Abstract
Aim
Mediterranean diet (MD) is a healthful dietary pattern with benefits for prevention of metabolic diseases including non-alcoholic fatty liver disease (NAFLD). In the current meta-analysis, we assessed the association between MD and liver steatosis and cardiometabolic risk factors in patients with NAFLD.
Methods
PubMed, Scopus, and Embase were searched to find observational and clinical studies on the issue. No restriction on date and language was made. Outcomes included body mass index (BMI), waist circumference, blood pressure, triglycerides (TG), cholesterol fractions, glucose, insulin, insulin resistance, and liver transaminases.
Results
Seven observational reports and 6 trials met our inclusion criteria and entered in the meta-analysis. In observational studies, there was an inverse association between MD and NAFLD (effect size (ES) = 0.95; 95% CI: 0.90, 1.00; P = 0.05). In trials, subgroup analysis based on the method of liver examination showed effectiveness of MD on steatosis examined by magnetic resonance spectroscopy (P < 0.002; n = 2) but not by ultrasound (P = 0.08; n = 2). MD also showed a significant decreasing effect on BMI (ES = −1.23 kg/m2; 95% CI: −2.38, −0.09), plasma triglycerides (ES = −33.01 mg/dL; 95% CI: −52.84, −13.18), and HOMA-IR (ES = -0.94; 95% CI: −1.29, −0.58) but no significant effect was observed in waist circumference, cholesterol fractions, glucose and insulin, and liver transferases.
Conclusion
Overall, available data from observational and clinical studies indicated a trend for the relationship between MD and hepatic steatosis. Improvement in the most important risk factors of NAFLD, i.e. BMI, serum triglycerides, and insulin resistance, may be involved in such relationship.
Electronic supplementary material
The online version of this article (10.1007/s40200-019-00475-2) contains supplementary material, which is available to authorized users.
Keywords: Mediterranean diet, Non-alcoholic fatty liver disease, Hepatic steatosis, Insulin resistance, Body mass index, Triglycerides, Cardiometabolic biomarkers
Introduction
Mediterranean diet (MD) is a diet traditionally used in Mediterranean region but it is now acknowledged as a healthful dietary pattern with benefits for prevention of chronic metabolic diseases [1]. MD is rich in vegetables, fruits, cereals, olive oil, nuts, legumes, and fish, contains moderate amounts of dairy, eggs, poultry, and red wine, and is limited in red meat, sweets, and sweetened beverages [1]. This multifarious diet composition along with reasonable restrictions on unhealthy items gives MD a peculiar potential for prevention of diseases. High amounts of fish, olive oil, nuts, and vegetables and low consumption of red meat increases unsaturated to saturated fat ratio and leads to the regulation of lipoprotein metabolism and prevention of cardiovascular events [2]. Whole grain cereals in the MD improve glycemic control following consumption of meals and thus reduces the risk of metabolic diseases such as obesity, type 2 diabetes, and cardiovascular diseases [3]. Moreover, antioxidants present in fruit and vegetables help in scavenging reactive oxygen species which are factors involved in exacerbating metabolic disorders [4].
Non-alcoholic fatty liver disease (NAFLD) is a prevalent hepatic disorder that can progress to non-alcoholic steatohepatitis (NASH), cell damage, and ultimately liver fibrosis and cirrhosis [5]. NAFLD is a metabolic disorder accompanied with obesity, insulin resistance, hypertension, and dyslipidemia, all of which are components of metabolic syndrome [5, 6]. Indeed, NAFLD is nominated as hepatic manifestation of metabolic syndrome. Obesity, particularly if accompanied by accumulation of fat in visceral areas, is the key component in the pathophysiology of NAFLD. Excessive visceral fat mass release plenty of free fatty acids into blood which are gathered by the liver, packed in the form of very low-density lipoprotein (VLDL), and released into circulation. High VLDL concentrations are associated with elevated plasma triglycerides and suppressed high-density lipoprotein levels, both of which are considered as important risk factors of metabolic syndrome and cardiovascular disease [5, 6].
A number of observational and interventional studies have documented the association between MD and incidence or severity of NAFLD [7] but no meta-analysis has yet been performed on these reports. A number of meta-analyses have evaluated the association between MD and metabolic diseases [8, 9]. Evidence derived from meta-analyses of cross-sectional, prospective cohorts, and randomized controlled trials (RCT) has shown that adherence to MD is associated with reduced risk of overall mortality, cardiovascular diseases, coronary heart disease, myocardial infarction, diabetes, neurodegenerative diseases, and overall cancer incidence [8]. In the current meta-analysis, we assessed the association between MD and liver steatosis in patients with NAFLD using both observational and clinical investigations. In RCTs, we also investigated the effect of MD on cardiometabolic risk factors of NAFLD patients.
Methods
Search
PubMed, Scopus, and Embase were searched from the earliest online available date through May 2019 to find observational studies investigating (1) the association between MD and hepatic steatosis in observational studies or (2) examining the effect of MD on cardiometabolic risk factors of NAFLD patients in RCT. Main search terms included Mediterranean diet, non-alcoholic fatty liver, liver steatosis, hepatic steatosis, steatohepatitis, and NAFLD. In PubMed search, MeSH terms were used where possible. There was no restriction on date and language. Review papers were also checked to find possible undetected references. The search and screening were performed by two independent investigators. The disagreements were resolved by discussion.
Eligibility criteria
Original articles pertaining MD and NAFLD were assessed for eligibility. For observational studies, any cross-sectional, case-control, or cohort study that assessed the relationship with adherence to MD and the prevalence or development of NAFLD was considered eligible. Studies were excluded if (1) components of MD were examined instead of MD as a whole, and (2) there was inadequate information for odds ratio (OR) and confidence interval (CI). For RCTs, trials that examined the effect of MD on NAFLD patients were considered unless in the case of the following: (1) inappropriate study design such as the lack of control group; (2) MD combined with other interventions, such as exercise or ketogenic MD; (3) testing components of MD instead of the whole diet; (4) administration of non-isocaloric diets to treatment and control groups; (5) no report or reporting insufficient information on parameters of interest, such as mean and standard deviation (SD) or the number of participants in each group; (6) repeated publications. In any type of study, NAFLD needed to be diagnosed by either imaging or histological methods. Also, cases of alcoholic fatty liver should have been ruled out.
Outcomes
For both observational and clinical studies, hepatic steatosis was our main goal. In RCTs we also collected data on the following outcomes: weight, body mass index (BMI), waist circumference, blood pressure, triglycerides, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, fasting blood glucose, fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT).
Quality assessment
The quality of cohort and case-control studies was assessed with Newcastle-Ottawa Scale (NOS) which assesses selection bias, comparability of the groups for important factors, and outcome [10]. For cross-sectional studies an adapted form of the Newcastle-Ottawa Scale (NOS) was used [11]. For RCTs, the quality of the trials was assessed according to the Cochrane’s instructions for risk of bias assessment [12]. The criteria used by this tool include: random sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias.
Statistical analysis
For RCTs, Mean and SD of the difference between pre- and post-intervention data for control and treatment groups were used to calculate pooled effects. The random-effects inverse-variance model was used to calculate weighted mean difference and 95% CI. For observational studies, OR and 95% CI were used. Where possible, the highest quantile was compared to the lowest quantile (set as reference). ORs and 95% CIs were log-transformed and then DerSimonian and Laird random-effect method was used to calculate the effect size. Between-study heterogeneity was evaluated using Cochrane χ2 test and I2. Publication bias was determined by visual evaluation of funnel plots and Egger’s test [13]. STATA software version 12.0 (StataCorp, USA) was used for data analysis. P < 0.05 was considered statistically significant.
Results
Search
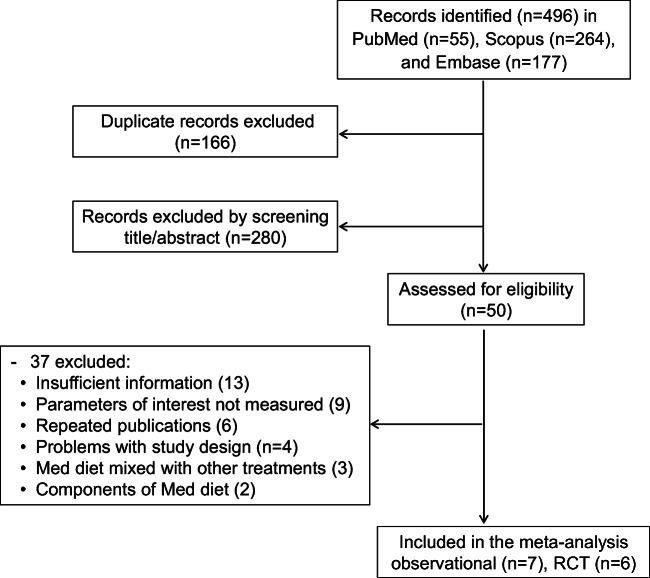
After searching the databases, 496 articles were found, screened through reading their titles and abstracts, and carefully assessed according to the inclusion/exclusion criteria. Finally, 7 observational studies (5 cross-sectional, 1 case-control, and 1 prospective cohort) and 6 trials passed the eligibility stage and entered in the meta-analysis. The flowchart of the study selection is presented in Fig. 1. The number of participants in observational studies was 16,823 (7732 males and 9091 females) and that in RCTs was 272 (179 males and 93 females). Summary characteristics of the selected articles are presented in Table 1.
Fig. 1.
Flowchart of the study selection
Table 1.
Characteristics of the studies included in the meta-analysis of Mediterranean diet for patients with NAFLD
| First author, year | Location | Design | N | Age | BMI | Duration | Test diet | Control diet | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Observational | |||||||||
| Aller, 2019 [14] | Spain | Cross-sectional | 115/881 | 47.4 ± 37.2 | 34.3 ± 5.8 | – | – | – | Low vs. high steatosis |
| Baratta, 2017 [15] | Italy | Cross-sectional | 361/223 | 56.2 ± 12.4 | 30.0 ± 5.1 | – | – | – | HS |
| Chan, 2015 [16] | China | Cross-sectional | 332/465 | 48.1 ± 10.6 | 23.6 ± 3.3 | – | – | – | HS |
| Khalatbari, 2019 [17] | England | Cross-sectional | 4398/5247 | 48.9 ± 7.4 | 26.5 ± 4.5 | – | – | – | HS |
| Khalatbari, 2019 [17] | Switzerland | Cross-sectional | 1709/2248 | 57.0 ± 10.4 | 25.7 ± 4.2 | – | – | – | HS |
| Kontogianni, 2014 [18] | Greece | Case-control | 72/44 | 44.6 ± 12.0 | 28.2 ± 3.8 | – | – | – | HS |
| Ma, 2018 [19] | USA | Prospective cohort | 745 /776 | 51 ± 10 | 27.6 ± 5.1 | 6 y | – | – | HS |
| RCT | |||||||||
| Abenavoli, 2017 [20] | Italy | Parallel | 20/102 | 43 ± 23.7 | 30 ± 4.44 | 6 mon | Hypocaloric Med diet | Hypocaloric diet | Weight, BMI, WC, BP, TC, TG, LDL, FBG, insulin, HOMA-IR, ALT, AST, GGT, HS |
| Biolato, 2019 [21] | Italy | One-arm crossover | 18/12 | 42.7 | 30.9 ± 3.6 | 16 wk | Hypocaloric Med diet | Hypocaloric low-fat diet | Weight, BMI, WC, BP, TC, TG, LDL, HDL, FBG, HOMA-IR, ALT, AST, GGT |
| Katsagoni, 2018 [22] | Greece | Parallel | 21/21 | 45.5 ± 14.7 | 29.8 ± 1.9 | 6 mon | Med diet | Healthy diet | Weight, BMI, TC, TG, LDL, HDL, FBG, insulin, HOMA-IR, ALT, GGT |
| Misciagna, 2017 [23] | Italy | Parallel | 50/48 | – | – | 6 mon | Low-glycemic index Med diet | Control diet | HS |
| Properzi, 2018 [24] | Australia | Parallel | 24/24 | 52 ± 11.3 | 30.9 ± 4.9 | 12 wk | Med diet | Low-fat diet | Weight, BMI, WC, BP, TC, TG, LDL, HDL, FBG, insulin, HOMA-IR, ALT,GGT, HS |
| Ryan, 2017 [25] | Australia | Crossover | 12/12 | 55 ± 14 | 32.0 ± 4.2 | 6 wk | Med diet | Low-fat high-carbohydrate diet | Weight, BMI, WC, BP, TG, HDL, FBG, insulin, HOMA-IR, ALT, GGT, HS |
1Numbers are related to the males and females, respectively. 2Numbers are related to Mediterranean diet and control groups, respectively
Abbreviations: ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BP blood pressure, FBG fasting blood glucose, GGT gamma-glutamyl transferase, HDL high-density lipoprotein cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, LDL low-density lipoprotein cholesterol, TC total cholesterol, TG triglycerides, WC waist circumference
Observational studies
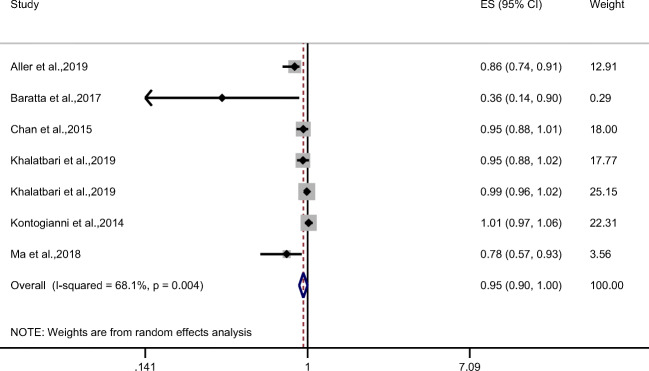
In observational studies, there was an approximately significant inverse association between MD and NAFLD (combined effect size (ES) = 0.95; 95% CI: 0.90, 1.00; P = 0.05) with a high heterogeneity between results (I2 = 68.1%, P = 0.004) (Fig. 2).
Fig. 2.
Forest plot of observational studies evaluating the association of Mediterranean diet and NAFLD
Clinical trials
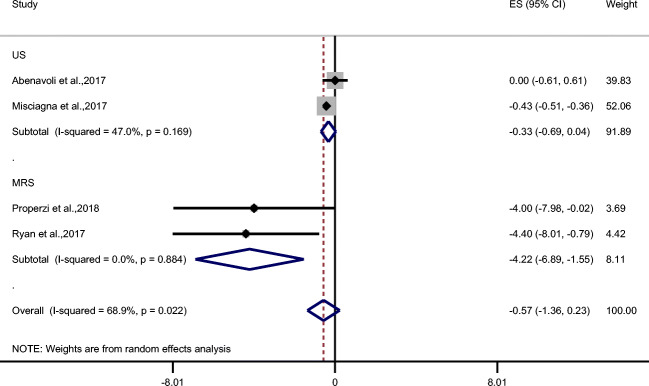
Only 4 trials tested the effect of MD on liver steatosis (Fig. 3), but the method of the evaluation was not identical; Abenavoli et al. [20] and Misciagna et al. [23] used liver ultrasound while Properzi et al. [24] and Ryan et al. [25] used magnetic resonance spectroscopy (MRS). Combined effects of MD on all 4 trials did not show a significant effect (ES = −0.57; 95% CI: −1.36, 0.23; P = 0.16) but in subgroup analysis, studies that examined liver steatosis by MRS (n = 2) revealed the effectiveness of MD on liver steatosis (ES = −4.22; 95% CI: −6.89, −1.55; P < 0.002) but trials that used ultrasound (N = 2) showed a trend towards significance (ES = −0.33; 95% CI: −0.69, 0.04; P = 0.08).
Fig. 3.
Forest plot of trials examining the effect of Mediterranean diet on liver steatosis in NAFLD patients with subgroup analysis based on the method of hepatosteatosis evaluation
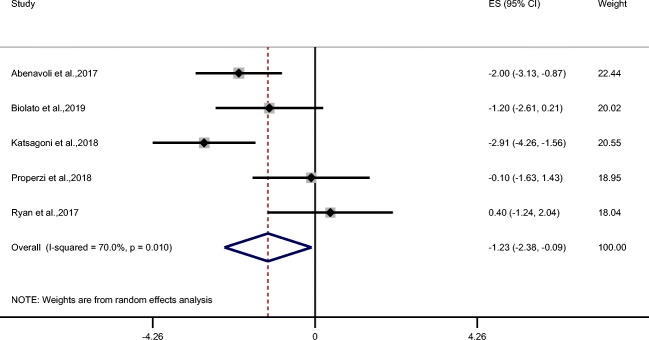
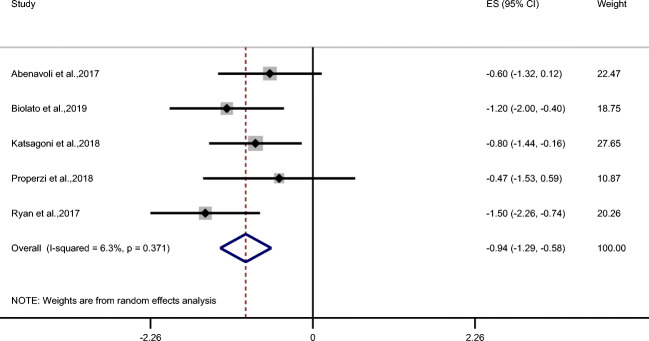
MD showed a significant decreasing effect on BMI (ES = −1.23 kg/m2; 95% CI: −2.38, −0.09 kg/m2; P = 0.034; n = 5) (Fig. 4) and weight (ES = −4.13 kg; 95% CI: −8.06, −0.20 kg; P = 0.039; n = 5) (Table 2) but not on waist circumference (Table 2). MD also demonstrated improvement in triglycerides (ES = −33.01 mg/dL; 95% CI: −52.84, −13.18 mg/dL; P = 0.001; n = 5) and total cholesterol (ES = −6.89 mg/dL; 95% CI: −14.90, 1.12 mg/dL; P = 0.09; n = 4) (Fig. 5a, b) but no effect was observed on LDL and HDL cholesterol, systolic and diastolic blood pressure, fasting blood glucose, and insulin (Table 2). Despite that MD did not change insulin but it revealed a significant effect on HOMA-IR (ES = −0.94; 95% CI: −1.29, −0.58; P < 0.001; n = 5) (Fig. 6).
Fig. 4.
Forest plot of trials examining the effect of Mediterranean diet on BMI of NAFLD patients
Table 2.
Effects of Mediterranean diet on some of cardiometabolic biomarkers of NAFLD patients
| Outcomes | Studies (n) | ES (95% CI)1 | P value | Heterogeneity | P for heterogeneity |
|---|---|---|---|---|---|
| Weight (kg) | 5 | −4.13 (−8.06, −0.20) | 0.04 | 75.5% | 0.003 |
| Waist circumference (cm) | 4 | −1.85 (−5.01, 1.32) | 0.25 | 63.7% | 0.04 |
| Fasting blood glucose (mg/dL) | 5 | −0.94 (−4.24, 2.37) | 0.58 | 69.9% | 0.01 |
| Fasting insulin (mU/L) | 4 | −1.06 (−6.33, 3.13) | 0.51 | 91.6% | < 0.001 |
| LDL cholesterol (mg/dL) | 4 | −0.09 (−18.03, 17.85) | 0.99 | 88.4% | < 0.001 |
| HDL cholesterol (mg/dL) | 4 | 0.25 (−1.57, 2.06) | 0.79 | 10.3% | 0.34 |
| Systolic blood pressure (mmHg) | 4 | 0.32 (−5.30, 5.93) | 0.91 | 71.6% | 0.01 |
| Diastolic blood pressure (mmHg) | 4 | −1.23 (−6.34, 2.07) | 0.32 | 75.4% | 0.007 |
| Alanine aminotransferase (U/L) | 5 | −8.87 (−19.45, 1.71) | 0.1 | 79.4% | 0.001 |
| Aspartate aminotransferase (U/L) | 2 | −5.96 (−11.74, −0.18) | 0.04 | 55.8% | 0.13 |
| Gamma-glutamyl transferase (U/L) | 5 | −4.73 (−13.78, 4.32) | 0.31 | 36.0% | 0.18 |
1Effect size and 95% confidence interval
Fig. 5.
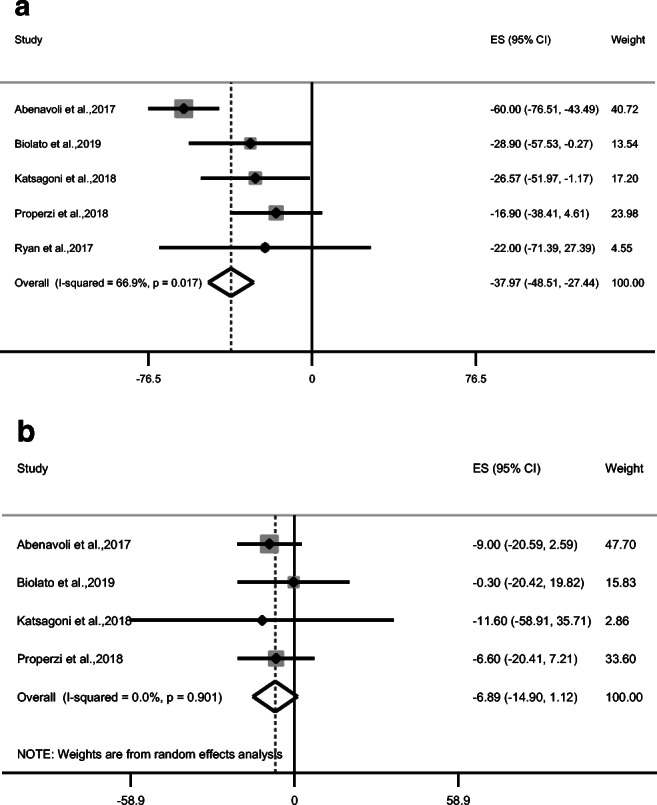
Forest plot of trials examining the effect of Mediterranean diet on a triglycerides and b total cholesterol of NAFLD patients
Fig. 6.
Forest plot of trials examining the effect of Mediterranean diet on HOMA-IR of NAFLD patients
MD showed reductions in liver transferases; ALT (ES = −8.87 U/L; 95% CI: −19.45, 1.71 U/L; P = 0.10; n = 5), AST (ES = −5.96 U/L; 95% CI: −11.74, −0.18 U/L; P = 0.04; n = 2), and GGT (ES = −4.73 U/L; 95% CI: −13.78, 4.32 U/L; P = 0.31; n = 5), although the reductions were not significant unless for AST, for which due to insufficient number of trials (n = 2) the result is inconclusive (Table 2).
With the exception of total cholesterol, HDL cholesterol, HOMA-IR, AST and GGT, there was high heterogeneity between data ranging from 63.7% to 91.6%. Due to low number of trials, performing meta-regression and subgroup analysis was not possible. The heterogeneity was also observed between results of observational investigations (79%).
Quality assessment
Risk of bias for observational studies (Supplementary Table 1) and RCTs (Supplementary Table 2) was assessed according to the criteria described in the Methods. Case-control and cohort studies had high quality score but cross-sectional studies lacked report of non-response rate but they all had used good statistical tests and appropriate confounders control. RCTs had mostly high risk of bias due to lack of blinding patients and/or personnel.
Publication bias
There was a significant publication bias inspected by Egger’s test in the observational studies (P = 0.002) but no publication bias was detected in the investigated outcomes of RCTs.
Discussion
Evidence from observational and clinical investigations puts forward an inverse association between MD and NAFLD. Among the assessed biomarkers, BMI, triglycerides, and HOMA-IR showed significant reduction following consumption of MD, total cholesterol (P = 0.09) and ALT (P = 0.10) indicated trends for reduction, but no specific effect was observed for LDL and HDL cholesterol and systolic blood pressure (P = 0.8–0.99).
Although the evaluated outcomes were all related to NAFLD, the improvement of NAFLD following MD was not concordant with betterment of all parameters. BMI, triglycerides, and HOMA-IR were the only parameters with significant reduction following MD, suggesting that the possible beneficial effect of MD on NAFLD may be exerted through improvement of these parameters. Previous studies have shown that obesity, hypertriglyceridemia, and insulin resistance are the most important and crucial risk factors in the pathogenesis of NAFLD, with obesity being the most underlying element [5, 6]. Release of fatty acids from superfluous fat masses particularly in the visceral areas induces systemic and hepatic insulin resistance which successively intensifies liberation of free fatty acids from adipose tissues. Excessive amounts of circulating free fatty acids ultimately leads to hypertriglyceridemia and consequently NAFLD [5, 6].
MD did not have a significant effect on cholesterol and blood pressure, likely because compared to weight, plasma triglycerides, and insulin resistance, these parameters have less direct relationship to NAFLD [5, 6]. Similarly, fasting blood glucose and serum insulin concentrations were not affected by MD because the net amount of glucose and insulin in the blood is less likely to directly relate to NAFLD. Contrariwise, insulin resistance which is one of the most important contributors of NAFLD significantly decreased. The influence of MD on liver transaminases largely depends on the degree by which these enzymes are affected by NAFLD. Since the extent of change in these enzymes is not accordant to the development or degree of NAFLD, their alteration following consumption of MD is not expected. Studies have shown that hepatic steatosis may not be associated with elevation of liver transaminases [26]. ALT is generally used as a non-invasive tool for detection of hepatosteatosis [27, 28]. However, approximately 80% of NAFLD patients have normal ALT [29]. It has been suggested that in individuals with normal ALT, evaluation of glucose metabolism and insulin resistance should be used as a complementary tool for detection of NAFLD [30].
Having a look at the mechanisms involved in the development of NAFLD, the above-mentioned justifications become more clear. There are numerous mechanisms through which MD might prevent hepatic steatosis. Some of these mechanisms directly affect hepatic fat oxidation and/or lipogenesis, some rectify metabolic causes or consequences of NAFLD, and some prevent progression of NAFLD to its more severe form, nonalcoholic steatohepatitis (NASH). Here, we state some of the most important and relevant mechanisms.
A part of the beneficial effects of MD is due to its fatty acid composition; low saturated (SFA) and high poly- (PUFA) and mono- (MUFA) unsaturated fatty acids. In MD, consumption of nuts and fish provides high quantities of PUFA whereas olive oil affords sufficient amount of MUFA. SFA intensify hepatic steatosis but PUFA and MUFA are protective against liver fat accumulation [31, 32]. Long-chain n-3 fatty acids are the most beneficial PUFA for NAFLD. They prevent liver adipogenesis by modifying transcription of peroxisome proliferator-activated receptor α (PPARα) and sterol regulatory element-binding protein (SREBP)-1 genes, which regulate hepatic fatty acid oxidation and synthesis, respectively [33, 34]. Long-chain n-3 fatty acids also suppress direct uptake of fatty acids from blood into hepatocytes [35].
Vegetables, fruits, nuts, legumes, and cereals provide great quantities of fiber for MD. Bacterial fermentation of fiber may contribute to the beneficial metabolic effects of MD. Fermentation metabolites, such as butyrate and propionate, have shown anti-obesity, insulin-sensitizing, and anti-inflammatory potential although the direct effect of fiber on hepatic steatosis has not been indicated in clinical trials [36]. Whole grains have also phytochemicals such as betaine, ferulic acid, and alkylresorcinols that may prevent fat accumulation in the liver [37].
MD has also potential to block or slow down the progression of NAFLD to more severe stages of fatty liver such as NASH and liver fibrosis. Fruit and vegetables, olive oil, nuts, whole grains, and red wine in MD contain antioxidant vitamins such as vitamins C and E, and antioxidant phytochemicals such as polyphenols and flavonoids. These antioxidants can attenuate oxidative stress induced by accumulated fat in the liver [38]. Moreover, such ingredients exhibit additional activities independent of their antioxidant effects. For instance, flavonoids are suggested to decrease body weight as well as fat deposition in both visceral tissues and in the liver, in the later at least partly by increasing fatty acid β-oxidation and suppressing lipogenesis (through down-regulation of SREBP-1) [6].
Thanks to its high nuts and vegetable content, MD contains plenty of n-6 fatty acids; but it also has fish with abundant n-3 fatty acids, yielding a proper balance between n-6 and n-3 fatty acids. Considering distinct and sometimes contrary effects of these fats on systemic inflammation and adipogenesis, this balance is crucial for health and prevention of obesity [39]. For instance, sufficient quantity of n-3 fatty acids in membranes is needed for appropriate membrane functionality. As an example, long-chain n-3 fatty acids improve insulin sensitivity by maintaining membrane fluidity and regulating expression and activity of insulin receptors [40].
Limitations
This was the first meta-analysis conducted to demonstrate the association between MD and NAFLD. Although the results were largely promising, more studies in both observational and interventional types are needed to make firmer conclusions. There were limitations in either form of studies. For instance, various factors were used as confounders in the analysis of epidemiological investigations; this could have affected comparability of the results. Also, studies used different methods for detection or estimation of NAFLD severity or recruited patients with various levels of liver steatosis, and these differences may have also compromised homogeneity of the findings. The heterogeneity was also observed in dietary intervention: there was difference between RCTs in definition of MD, diet strategy for MD (e.g., MD with or without calorie restriction), diet strategy in the control group (e.g., low fat or regular diet), and intervention periods. These heterogeneities could be amended by subgroup analysis if there were sufficient number of RCTs, but limited number of trials did not allow to perform such analysis. More investigations need to be conducted in this area.
Conclusion
Overall, available data from observational and clinical studies indicated trends for the relationship between MD and hepatic steatosis. RCTs showed significant improvement in BMI, serum triglycerides, and insulin resistance following consumption of MD but less remarkable or no alteration was observed in other outcomes. This suggests that improvement in the most important risk factors of NAFLD, i.e. BMI, serum triglycerides, and insulin resistance, may be involved in such relationship.
Electronic supplementary material
(DOCX 14 kb)
Compliance with ethical standards
Conflict of interest
Authors have no conflict of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients. 2015;7(11):9139–9153. doi: 10.3390/nu7115459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godos J, Federico A, Dallio M, Scazzina F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int J Food Sci Nutr. 2017;68(1):18–27. doi: 10.1080/09637486.2016.1214239. [DOI] [PubMed] [Google Scholar]
- 3.Jones JL, Fernandez ML, McIntosh MS, Najm W, Calle MC, Kalynych C, Vukich C, Barona J, Ackermann D, Kim JE, Kumar V, Lott M, Volek JS, Lerman RH. A Mediterranean-style low-glycemic-load diet improves variables of metabolic syndrome in women, and addition of a phytochemical-rich medical food enhances benefits on lipoprotein metabolism. J Clin Lipidol. 2011;5(3):188–196. doi: 10.1016/j.jacl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Billingsley HE, Carbone S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: an in-depth review of the PREDIMED. Nutr Diabetes. 2018;8(1):13. doi: 10.1038/s41387-018-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018;22(1):23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Akhlaghi M. Non-alcoholic fatty liver disease: beneficial effects of flavonoids. Phytother Res. 2016;30(10):1559–1571. doi: 10.1002/ptr.5667. [DOI] [PubMed] [Google Scholar]
- 7.Suárez M, Boqué N, Del Bas JM, Mayneris-Perxachs J, Arola L, Caimari A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients. 2017;9(10):pii: E1052. doi: 10.3390/nu9101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72(1):30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 9.Galbete C, Schwingshackl L, Schwedhelm C, Boeing H, Schulze MB. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol. 2018;33(10):909–931. doi: 10.1007/s10654-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 11.Newcastle-Ottawa Scale adapted for cross-sectional studies. Available at: https://journals.plos.org/plosone/article/file?type=supplementary&id=info:doi/10.1371/journal.pone.0147601.s001
- 12.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chapter 16.4.5: Methods for incorporating cross-over trials into a meta-analysis [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2016 Sep 20]. Available from: http://handbook.cochrane.org/chapter_16/16_4_5_methods_for_incorporating_cross_over_trials_into_a.htm
- 13.Sedgwick P. What is publication bias in a meta-analysis? BMJ. 2015;351:h4419. doi: 10.1136/bmj.h4419. [DOI] [PubMed] [Google Scholar]
- 14.Aller R, Burgueño Gomez B, Sigüenza R, Fernández-Rodríguez C, Fernández N, Antolín B, Durà M, Pina M, Lorenzo S, García C, de Luis Román D. Comparative study of overweight and obese patients with nonalcoholic fatty liver disease. Rev Esp Enferm Dig. 2019;111(4):256–263. doi: 10.17235/reed.2019.5926/2018. [DOI] [PubMed] [Google Scholar]
- 15.Baratta F, Pastori D, Polimeni L, Bucci T, Ceci F, Calabrese C, Ernesti I, Pannitteri G, Violi F, Angelico F, Del Ben M. Adherence to Mediterranean diet and non-alcoholic fatty liver disease: effect on insulin resistance. Am J Gastroenterol. 2017;112(12):1832–1839. doi: 10.1038/ajg.2017.371. [DOI] [PubMed] [Google Scholar]
- 16.Chan R, Wong VW, Chu WC, Wong GL, Li LS, Leung J, Chim AM, Yeung DK, Sea MM, Woo J, Chan FK, Chan HL. Diet-quality scores and prevalence of nonalcoholic fatty liver disease: a population study using proton-magnetic resonance spectroscopy. PLoS One. 2015;10(9):e0139310. doi: 10.1371/journal.pone.0139310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalatbari-Soltani S, Imamura F, Brage S, De Lucia Rolfe E, Griffin SJ, Wareham NJ, Marques-Vidal P, Forouhi NG. The association between adherence to the Mediterranean diet and hepatic steatosis: cross-sectional analysis of two independent studies, the UK Fenland Study and the Swiss CoLaus Study. BMC Med. 2019;17(1):19. doi: 10.1186/s12916-019-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, Fragopoulou E, Zafiropoulou R, Manios Y, Papatheodoridis G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. 2014;33(4):678–683. doi: 10.1016/j.clnu.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, Lichtenstein AH, Hu FB, Levy D. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155(1):107–117. doi: 10.1053/j.gastro.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abenavoli L, Greco M, Milic N, Accattato F, Foti D, Gulletta E, Luzza F. Effect of Mediterranean diet and antioxidant formulation in non-alcoholic fatty liver disease: a randomized study. Nutrients. 2017;9(8):pii: E870. doi: 10.3390/nu9080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biolato M, Manca F, Marrone G, Cefalo C, Racco S, Miggiano GA, Valenza V, Gasbarrini A, Miele L, Grieco A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25(4):509–520. doi: 10.3748/wjg.v25.i4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, Papageorgiou MV, Fragopoulou E, Kontogianni MD. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br J Nutr. 2018;120(2):164–175. doi: 10.1017/S000711451800137X. [DOI] [PubMed] [Google Scholar]
- 23.Misciagna G, Del Pilar DM, Caramia DV, Bonfiglio C, Franco I, Noviello MR, Chiloiro M, Abbrescia DI, Mirizzi A, Tanzi M, Caruso MG, Correale M, Reddavide R, Inguaggiato R, Cisternino AM, Osella AR. Effect of a low glycemic index Mediterranean diet on non-alcoholic fatty liver disease. A randomized controlled clinical trial. J Nutr Health Aging. 2017;21(4):404–412. doi: 10.1007/s12603-016-0809-8. [DOI] [PubMed] [Google Scholar]
- 24.Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, Tibballs J, MacQuillan GC, Garas G, Adams LA. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. 2018;68(5):1741–1754. doi: 10.1002/hep.30076. [DOI] [PubMed] [Google Scholar]
- 25.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O'Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Calvaruso V, Craxì A. Implication of normal liver enzymes in liver disease. J Viral Hepat. 2009;16(8):529–536. doi: 10.1111/j.1365-2893.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 27.Yki-Järvinen H. Diagnosis of non-alcoholic fatty liver disease (NAFLD) Diabetologia. 2016;59(6):1104–1111. doi: 10.1007/s00125-016-3944-1. [DOI] [PubMed] [Google Scholar]
- 28.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5(3):211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 30.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 31.Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, Risérus U. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63(7):2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Li L, Liu X, Luo R, Liao G, Li L, Liu J, Cheng J, Lu Y, Chen Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018;203:291–304. doi: 10.1016/j.lfs.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Clarke SD. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am J Physiol Gastrointest Liver Physiol. 2001;281(4):G865–G869. doi: 10.1152/ajpgi.2001.281.4.G865. [DOI] [PubMed] [Google Scholar]
- 34.Scorletti E, Byrne CD. Omega-3 fatty acids and non-alcoholic fatty liver disease: evidence of efficacy and mechanism of action. Mol Asp Med. 2018;64:135–146. doi: 10.1016/j.mam.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka N, Zhang X, Sugiyama E, Kono H, Horiuchi A, Nakajima T, Kanbe H, Tanaka E, Gonzalez FJ, Aoyama T. Eicosapentaenoic acid improves hepatic steatosis independent of PPARα activation through inhibition of SREBP-1 maturation in mice. Biochem Pharmacol. 2010;80(10):1601–1612. doi: 10.1016/j.bcp.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. 2015;6(4):110–119. doi: 10.4291/wjgp.v6.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37(7):936–949. doi: 10.1111/liv.13435. [DOI] [PubMed] [Google Scholar]
- 38.Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24(19):2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D, Wahlqvist ML, Sinclair AJ. Advances in n-3 polyunsaturated fatty acid nutrition. Asia Pac J Clin Nutr. 2019;28(1):1–5. doi: 10.6133/apjcn.201903_28(1).0001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)