Abstract
Several investigations have been conducted during the past years to examine the correlation between dysbiosis and both intestinal and extra-intestinal diseases such as inflammatory bowel disease (IBD) and ulcerative colitis (UC).E. coli Nissle 1917 (EcN) is a nonpathogenic gram-negative strain utilized in numerous gastrointestinal issues, consisting of diarrhea, uncomplicated diverticular malady, IBD and specifically UC. Many investigations have been done to examine the capability of assertive bacteria, inclusive of commensal and probiotic strains to enhance IBD in clinical testing. Bacterial secreted factors have been investigated to detect the EcN agents that facilitate the regulation of tight junction. These agents candiffuse smoothly through the mucin layer before reaching intestinal epithelial cells. Outer membrane vesicles (OMVs) are known as intercellular communicasomes as they facilitate the distal transfer of active compounds between cells. A few investigations have detailed immune-modulatory attributes for EcN through various systems that could be liable for its clinical viability in IBD. Today, the function of gut microbiota extracellular vesicles in health and disease has become a focus of attention as they serve as vehicles for the transmission of microorganisms to distal tissues of many bacterial effectors.
Introduction
More than thousands of various species, exceeding from 15,000 diverse bacterial strains with the average weight of 1 kg form the human microflora, termed as microbiota. Several studies have been conducted during the past years to examine the correlation between dysbiosis and both intestinal and extra-intestinal diseases such as inflammatory bowel disease (IBD) and ulcerative colitis (UC) [1]. The term IBD applies specifically to the illness of UC and Crohn’s disease (CD). It was shown that these chronic inflammatory diseases may result in serious complications due to their increasing universal occurrence [2]. Microbiota studies in UC showed that the severity of intestinal tract inflammation was related to the number of bacteria. In this regard, some results showed that surgical removal of bacteria improved the inflammation, and also inflammation did not manifest in germ-free animals [3].
Recent studies showed that the emergence of several chronic inflammatory diseases and systemic malfunctions are resulting from microbiota dysbiosis and intestinal barrier impairment [4].
The occurrence of dysbiosis in IBD [4] has been proposed in some investigations. As a result of dysbiosis, the microbial pattern was modified extensively; the diversity and the riches of the bacterial taxa within the Phyla of Firmicutes and Bacteroides diminished while it improved in the Gammaproteobacteria [5, 6]. In addition, an α diversity reduction in the fecal microbiome was observed in patients suffering from CD compared with healthy controls, and [7] similar data was noticed in monozygotic twins discordant for CD [8]. IBD treatment is complicatedand did not seem to be effective for all the patients who underwent commercially available medications to cure intestinal disorders, consisting of salicylates, corticoids, immune-suppressants and biological agents [9]. Many attempts have been made to modulate the gut microbiota due to the prevalence of dysbiosis in IBD. [10]. In this regard, many investigations have been done to examine the capability of assertive bacteria inclusive of commensal and probiotic strains with the aim of enhancing IBD in clinical testing [11, 12] or animal models of colitis [2]. It was found that certain probiotics and gut beneficial microbes inhibited the barrier disruption caused by the enteric pathogenic agents [13–15].
Escherichia coli Nissle 1917 (EcN) has been proven to be operative for curing the inflammatory intestinal illnesses [16–18] and acute diarrhea [19]. EcN is a nonpathogenic gram-negative strain utilized in numerous gastrointestinal issues consisting of diarrhea [20], uncomplicated diverticular malady [21] and IBD, specifically UC [22]. Bacterial secreted factors have been investigated to detect the EcN agents that facilitate the regulation of tight junctions (TJ). These agents can diffuse smoothly through the mucin layer before reaching intestinal epithelial cells [23, 24].
Today, it is known that outer membrane vesicles (OMV) produced by gut bacteria play an important role in microbiota-host communication, as they facilitate the long-distance transfer of microorganisms to the host [25]. A combination of adhesins, sulfatases,and proteases enables OMVs to interact with host epithelial cells by providing various pathways such as micropinocytosis, lipid raft- and clathrin-dependent endocytosis [26]. Today, it is known that microbiota vesicles exert a positive influence on immunity and signaling pathways at the intestinal mucosa [27, 28]. However, there is limited data available in this area, and to the best of our knowledge, there is no report on the mechanism of vesicles secreted by gut microbiota or probiotic species [29].
Mechanism of action in E. coli Nissle1917
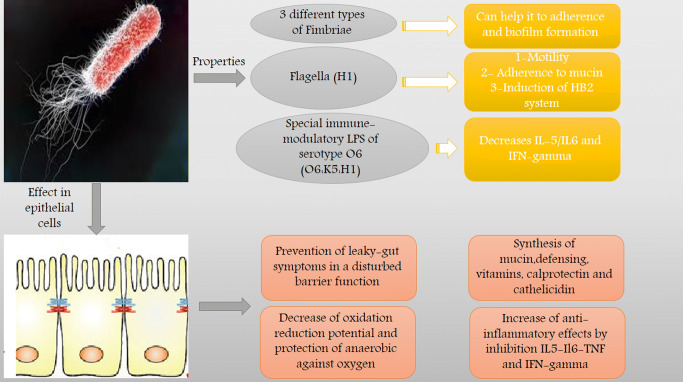
In 1917, during World War I, Alfred Nissle from Freiburg, Germany isolated EcN 1917 (O6:K5:H1) from the intestinal microflora of a young soldier who did not develop infectious diarrhea during a severe outbreak of shigellosis Mutaflor® as a probiotic medication was formulated by Prof. Nissle. Since then, this medicine is commercially available in Germany and lately in Italy as EcN [30]. The probiotics efficiency has been reported to be uncertain due to their dependence on the type of IBD (UC, CD, or pouchitis) or the phase (exacerbation or remission) of the intestinal condition when the probiotics were applied [31]. A few investigations have detailed the immunomodulatory attributes of EcN through various systems that could be liable for its clinical viability in IBD. In order to balance the cytokine generation in immune cells to intervene with various cell pathways such as various transcription factors like Nuclear factor kappa beta (NF-kB) and modulating the activity of the mitogen-activated protein kinase (MAPKs), these systems are mostly connected with the capacity of this probiotic [3, 32]. Further, the antimicrobial attributes of this probiotic, which includes both an immediate impact and the incitement of defensins creation by intestinal epithelial cells, can likewise add to its positive impacts in trial colitis just as in human IBD [33]. As affirmed by Wehkamp et al., [33] EcN was found to cause induction in human beta-defensin-2 (hBD-2) in intestinal epithelial cells through NF-kB and activator protein 1 (AP1).
Antigenic attributes of flagellin resulted in the expression of hBD-2. [34]. The expression of hBD-2 is generally observed in inflammation, as in UC and to a lower extent in CD, [35] which may express the effective application of EcN in UC [36]. In addition, EcN appeared to have a direct impact on the paracellular pathway, leading to the revival of an impaired epithelial barrier. TJ breakdown occurs as a result of T84-cells with enteropathogenic incubation.
While coincubation with EcN leads to an increase of zonula occludens-2 (ZO-2) expression, its redistribution toward cell boundaries and silencing of protein kinase C (PKC) isotypes results in tight junction and epithelial barrier repair (Fig. 1).
Fig. 1.
Properties and mechanism of action E. coli Nissle 1917 in gut
OMV of E. coli Nissle1917
In order to communicate with host cells, commensal and pathogenic gram-negative bacteria implement various mechanisms. Production of the membrane vesicles is one of the procedures that enable the cargo to be transferred to distant targets in the host [37–40]. OMVs are known as intercellular communicasomes as they facilitate the distal transfer of active compounds between cells. Vesicular microbe-associated molecular pattern (MAMPs) including Lipopolysaccharide (LPS), peptidoglycan, lipoproteins, Deoxynucleic acid (DNA) and Ribonucleic acid (RNA) enables OMV to interact with host cells with the aid of pattern recognition receptor (PRRs), and activate signaling pathways leading to the modification of cytokine/chemokine [27, 41]. Commensal bacteria not only perform in the recognition of extracellular Toll-Like receptors (TLRs) but also in the signaling process through Nod like receptors.
(NOD1 and NOD2) which are two components of NLRs [42, 43]. Consequently, OMVs discharged by commensal bacteria are considered to play an important role in signaling pathways of intestinal mucosa [27]. The capacity of bacterial OMVs to communicate and join host cells has favored their potential for novel medical and biotechnological applications [44–46]. Investigations regarding the common gram-negative bacteria residing in the human gut manifested that the secretion of OMVs by Bacteroides fragilis and Akkermansia muciniphila enhanced the immunomodulatory consequences, and also inhibited the gut inflammation in mice models of experimental colitis [28, 47]. The probiotic EcN as a decent intestinal colonizer has been authorized for use in human medicineto treat few diseases gastrointestinal tract diseases. For example, EcN has been well-documented to relieve UC [18, 30]. Further, EcN as a strong intestinal colonizer has been approved for use in human medicine to treat a variety of gastrointestinal disorders [3, 48–50].
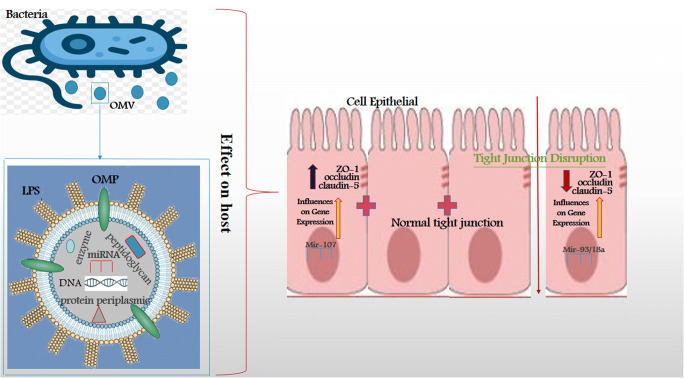
The capacity of EcN to diminish intestinal penetrability and fix defective gut might be associated with its capability to strengthen TJ.
In vivo upregulation of ZO-1 by EcN suspensions was observed in both healthy and dextran sodium sulfate-treated mice [50], while in vitro upregulation of ZO-2 [51] or claudin-14 [52], was examined based on the applied epithelial cell line. Along these lines, MVs produced by commensal strains are rising as crucial factors in signaling procedures in the intestinal mucosa. Recent investigations indicated that EcN OMVs, just as vesicles created by other commensal E. coli strains, are disguised by epithelial cells through clathrin-mediated endocytosis [53], and intervene signaling to the immune system through the intestinal epithelial barrier [54]. In this regard, investigations confirmed that OMVs from the probiotic EcN and other commensal E. coli strains, transfer mediators that activate the host immunity. OMVs from E. coli strains display immunomodulatory actions on various in vitro models of the intestinal barrier and human colonic explants, managing the antimicrobial peptides and inflammatory biomarkers expression toward a calming profile [54]. Inflammation and colitis progression in dextran sodium sulfate (DSS)-treated mice improved when OMVs derived from the probiotic EcN applied orally, correspondingly to the implementation of probiotic suspensions (Fig. 2) [2].
Fig. 2.
Role of bacteria and its OMV in tight junctions expression genes in the gut
The efficiency of E. coli Nissle 1917 in IBD
The etiology of IBD has not been explained so far. In any case, it has been suggested that intestinal dysbiosis is essential since it can advance a wrong invulnerable reaction in people who are suffering from genetic disorders, prompting the inflammatory intestinal conditions [55]. Not all patients showed total satisfactory impacts when treated with any of the commercially available medications due to the complexity of these drugs [9].
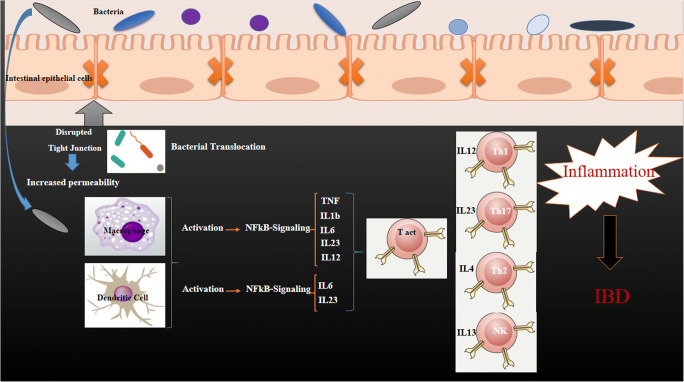
Numerous examinations have assessed the restorative capability of specific microbes, including commensal and probiotic strains, to enhance IBD in clinical preliminaries [11, 12] or in vitro models of colitis [28, 47, 48, 56, 57]. All in all, these examinations have confirmed that these bacteria affect the gut performance by the means of enhancing the gut permeability and histological modifications and decreasing inflammatory cytokine generation. EcN as one of the probiotics has been known for its positive effects in IBD treatment. The usage of this probiotic has been validated by the latest American recommendation in 2011; “A” recommendations to support remission in human UC [58]. [59] Altenhoefer examined the intervention of EcN with Salmonella invasion of human embryonic intestinal epithelial INT407 cells. A 70% reduction in invasion performance of salmonella was observed when EcN 1917 and Salmonella enterica serovar Typhimurium strain C17 were applied concurrently. In addition, the pathogenicity of Yersinia enterocolitica, Shigella flexneri, Legionella pneumophila, and even Listeria monocytogenes was blocked by EcN without influencing the viability of the pathogen. The effectiveness of the EcN in patients with IBD has been confirmed in many investigations. EcN therapies have been found as effective as gold standard therapies mesalazine for the maintenance of remission in UC. [18, 36, 60, 61]. On the other side, there is only one clinical research that manifested the beneficial effect of EcN in maintaining remission in people who suffer from colonic CD [62]. Implementing EcNdecreased the risk of relapse and also lowered the need of glucocorticoids [63]. In the gut, the probiotics can balance the cytokine generation by the immune system [64, 65] and recover the impaired epithelium by the means of TJ and zonula occludens proteins modulation [50, 51]. Although interrelationship between the EcN bacteria and the immune or epithelial cells may play the main role, other mechanisms including the development and release of secreted bacterial factors should not be underestimated. It is assumed that OMVs have a key job in bacteria-host interactions comparing with other discharged elements, which permits the transfer of effector molecules upon communication and incorporation into the host cells (Fig. 3) [2].
Fig. 3.
Effect of intestinal microbes in inflammatory bowel diseases by disruption of tight junctions and induction of pro-inflammatory. After damaging tight-junction proteins between epithelial cells, bacteria can enter to the down layer of epithelial cells and encounter different immune cells in the first line of immune system defense. The immune cells have responses to it. Several inflammatory responses such as NF-kB signaling can be activated, leading to the production of many different inflammatory cytokines such as Tumor Necrosis Factor (TNF), interleukin 1 beta (IL-1b), interleukin 8 (IL8) and something else. As a result, it can lead to different inflammatory diseases such as IBD
The miRNA-bacteria gene interaction
MicroRNAs (miRNAs) as specific mediators, contribute to the development of the inflammatory response. Some miRNAs play a role in key cell activities in the immune system, comprising differentiation, expansion, signal transduction, and apoptosis [66]. Several miRNAs, such as miR-21, miR-122a, miR-155, and miR-150 were found to be engaged in the impairment of TJ proteins and increase of intestinal epithelial permeability [67, 68].
Another type of miRNA is extracellular RNAs (exRNAs) that are believed to be largely enclosed inside extracellular vesicles (EVs) or firmly bound to cell proteins and lipids [69].
The EV RNA content mainly includes small RNAs (sRNAs), and miRNA-sized sRNAs (msRNAs) [70, 71]. These structures have been a focus of attention due to their gene-regulatory functions [72], for instance, msRNAs contained in EVs have been as of late recommended as biomarkers for malignancy and different maladies [73, 74]. Free exRNAs that are not inside EVs have likewise been appeared to connect with EV films [75, 76], proposing the fusion of microbial exRNAs into host EVs, or inversely [77].
The existence and characterization of sRNAs in OMVs generated by intracellular bacteria have been depicted in the latest examinations. [71, 78, 79]However, the function of sRNAs in host cell biology has not thus far been reported [78–81]. 22 nt sRNA in Mycobacterium marinum was found by Furuse et al., yet intracellular content of the sRNA was too low to even repress the target mRNA in cultivated cells. It is estimated that other clinically pertinent gram-negative bacteria like Salmonella, E. coli, Yersinia pestis, Klebsiella, Shigella, Moraxella, Helicobacter, Acinetobacter, Campylobacter, Legionella, Neisseria,and Hemophilus may likewise utilize OMV-sRNA based systems, furthering their potential benefit throughout infection [82].
Fecal miRNAs have not been described in normal human and animal feces. Studies found that miRNAs are a typical segment of excrement both in mice and people and certain gut epithelial cells [83]. Fecal miRNAs were found to be available in OMV as reported by Liu et al. They noticed that miRNAs could penetrate bacteria and co-localize with bacterial nucleic acids, providing a spatial reason for miRNA-bacteria gene communication. They have also found that distinctive miRNAs had various abilities to access bacteria, manifesting their various regulatory functions. Nevertheless, the process that regulates the penetration of miRNAs into bacteria, along with the mechanisms that process miRNAs after they get into bacteria, require further analysis [84]. The RNA substance of OMVs emitted by E. coli, Porphyromonas gingivalis and Vibrio cholera has been lately reported [71, 85, 86], however, no biological effects on host cells were reported .
The transfer of sRNAs to host cells by the means of extracellular bacteria OMV has been exhibited by Koeppen et al for the first time. The relative abundance of sRNAs in subsequent transfer host cells does not exactly reflect their relative abundance in OMVs. Such inconsistency can be explained by discrepancies in the efficacy or stability of RNA transfer after transmission to the host. OMVs produced by E. coli was enhanced in short RNAs as reported by Ghosal et al. (15–40 nt), and there was a distinction in the profiles of intracellular, OMV-related and without OMV extracellular RNA. The OMV-interceded transfer of sRNAs to host cells may be basic to all gram-negative microscopic organisms [71]. Looking beyond the activity of the host-pathogen, OMV sRNAs can also be a process by which gram-negative bacteria contend with other microbes in the same ecological niche. In order to fully understand the mechanisms of action of microbe-microbe, and to possibly recognize new medications for control of bacterial contaminations, more research is needed. Once these mechanisms have been better comprehended, the targeted design of sRNA antagonists or blockage of OMV development or fusion with host cells may open new avenues of therapies and infection prevention in the face of rising antibiotic resistance [82].
Conclusion
In 1917, Escherichia coli Nissle was isolated from the feces of a German soldier who claimed to be covered from infectious diarrheal sicknesses. From that point forward, EcN has been widely studied, and a specific trend of fitness factors as the undeniable absence of harmfulness factors have risen. This, combined with noteworthy probiotic properties extending from opposing impacts toward different individuals from the intestinal microbiota to immunomodulation and support of the intestinal barrier work, make this strain an alluring possibility for probiotic treatment in IBDs. Two main elements in inter-species interactions are microbiota-derived vesicles and discharged components. Today, the function of gut microbiota extracellular vesicles in health and disease has become a focus of attention, as they serve as vehicles for the transmission of microorganisms to the distal tissues of many bacterial effectors. Consequently, their responsibilities are not only limited to neighborhood intestinal conditions.
Acknowledgments
We would like to thank all the personnel of Mycobacteriology and Pulmonary Research Department, Pasteur Institute of Iran, for their assistance in this project.
Authors’ contributions
AB, HM and ZHT wrote the main manuscript text. AB design of Fig. SF edit of manuscript. SD and AM edit of final manuscript. All authors read and approve final manuscript.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict interests.
Data availability
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ava Behrouzi and Hoora Mazaheri contributed equally to this work.
References
- 1.Scaldaferri F, et al. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. doi: 10.1155/2013/435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabrega MJ, et al. Intestinal anti-inflammatory effects of outer membrane vesicles from Escherichia coli Nissle 1917 in DSS-experimental colitis in mice. Front Microbiol. 2017;8:1274. doi: 10.3389/fmicb.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabig A, Paclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, Raupach B, Sonnenborn U, Eckert J, Schumann RR, Wiedenmann B, Dignass AU, Sturm A. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun. 2006;74(7):4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putignani L, et al. Gut microbiota Dysbiosis as risk and premorbid factors of IBD and IBS along the childhood-adulthood transition. Inflamm Bowel Dis. 2016;22(2):487–504. doi: 10.1097/MIB.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 5.Frank DN, et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dicksved J, Halfvarson J, Rosenquist M, Järnerot G, Tysk C, Apajalahti J, Engstrand L, Jansson JK. Molecular analysis of the gut microbiota of identical twins with Crohn's disease. Isme j. 2008;2(7):716–727. doi: 10.1038/ismej.2008.37. [DOI] [PubMed] [Google Scholar]
- 9.Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20(7):1082–96. [DOI] [PubMed]
- 10.Qiao YQ, Cai CW, Ran ZH. Therapeutic modulation of gut microbiota in inflammatory bowel disease: more questions to be answered. J Dig Dis. 2016;17(12):800–810. doi: 10.1111/1751-2980.12422. [DOI] [PubMed] [Google Scholar]
- 11.Fedorak RN. Probiotics in the management of ulcerative colitis. Gastroenterology & hepatology. 2010;6(11):688–690. [PMC free article] [PubMed] [Google Scholar]
- 12.Wasilewski A, Zielińska M, Storr M, Fichna J. Beneficial effects of probiotics, prebiotics, Synbiotics, and Psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(7):1674–1682. doi: 10.1097/MIB.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 13.Johnson-Henry KC, et al. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun. 2008;76(4):1340–1348. doi: 10.1128/IAI.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu ZH, Shen TY, Zhang P, Ma YL, Moyer MP, Qin HL. Protective effects of lactobacillus plantarum against epithelial barrier dysfunction of human colon cell line NCM460. World J Gastroenterol. 2010;16(45):5759–5765. doi: 10.3748/wjg.v16.i45.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin H, et al. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;9:63. doi: 10.1186/1471-2180-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chibbar R, Dieleman LA. Probiotics in the Management of Ulcerative Colitis. J Clin Gastroenterol. 2015;49(Suppl 1):S50–S55. doi: 10.1097/MCG.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 17.Kruis W, et al. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Color Dis. 2012;27(4):467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henker J, et al. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur J Pediatr. 2007;166(4):311–318. doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henker J, Laass MW, Blokhin BM, Maydannik VG, Bolbot YK, Elze M, Wolff C, Schreiner A, Schulze J. Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. Pediatr Infect Dis J. 2008;27(6):494–499. doi: 10.1097/INF.0b013e318169034c. [DOI] [PubMed] [Google Scholar]
- 21.Fric P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol. 2003;15(3):313–315. doi: 10.1097/01.meg.0000049998.68425.e2. [DOI] [PubMed] [Google Scholar]
- 22.Schultz M, Clinical use of E. coli Nissle In inflammatory bowel disease. Inflamm Bowel Dis, 2008. 1917;14(7):1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 23.Hevia A, et al. Molecular players involved in the interaction between beneficial Bacteria and the immune system. Front Microbiol. 2015;6:1285. doi: 10.3389/fmicb.2015.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez B, Urdaci MC, Margolles A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology. 2010;156(Pt 11):3232–3242. doi: 10.1099/mic.0.044057-0. [DOI] [PubMed] [Google Scholar]
- 25.Hickey CA, et al. Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a Sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe. 2015;17(5):672–680. doi: 10.1016/j.chom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho AL, Fonseca S, Miquel-Clopés A, Cross K, Kok KS, Wegmann U, Gil-Cordoso K, Bentley EG, al Katy SHM, Coombes JL, Kipar A, Stentz R, Stewart JP, Carding SR. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J Extracell Vesicles. 2019;8(1):1632100. doi: 10.1080/20013078.2019.1632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12(4):509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen AM, et al. Treatment of inflammatory bowel disease associated E. coli with ciprofloxacin and E. coli Nissle in the streptomycin-treated mouse intestine. PLoS One. 2011;6(8):e22823. doi: 10.1371/journal.pone.0022823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scaldaferri F, et al. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: an update. World J Gastroenterol. 2016;22(24):5505–5511. doi: 10.3748/wjg.v22.i24.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen N, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2019;5:Cd001176. doi: 10.1002/14651858.CD001176.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafez M, Hayes K, Goldrick M, Grencis RK, Roberts IS. The K5 capsule of Escherichia coli strain Nissle 1917 is important in stimulating expression of toll-like receptor 5, CD14, MyD88, and TRIF together with the induction of interleukin-8 expression via the mitogen-activated protein kinase pathway in epithelial cells. Infect Immun. 2010;78(5):2153–2162. doi: 10.1128/IAI.01406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wehkamp J, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72(10):5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlee M, et al. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75(5):2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehkamp J, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9(4):215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Rembacken BJ, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354(9179):635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 37.Ashrafian F, et al. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol Hepatol Bed Bench. 2019;12(2):163–168. [PMC free article] [PubMed] [Google Scholar]
- 38.Ashrafian F, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behrouzi A, Vaziri F, Riazi Rad F, Amanzadeh A, Fateh A, Moshiri A, et al. Comparative study of pathogenic and non-pathogenic Escherichia coli outer membrane vesicles and prediction of host-interactions with TLR signaling pathways. BMC research notes. 2018;11(1):539–9. [DOI] [PMC free article] [PubMed]
- 40.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patten DA, et al. Commensal-derived OMVs elicit a mild proinflammatory response in intestinal epithelial cells. Microbiology. 2017;163(5):702–711. doi: 10.1099/mic.0.000468. [DOI] [PubMed] [Google Scholar]
- 42.Behrouzi A, Nafari AH, Siadat SD. The significance of microbiome in personalized medicine. Clinical and translational medicine. 2019;8(1):16–6. [DOI] [PMC free article] [PubMed]
- 43.Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy K, Verdu EF. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1−/−; Nod2−/− mice. Inflamm Bowel Dis. 2012;18(8):1434–1446. doi: 10.1002/ibd.22848. [DOI] [PubMed] [Google Scholar]
- 44.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177(14):3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieves W, et al. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol. 2014;21(5):747–754. doi: 10.1128/CVI.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nokleby H, et al. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine. 2007;25(16):3080–3084. doi: 10.1016/j.vaccine.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, Rodríguez-Cabezas ME, Gálvez J. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem Pharmacol. 2011;82(12):1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Olier M, Marcq I, Salvador-Cartier C, Secher T, Dobrindt U, Boury M, Bacquié V, Pénary M, Gaultier E, Nougayrède JP, Fioramonti J, Oswald E. Genotoxicity of Escherichia coli Nissle 1917 strain cannot be dissociated from its probiotic activity. Gut Microbes. 2012;3(6):501–509. doi: 10.4161/gmic.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2(12):e1308–8. [DOI] [PMC free article] [PubMed]
- 51.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9(3):804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 52.Hering NA, Richter JF, Fromm A, Wieser A, Hartmann S, Günzel D, Bücker R, Fromm M, Schulzke JD, Troeger H. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCzeta and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014;7(2):369–378. doi: 10.1038/mi.2013.55. [DOI] [PubMed] [Google Scholar]
- 53.Canas MA, et al. Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via Clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS One. 2016;11(8):e0160374. doi: 10.1371/journal.pone.0160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabrega MJ, et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 2016;7:705. doi: 10.3389/fmicb.2016.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247–7. [DOI] [PMC free article] [PubMed]
- 56.Ewaschuk JB, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 57.Martin R, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Floch MH, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011;45(Suppl):S168–S171. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]
- 59.Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, Hacker J, Oelschlaeger TA. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol. 2004;40(3):223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 60.Kruis W, et al. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11(5):853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 61.Matthes H, et al. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN) BMC Complement Altern Med. 2010;10:13. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malchow HA. Crohn's disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn's disease? J Clin Gastroenterol. 1997;25(4):653–658. doi: 10.1097/00004836-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Dembiński A, et al. Synergic interaction of Rifaximin and Mutaflor (Escherichia coli Nissle 1917) in the treatment of acetic acid-induced colitis in rats. Gastroenterol Res Pract. 2016;2016:3126280–0. [DOI] [PMC free article] [PubMed]
- 64.Guzy C, Paclik D, Schirbel A, Sonnenborn U, Wiedenmann B, Sturm A. The probiotic Escherichia coli strain Nissle 1917 induces gammadelta T cell apoptosis via caspase- and FasL-dependent pathways. Int Immunol. 2008;20(7):829–840. doi: 10.1093/intimm/dxn041. [DOI] [PubMed] [Google Scholar]
- 65.Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazalé T, Raupach B, Eckert J, Schumann RR, Enders C, Sonnenborn U, Wiedenmann B, Dignass AU. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infect Immun. 2005;73(3):1452–1465. doi: 10.1128/IAI.73.3.1452-1465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu XM, Zhang HJ. miRNAs as new molecular insights into inflammatory bowel disease: crucial regulators in autoimmunity and inflammation. World J Gastroenterol. 2016;22(7):2206–2218. doi: 10.3748/wjg.v22.i7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bian Z, et al. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol. 2011;225(4):544–553. doi: 10.1002/path.2907. [DOI] [PubMed] [Google Scholar]
- 68.Ye D, et al. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141(4):1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patton JG, et al. Biogenesis, delivery, and function of extracellular RNA. J Extracell Vesicles. 2015;4:27494. doi: 10.3402/jev.v4.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, Ricordi C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration type 1 diabetes. Sci Rep. 2017;7(1):5998. doi: 10.1038/s41598-017-05787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosal A, Upadhyaya BB, Fritz JV, Heintz-Buschart A, Desai MS, Yusuf D, Huang D, Baumuratov A, Wang K, Galas D, Wilmes P. The extracellular RNA complement of Escherichia coli. Microbiologyopen. 2015;4(2):252–266. doi: 10.1002/mbo3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi JW, et al. Tiny RNAs and their voyage via extracellular vesicles: secretion of bacterial small RNA and eukaryotic microRNA. Exp Biol Med (Maywood) 2017;242(15):1475–1481. doi: 10.1177/1535370217723166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michael A, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16(1):34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie Z, et al. Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS One. 2013;8(4):e57502. doi: 10.1371/journal.pone.0057502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bryniarski K, Ptak W, Martin E, Nazimek K, Szczepanik M, Sanak M, Askenase PW. Free extracellular miRNA functionally targets cells by transfecting Exosomes from their companion cells. PLoS One. 2015;10(4):e0122991. doi: 10.1371/journal.pone.0122991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stremersch S, Brans T, Braeckmans K, de Smedt S, Raemdonck K. Nucleic acid loading and fluorescent labeling of isolated extracellular vesicles requires adequate purification. Int J Pharm. 2018;548(2):783–792. doi: 10.1016/j.ijpharm.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 77.Lee H-J. Microbe-host communication by small RNAs in extracellular vesicles: vehicles for Transkingdom RNA transportation. Int J Mol Sci. 2019;20(6):1487. doi: 10.3390/ijms20061487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furuse Y, et al. Search for microRNAs expressed by intracellular bacterial pathogens in infected mammalian cells. PLoS One. 2014;9(9):e106434. doi: 10.1371/journal.pone.0106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortega AD, et al. Non-coding RNA regulation in pathogenic bacteria located inside eukaryotic cells. Front Cell Infect Microbiol. 2014;4:162. doi: 10.3389/fcimb.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sesto N, Koutero M, Cossart P. Bacterial and cellular RNAs at work during Listeria infection. Future Microbiol. 2014;9(9):1025–1037. doi: 10.2217/fmb.14.79. [DOI] [PubMed] [Google Scholar]
- 81.Singh PP, Li L, Schorey JS. Exosomal RNA from Mycobacterium tuberculosis-infected cells is functional in recipient macrophages. Traffic. 2015;16(6):555–571. doi: 10.1111/tra.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koeppen K, et al. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 2016;12(6):e1005672. doi: 10.1371/journal.ppat.1005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jung M, Schaefer A, Steiner I, Kempkensteffen C, Stephan C, Erbersdobler A, Jung K. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem. 2010;56(6):998–1006. doi: 10.1373/clinchem.2009.141580. [DOI] [PubMed] [Google Scholar]
- 84.Liu S, et al. The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe. 2016;19(1):32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ho MH, et al. Functional advantages of Porphyromonas gingivalis vesicles. PLoS One. 2015;10(4):e0123448. doi: 10.1371/journal.pone.0123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sjostrom AE, et al. Membrane vesicle-mediated release of bacterial RNA. Sci Rep. 2015;5:15329. doi: 10.1038/srep15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.