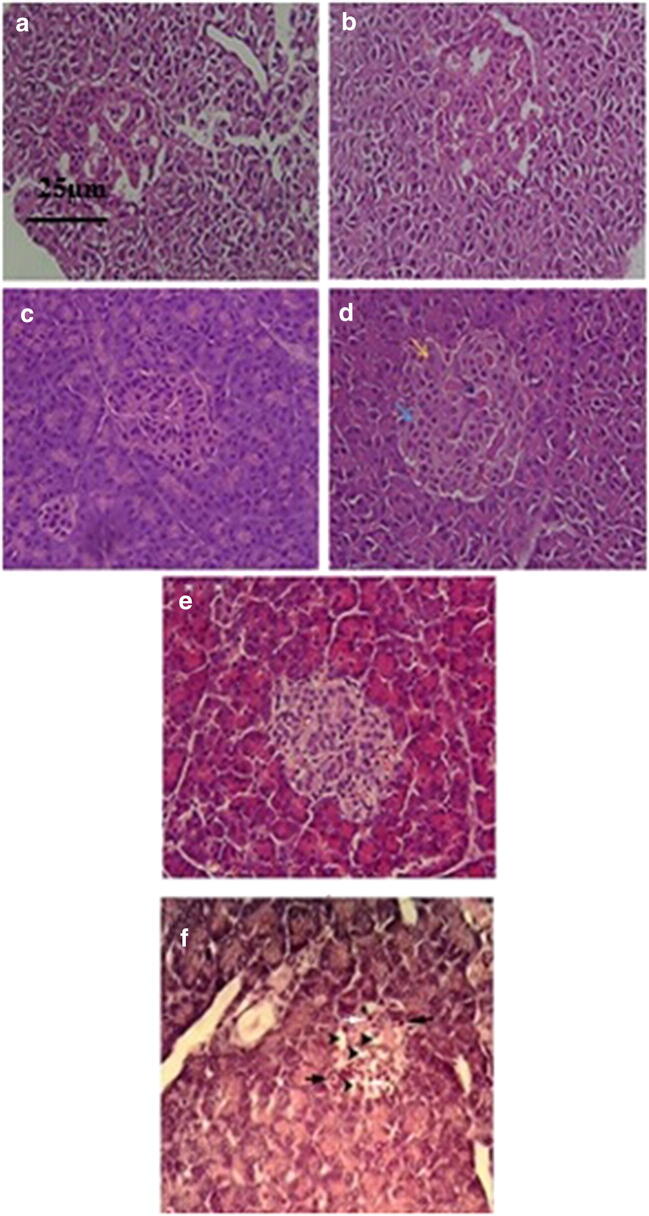
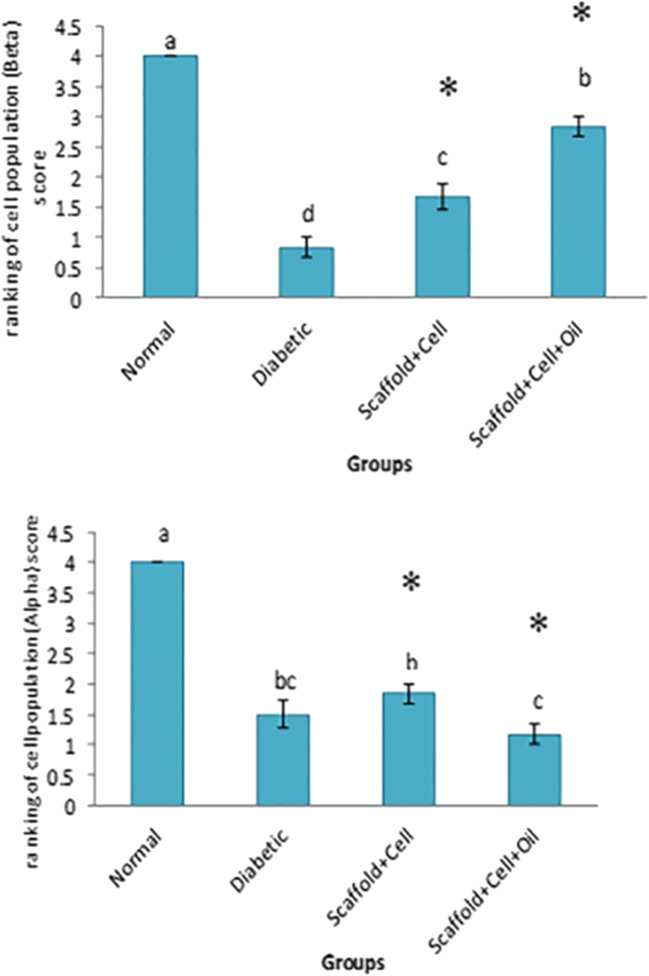
Pancreatic histological sections results showed that in cell + scaffold and oil + cell + scaffold groups, α cells were significantly (P ≤ 0.05) decreased and β cells increased compared to blank diabetic group (Figs. 4 and 5).
Fig. 4.
H & E ∗ 540 stainings of a histological section of islets of Langerhans (⋅ 400). A & B) cell + scaffold group, C & D) cell + oil + scaffold group (Black arrow indicates blood vessel, yellow arrow α cells, and blue arrow β cells), E) Normal islets of Langerhans, F) diabetic group, white arrow indicates necrotic cells and black arrow indicates inflammatory cells
Fig. 5.
Evaluation of α and β cells population in examined groups. Results are presented as mean (a-d) ± SD. *P < 0.05 was considered a significant difference as compared to control (blank diabetic group). Cell population 25% (score 1), 25–50% (score 2), 50–75% (score 3), 75–100% (score 4)
Discussion
Originally, the focus of clinical translation for AMSCs was on their capacity to differentiate into multiple lineages of attention to the field of regenerative medicine, particularly for regenerating cartilage and bone defects [28]. AMSCs can differentiate to IPCs that expressed Ngn3 and Pdx1 by MiR-375 and Anti-MiR-9. Gabr et al. (2015) used nicotine amid and glucagon-like peptide 1 to differentiate MSCs to IPCs [5, 19, 34]. Allahverdi et al. [2] found that lentiviral vectors could deliver the PDX1 gene to MSCs and induce pancreatic differentiation [2]. In the present study, a novel differentiation protocol was used to produce IPCs without any growth factor and genetic manipulation as the same in our previous study [15]. Orthotropic transplantation in the same anatomical region of an organ is the best tool in organ graft [37]. In the anatomical region, the microenvironment of the cells and the paracrine secretion help to maintain cell behavior similar to cells present in healthy tissue [35]. On the other hand, the mimic of cells helps to proliferation and differentiation of cells by a natural signaling pathway manner. However, consider the microenvironment, type and be autologous of transplanted cells is very effective in this process [22]. Yamamoto et al. [39] used the spleen microenvironment to differentiate embryonic stem cells to hepatocytes [39]. One of the strategies in tissue engineering is the modification of the surface coating of the scaffolds by diverse biomaterials [40]. PLLA was well examined in the literature due to several of their physical, chemical, morphological, thermal and physical confidants [25]. PLLA coated by M. chamomilla L. oil, and their blends did not have any side effect on scaffold bioactivity or antigenicity [6, 11]. Chamomile ethanolic extract has antihyperglycemic and antioxidative activities in the diabetic rat model [7]. Also, Kato et al. [20] determined the effects of chamomile oil on reducing blood sugar and preventing diabetic complications [20]. In diabetic patients, hyperglycemia is considered as a.
Reactive oxygen species (ROS) elevator and antioxidant defense capacity depressor, through several ways such as glycolytic pathway. Chamomile oil with reducing blood sugar leads to decrease ROS and makes a balance between ROS and antioxidant capacity. Chamomile oil increases glycogen synthesis and sorbitol synthesis inhibition causes to decrease blood sugar [7]. Several types of research focused on diabetes treatment by herbal drugs such as Ale or [38], Cinnamon [21], Ginger [1]. According to one study, garlic extract contained antioxidant substances, capable of increasing insulin secretion and decreasing blood sugar [27]. In the present study, blood sugar decreased in scaffold + oil, scaffold + cell, and scaffold + cell + oil after 21 days. Interestingly, this decrease had a significant difference in cell + scaffold and cell + scaffold + oil compared to other groups. It may be because of the unknown growth factor and cytokines by differentiated cells loaded on the scaffold. These factors lead to cell migration in injured sites and improve recovery duration. Cheng et al., [9]showed MSCs co-cultured with damaged pancreatic cells can repair beta cells and effect insulin-secreting cells [9]. MSCs, secrete insulin-like growth factor 1 factor after co-culturing with damaged pancreatic cells. MSCs also activated the PI3K pathway that makes a cascade of molecules in vivo, and thus acts on muscle, fat, and liver. This pathway increases the uptake of glucose into the cells, glycogen synthesis, lipogenesis and decreases gluconeogenesis leading to a reduction of blood sugar levels [9]. Since, hyperglycemia associated with fat and vice versa, therefore, serum cholesterol, HDL and LDL of the diabetic rabbit model was evaluated. It was found that the amount of cholesterol and LDL in the cell + scaffold + oil significantly decreased compared to the cell + scaffold. Although the ratio of HDL levels in cell + scaffold + oil group was significantly lower than scaffold + cell groups, the LDL / HDL ratio which is considered a biomarker was already 2.5 and 4 in the cell + scaffold and cell + scaffold + oil, respectively. These results were following Saeb et al., research on the effect of pistachio oil on serum lipid rabbit, normal cholesterol 28 mg / dl and LDL 28 mg/dl reported [31]. In agreement with Saghahazrati et al. (2019), about the protective effects of cultured MSCs onto an electrospun PLLA scaffold coated with Matricaria chamomilla L. Oil, the results of our study showed that the Matricaria chamomilla L. Oil might improve survival and differentiation MSCs and serum insulin level. Also, a clinical trial study has been shown that Chamomile might have valuable effects on serum lipid profile and glycemic control in patients with type 2 diabetes mellitus [30]. In our investigation, it has been found an increase in insulin secretion with a lower level of HDL, LDL, and cholesterol compared with a diabetic condition. So, chamomile oil along with in vivo-differentiated stem cells has great potential for use to the treatment of diabetes although further genomics, proteomics, and metabolomics studies are needed and ongoing.
Conclusions
Taken together, M. chamomilla L. oil could affect the adhesion of cells to the scaffold and along with the cells decrease blood sugar and increase blood insulin in the rabbit. It is recommended that the effectiveness of chamomile oil + cell + scaffold and molecular pathways activated by these materials will be considered in future trends. More extensive studies are suggested to be carried out in this direction soon including the synergistic effect of stem cell and herbal therapy in humans and also the molecular mechanisms underlying their therapeutic effects in diabetes.
Abbreviations
- AMSCs
Adipose mesenchymal stem cells
- MSCs
Mesenchymal stem cells
- PLLA
Poly L-lactic acid
- IPCs
Insulin-producing cells
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- STZ
Streptozotocin
- FTIR
Fourier transform infrared
- SEM
Scanning electron microscopy
- ROS
Reactive oxygen species
- H&E
Hematoxylin and Eosin
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Amin et al., ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiberofficinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–6. [DOI] [PubMed]
- 2.Allahverdi et al., A, Abroun S, Jafarian A, Soleimani M, Taghikhani M, Eskandari F. Differentiation of Human Mesenchymal Stem Cells into Insulin-Producing Cells by Using A Lentiviral Vector Carrying PDX1. Cell J. 2015;17:231–42. [DOI] [PMC free article] [PubMed]
- 3.Aurich et al., H, Soda M, Kaltvaber P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570–81. [DOI] [PubMed]
- 4.Bakhtina et al., A, Tohfafarosh M, Lichtler A, Arinzeh TL. Characterization and differentiation potential of rabbit mesenchymal stem cells for translational regenerative medicine. In Vitro Cell Dev Biol Anim. 2014;50:251–60. [DOI] [PubMed]
- 5.Bertassoli et al., BM, Neto A, Oliveira FDD, Arroyo MAM, Ferrao JSP, Silva JBD, Pignatari GC, Braga PB. Mesenchymal stem cells: emphasis in adipose tissue. Brazilian Archives of Biology Technology. 2013;56:607–17.
- 6.Bianco et al., P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–21. [DOI] [PubMed]
- 7.Cemek et al., M, Kaga S, Şimsek N, Buyukokuroglu ME, Konuk M. Antihyperglycemic and antioxidative potential of Matricariachamomilla L. in streptozotocin-induced diabetic rats. Journal of natural medicines. 2008;62:284–93. [DOI] [PubMed]
- 8.Chao et al., PH, Grayson W, Vunjak-Novakovic G. Engineering cartilage and bone using human mesenchymal stem cells. Journal of Orthopaedic Science. 2007;12:398–404. [DOI] [PMC free article] [PubMed]
- 9.Cheng et al., Z, White MF. The AKT ion in non-canonical insulin signaling. Nat Med. 2012;18:351–3. [DOI] [PMC free article] [PubMed]
- 10.Corcione et al., A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. [DOI] [PubMed]
- 11.Cui et al., YL, Hou X, Qi AD, Wang XH, Wang H, Cai KY, Yin J, De Yao Y, K. Biomimetic surface modification of poly (L-lactic acid) with gelatin and its effects on articular chondrocytes in vitro. J Biomed Mater Res A. 2003;66:770–8. [DOI] [PubMed]
- 12.Das M. Chamomile: medicinal properties medicinal, biochemical, and agricultural aspects. Boca Raton: CRC Press; 2014. [Google Scholar]
- 13.Deans et al., RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–84. [DOI] [PubMed]
- 14.Dutta et al., RC, Dutta AK. Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv. 2009;27:334–9. [DOI] [PubMed]
- 15.Fazili A, Gholami S, Minaie Zangi B, Seyedjafari E, et al. In vivo differentiation of Mesenchymal stem cells into insulin producing cells on Electrospun Poly-L-Lactide acid scaffolds coated with Matricaria chamomilla L. Oil. Cell. 2016;18:310–21. doi: 10.22074/cellj.2016.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold spring harbor protocols. 2008:pdb. prot4986. [DOI] [PubMed]
- 17.Hammes et al., F, Berney M, Wang Y, Vital M, Koster O, Egli T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008;42:269–77. [DOI] [PubMed]
- 18.Helman A, Melton DA. A stem cell approach to cure type 1 diabetes. Cold Spring Harb Perspect Biol, 2020 [DOI] [PMC free article] [PubMed]
- 19.Jafarian A, Taghikani M, Abroun S, Allahverdi A, Lamei M, Lakpour N, Soleimani M, et al. The generation of insulin-producing cells from human Mesenchymal stem cells by MiR-375 and Anti-MiR-9. PLoS One. 2015;10:e0128650. doi: 10.1371/journal.pone.0128650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato et al., A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem. 2008;56:8206–11. [DOI] [PubMed]
- 21.Khan et al., A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–8. [DOI] [PubMed]
- 22.Le Blanc et al., K, Frassoni F, Ball L, Locatelii F, Roelofc H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. [DOI] [PubMed]
- 23.Lin Y, Zhang F, Lian XF, Peng WQ, Yin CY. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-beta1/Smad2 signaling pathway. Cell Mol Biol (Noisy-le-Grand). 2019;65:123–126. [PubMed]
- 24.Ling CQ, Yue XQ, Ling C, et al. Three advantages of using traditional Chinese medicine to prevent and treat the tumor. J Integr Med. 2014;12:331–5. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 25.Mashhadikhan M, Soleimani M, Parivar K, Yaghmaei P, et al. ADSCs on PLLA/PCL hybrid nanoscaffold and gelatin modification: cytocompatibility and mechanical properties. Avicenna J Med Biotechnol. 2015;7:32–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Memon B, Abdelalim EM. Stem cell therapy for diabetes: Beta cells versus pancreatic progenitors. Cells. 2020;9. [DOI] [PMC free article] [PubMed]
- 27.Padiya et al., R, Chowdhury D, Borkar R, Srinivas R, Pal Bhadra M, Banerjee SK. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS One. 2014;9:e94228. [DOI] [PMC free article] [PubMed]
- 28.Pendleton C, LI Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A, et al. Mesenchymal stem cells derived from adipose tissue vs. bone marrow: in vitro comparison of their tropism towards gliomas. PloS One. 2013;8:e58198. doi: 10.1371/journal.pone.0058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradhan S, Farach-Carson MC, et al. Mining the extracellular matrix for tissue engineering applications. Regen Med. 2010;5:961–70. doi: 10.2217/rme.10.61. [DOI] [PubMed] [Google Scholar]
- 30.Rafraf M, Zemestani M, Asghari-Jafarabadi M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J Endocrinol Investig. 2015;38:163–70. doi: 10.1007/s40618-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 31.Saeb et al., M, Nazifi S, Mirzaei A. Studies on the effects of Turpentine oil on the serum concentration of Lipids and Lipoproteins of female rabbits. J Shahid Sadoughi Uni Med Sci Health Serv. 2004;3:42–50.
- 32.Sheikhpour M, Abolfathi H, Khatami S, Meshkani R, Barghi TS. 2020. The Interaction between gene profile and obesity in type 2 diabetes: A review. Obes Med:100197.
- 33.Siu et al., WS, Ko CH, Lam KW, Wat E, Shum WT, Lau CB, Leung PC. 2015. Evaluation of a topical herbal agent for the promotion of bone healing. Evid Based Complement Alternat Med. 2015:905270. [DOI] [PMC free article] [PubMed]
- 34.Sun et al., Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, Tang KX, Wang B, Song J, Li H, Wang KX. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. (Engl) Chin Med J. 2007;120:771–6. [PubMed]
- 35.Sun et al., Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519–42. [DOI] [PMC free article] [PubMed]
- 36.Uccelli et al., A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. [DOI] [PubMed]
- 37.Vincent et al., CE, Chinagorom LO. Organ transplantation and its physiological implications. Animal Research International. 2013;10:1752–78.
- 38.Vincent CE, Chinagorom LO, et al. Organ transplantation and its physiological implications. Anim Res Int. 2013;10:1752–78. [Google Scholar]
- 39.Yamamoto et al., H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, Ochiya T. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–93. [DOI] [PubMed]
- 40.Yan et al., SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–69. [DOI] [PubMed]
- 41.Yeh et al., GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–94. [DOI] [PubMed]
- 42.Zhang Y, Chen W, Feng B, Cao H. The clinical efficacy and safety of stem cell therapy for diabetes Mellitus: A systematic review and meta-analysis. Aging Dis. 2020;11:141–53. doi: 10.14336/AD.2019.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]