Abstract
Purpose
Diabetes mellitus is associated with perturbations in brain biochemical parameters associated with dementia. This study aimed at comparing the effect of metformin and metformin/donepezil combination on oxidative stress, endoplasmic reticulum stress and inflammation in the brain of diabetic Wistar rats.
Methods
Diabetes was induced by single intraperitoneal injection of 40 mg/kg streptozotocin after administration of 10% fructose for 14 days. Animals were randomly assigned to four groups of five animals each. Group 1 was the normal control and received only distilled water. Groups 2 and 3 were diabetic rats treated with metformin/donepezil combination and metformin only respectively, while group 4 was diabetic control. Treatment lasted for 21 days after confirmation of diabetes. Activities of acetylcholinesterase (AchE), butyrylcholinesterase (BchE), superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase were evaluated in the brain of diabetic rats. Enzyme-linked immunosorbent assay was used to estimate brain levels of tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) malondialdehyde and glucose transporter-4 (GLUT4), while expression of endoplasmic reticulum stress markers – glucose regulated protein-78 (GRP78), activating transcription factor-4 (ATF4) and C/EBP homologous protein (CHOP) was determined using real-time PCR in the hippocampus of diabetic rats.
Results
Treatment with metformin/donepezil combination significantly reduced the activities of AchE, BchE as well as levels of malondialdehyde, TNF-α and IL-6, while the activities of SOD, GPx and catalase were significantly increased in the brain. Moreover, expression of ER stress markers was attenuated in the hippocampus.
Conclusion
Metformin/donepezil combination appeared more efficacious than metformin only and could be considered for managing diabetes-associated dementia.
Keywords: Dementia, Inflammation, Antioxidant, Acetylcholinesterase, Glucose
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder usually characterized by insulin deficiency, insulin resistance and general dysregulation of glucose metabolism [1]. Notably, prolonged T2DM leads to microvascular and macrovascular complications and eventually leads to end-organ damage in important organs, such as the brain, where such damages usually present with cognitive impairment and dementia [2]. Moreover, earlier studies revealed an association between diabetes and Alzheimer’s disease (AD), with risk of Alzheimer’s disease increasing with adult onset diabetes mellitus [3].
Low-grade systemic inflammation has been identified as the pathological link between diabetes and AD as various inflammatory pathways are connected to the association between T2DM and neurodegenerative diseases including AD [4, 5]. Moreover, activities of butyrylcholinesterase (BuChE) and acetylcholinesterase (AChE) have been suggested to increase in both T2DM and AD in a manner involving low grade systemic inflammation. [6]. These enzymes are involved in acetylcholine hydrolysis, however, AChE is responsible for most of the cholinesterase activities in the brain while BuChE accounts for only about 10%. These enzymes are considered important therapeutic targets for AD [7].
Both animal and human studies reported that disruption of insulin function is implicated in development of neurodegenerative diseases [8], moreso, insulin resistance was identified to be an important predisposing factor and contribute to the pathogenesis of dementia [9, 10]. In fact, brain insulin resistance usually occurs prior to AD and also accounts for reduced cognitive ability in AD more than other known causes of the disease [11]. Thus, insulin resistance in the brain seems to be an attractive drug target that could ameliorate the cognitive decline associated with AD [12]. It has been established that loss of function of glucose-4 transporter (GLUT4) in peripheral tissues is associated with insulin resistance [13]. Besides, it was recently reported that GLUT4 may be involved in glucose transport both in normal brains as well as diabetic brains, especially in the nerve cells. Moreover, levels of GLUT4 was reported to decrease in streptozotocin-induced diabetic rats [14].
The endoplasmic reticulum (ER) is a cellular organelle responsible for protein folding, quality control system, calcium storage, lipid synthesis and secretion. Several factors such as oxidative stress, disturbance in glucose level, imbalances in the secretory pathway, mitochondrial dysfunctions, and other failures in protein quality control may result in buildup of unfolded/misfolded proteins in the ER [15]. Accumulation of misfolded proteins in the ER leads to activation of an evolutionarily conserved pathway called unfolded protein response (UPR) with the aim of improving ER and cellular function. UPR functions through three main stress sensors which include protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α) and activating transcription factor-6 (ATF6). These stressors are ER transmembrane proteins whose luminal domains associate with glucose regulated protein-78 (Grp78), an ER-resident chaperone. Their interaction with the chaperone prevents their activation [16]. Upon accumulation of misfolded proteins, GRP78 dissociates from the ER stress sensors and associates with the misfolded proteins, thereby activating the UPR [17]. Specifically, UPR relieves the burden on the ER by decreasing protein synthesis (translation), increasing protein folding capacity of ER and also increasing the degradation of unfolded proteins via the ubiquitin-proteasome pathway or autophagy [18]. However, failure of UPR to relieve ER burden leads to chronic ER stress with its attendant deleterious effects such as apoptosis, as brought about by the pro-apoptotic CCAAT-enhancer-binding protein homologous protein (CHOP), which is under the control of activating transcription factor 4 (ATF4), both of which are downstream mediators of UPR [19].
Neurons are highly susceptible to several insults that affect ER homeostasis as a result of redox imbalances and accumulation of unfolded proteins [20]. Moreover, studies have shown that an important factor in the onset of AD is increased production of amyloid-β peptides as well as its oligomerization and aggregation [21]. Previous studies showed that in AD brains, expression of GRP78 is increased in addition to significant increase in levels of other ER stress markers [22]. Moreso, it was earlier reported that activation of UPR correlates with age even without any pathology [23]. Thus, high level of ER stress markers apparently is an early event prior to observable protein deposition in AD [24]. The continuous hyperglycemia associated with T2DM may cause β-cell stress and diminish its function as a result of glucotoxicity. Under this condition, increased need for proinsulin biosynthesis may inundate the protein folding capacity of the ER and thereby lead to activation of UPR. Both long- and short term exposure of β-cells to hyperglycemia has been reported to cause hyperactivation of IRE1α [25].
Donepezil, a synthetic AChE inhibitor increases the availability of acetylcholine as well as its interaction with cholinergic receptors. It is one of the drugs approved for treating AD [26, 27]. While it selectively inhibits Ache, the inhibitory effect of donepezil on BuChe is much lower [28]. Conversely, metformin is an antidiabetic drug belonging to the class of the biguanides and it has become the most prescribed antidiabetic drug due to its safety [29]. Ability of metformin to reduce inflammation in different types of cell suggest that it could also protect against inflammation of nervous tissues [30]. It was previously reported that metformin reduced the risk of cognitive deficit [31], while T2DM patients on metformin treatment were less likely to have dementia [32]. Moreover, metformin was proposed to improve the efficacy of several drugs [33], underlining its relevance in combination therapy. The present study therefore aims to compare the effect of metformin/donepezil combination and metformin only on oxidative stress, endoplasmic reticulum stress and inflammation in the brain of fructose-fed type 2 diabetic rats.
Materials and methods
Chemicals
Streptozotocin, metformin, fructose, citric acid, epinephrine, and sodium citrate were procured from Sigma-Aldrich (St-Louis, MO, USA). Enzyme-linked immunosorbent assay (ELISA) kits for tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were purchased from MyBioSource Biotechnology company (San Diego, U.S.A). All other chemicals and reagents used were of analytical grade.
Experimental design and induction of diabetes
Twenty male Wistar rats weighing 180 − 200 g were obtained from the animal house, Afe Babalola University, Ado-Ekiti, Nigeria, after which they were maintained under standard conditions. Animals were fed ad libitum for 7 days before and throughout the course of the study. The rats were randomly grouped into four of 5 animals in each. Group 1 served as the normal control and received only distilled water while the study lasted. Groups 2 and 3 were diabetic animals treated with 100 mg/kg metformin/ 10 mg/kg donepezil combination and 100 mg/kg metformin only respectively, while group 4 was diabetic control and received distilled water only. Treatment of animals was done orally and lasted for 21 days after confirmation of diabetes. Rats were made diabetic by first giving them drinking water containing 10% fructose for 14 days, followed by injection of 40 mg/kg body weight streptozotocin dissolved in ice-cold 0.1 M citrate buffer (pH 4.5) intraperitoneally [34]. Diabetes was confirmed 72 h after injection of streptozotocin using Accu-check® glucometer and animals with blood glucose ≥ 250 mg/dl were considered diabetic and were used for the study.
Preparation of serum and homogenates
After the administration of the last doses of treatment, animals were made to fast for 12 h, and later sacrificed by decapitation under mild anesthesia. Blood was taken from the heart and spun at 3000 rpm for 5 min to obtain the serum which was used to analyze for biochemical parameters. The whole brain of each animal was rapidly dissected, hippocampus was removed and immediately stored at -80 ºC until used. The remaining portion of the brain was rinsed with isotonic saline, weighed and thereafter homogenized (1:10 w/v) in ice-cold 50 mmol/l Tris-HCl (pH 7.4) buffer containing 300 mmol/l sucrose [35]. The resultant homogenate was spun at 3000 rpm for 10 min at 4ºC and the resulting supernatant was used for analyses of biochemical parameters. Animal studies adhered to the Principles of Laboratory Animal Care [36]. All animal experiments were approved by the Animal Care Committee of the Afe Babalola University Research Directorate, Ado-Ekiti, Nigeria, with ethical approval number ABUAD-SCI19/03/102.
Biochemical analyses
Activities of AChE, BuChE as well as levels of GLUT4, IL-6 and TNF-α in the brain were determined using ELISA kits by following manufacturer’s instruction. Serum glucose level was estimated according to the protocol provided by the kit manufacturer (Randox Laboratories Crumlin, United Kingdom). Brain malondialdehyde (MDA) level was evaluated as described by Varshney and Kale (1990) [37]. Activities of superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) in the brain were evaluated using the methods of Misra and Fridovich (1972) [38], Sinha (1972) [39] and Rotruck et al., (1973) [40] respectively.
Gene expression analysis by reverse-transcription polymerase chain reaction (RT-PCR)
Isolation of total RNAs from hippocampus of diabetic rats as well as PCR amplification were done using OneTaq® 2X Master Mix (New England BioLabs, Massachusetts, USA) according to the manufacturer’s protocol. The reaction was run on a Labgene thermocycler. Primers for activating transcription factor-4 (ATF4), glucose regulated protein (GRP78), C/EBP homologous protein (CHOP) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Inqaba Biotec (Hatfeild, South Africa). Reverse Transcription–PCR reaction was performed in a 30 µl final volume. Amplification conditions were pre-denaturation at 94 °C for 5 min, denaturation at 94 °C for 30 s annealing at 55 °C for 30 s and extension 72 °C for 30 s and then 72 °C for 5 min by 30 cycles. Assessment of Polymerase Chain Reaction products (amplicons) were electrophoresed in 0.2% of agarose gel using 0.5 × TBE buffer (2.6 g of Tris base, 5 g of Tris boric acid and 2 ml of 0.5M EDTA and adjusted to pH 8.3 with the sodium hydroxide pellet) with 5 µl EZ-vision (VWR Life Science). The relative amount of complimentary DNA (cDNA) was quantified using ImageJ software, and the gene expression was normalized with GAPDH gene as housekeeping gene. The intensities of the bands form agarose gel electrophoresis were quantified densitometrically using ImageJ software (Table 1).
Table 1.
Sequence of primers for polymerase chain reaction
| Gene name | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| GRP78 | AGCCCACCGTAACAATCAAG | TCCAGCCATTCGATCTTTTC |
| ATF-4 | GTTGGTCAGTGCCTCAGACA | CATTCGAAACAGAGCATCGA |
| GAPDH | AGACAGCCGCATCTTCTTGT | CTTGCCGTGGGTAGAGTCAT |
| CHOP | CCAGCAGAGGTCACAAGCAC | CGCACTGACCACTCTGTTTC |
Data analysis
All results were expressed as mean value ± standard deviation (SD). Data was analyzed with one-way analysis variance (ANOVA) using Graphpad prism 5 software and SPSS package 16. Means were compared with Tukey test. p ˂ 0.05 was considered statistically significant.
Results
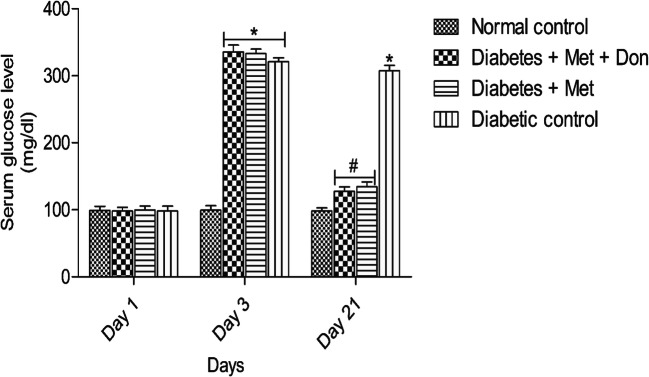
Result presented in Fig. 1 showed that treatment of diabetic rats with metformin/donepezil combination as well as metformin only for 21 days significantly (p < 0.05) reduced serum glucose levels when compared with rats in diabetic control group. There was however no significant (p < 0.05) difference in glucose levels in both treatment groups by the end of the study.
Fig. 1.
Effect of metformin/donepezil combination and metformin only on serum glucose level in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Met = Metformin, Don = Donepezil
As presented in Fig. 2, co-administration of metformin with donepezil showed a significantly (p < 0.05) higher inhibitory effect on acetylcholinesterase than metformin only in the brain of diabetic rats. However, both treatments significantly (p < 0.05) inhibited the enzyme when compared with the diabetic control group.
Fig. 2.
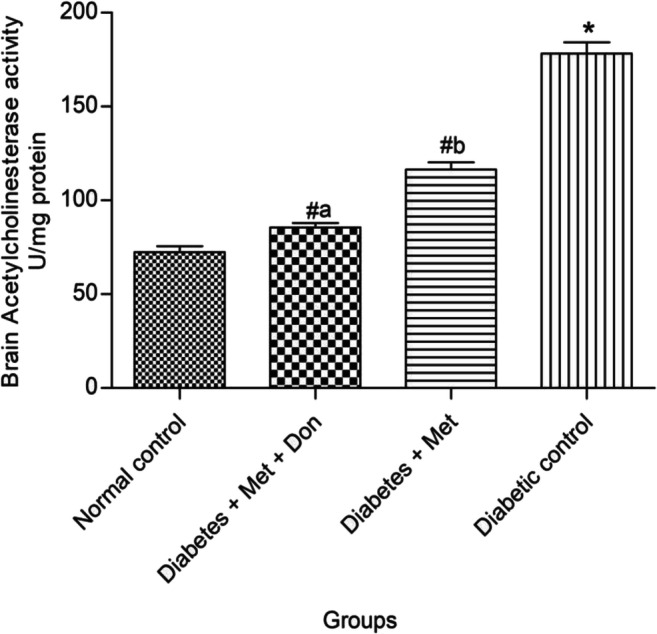
Effect of metformin/donepezil combination and metformin only on brain acetylcholinesterase activity in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
In Fig. 3, it was observed that treatment with both metformin only and combination of metformin and donepezil significantly (p < 0.05) inhibited butyrylcholinesterase in the brain of diabetic rats when compared with the diabetic control. However, there was no significant (p < 0.05) difference in the inhibitory effect of metformin only and metformin/donepezil combination.
Fig. 3.
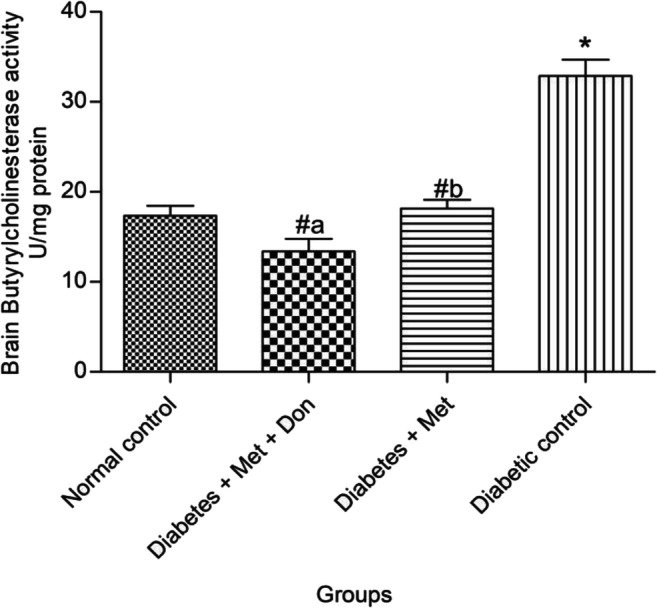
Effect of metformin/donepezil combination and metformin only on brain butyrylcholinesterase activity in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
Treatment of diabetic rats with metformin only and metformin/donepezil combination significantly (p < 0.05) increased brain level of GLUT4 (Fig. 4). Remarkably, brain GLUT4 level was significantly (p < 0.05) higher in diabetic rats treated with combination therapy of metformin and donepezil when compared with metformin only.
Fig. 4.
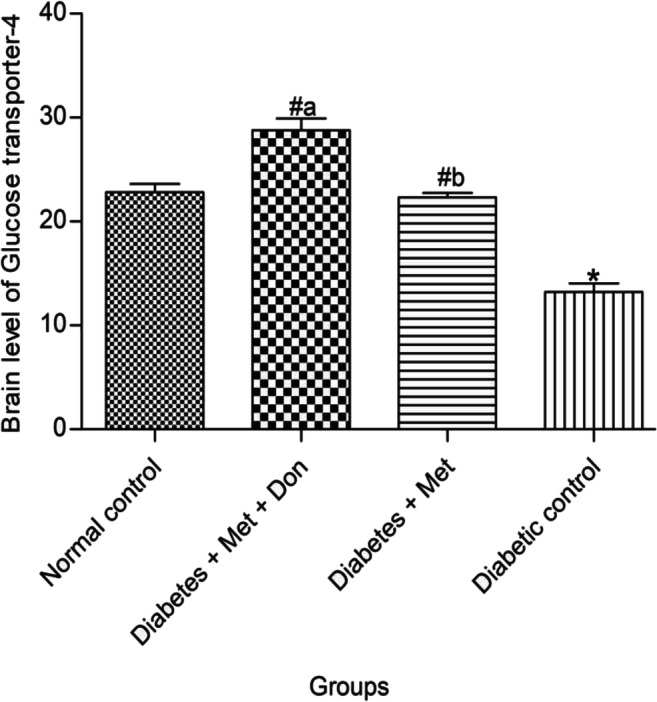
Effect of metformin/donepezil combination and metformin only on brain level of GLUT4 in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
Figure 5 shows that there was no significant (p < 0.05) difference in brain SOD activity of diabetic rats treated with metformin only and combination of metformin and donepezil. However, both treatments significantly (p < 0.05) increased brain SOD activity when compared with diabetic control group.
Fig. 5.
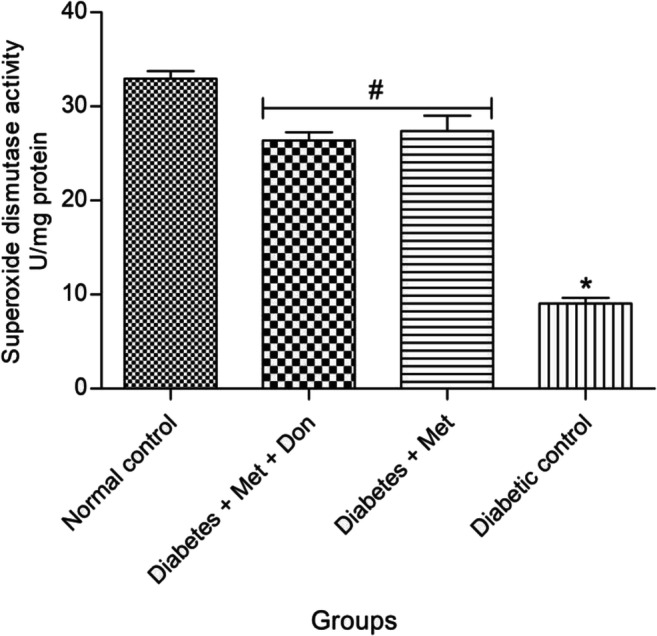
Effect of metformin/donepezil combination and metformin only on activity of SOD in the brain of diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Met = Metformin, Don = Donepezil
From result presented in Fig. 6, it was observed that diabetic rats treated with combination of metformin and donepezil had a significantly (p < 0.05) higher catalase activity in the brain when compared with those treated with metformin only. In addition, both treatments significantly (p < 0.05) increased catalase activity in the brain of diabetic rats when compared with the diabetic control group.
Fig. 6.
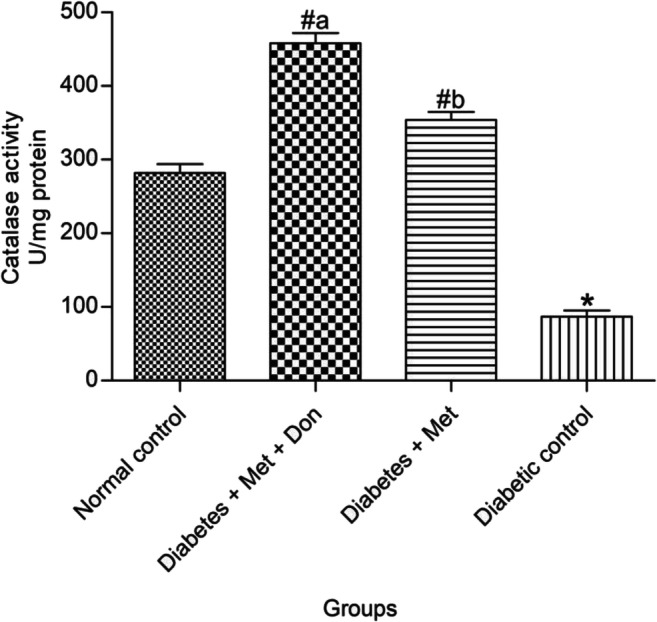
Effect of metformin/donepezil combination and metformin only on activity of catalase in the brain of diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
Figure 7 shows that activity of glutathione peroxidase in the brain of diabetic rats treated with combination of both metformin and donepezil was significantly (p < 0.05) higher than that of metformin only-treated diabetic rats. However, both treatments significantly (p < 0.05) increased brain glutathione peroxidase activity when compared with diabetic control group.
Fig. 7.
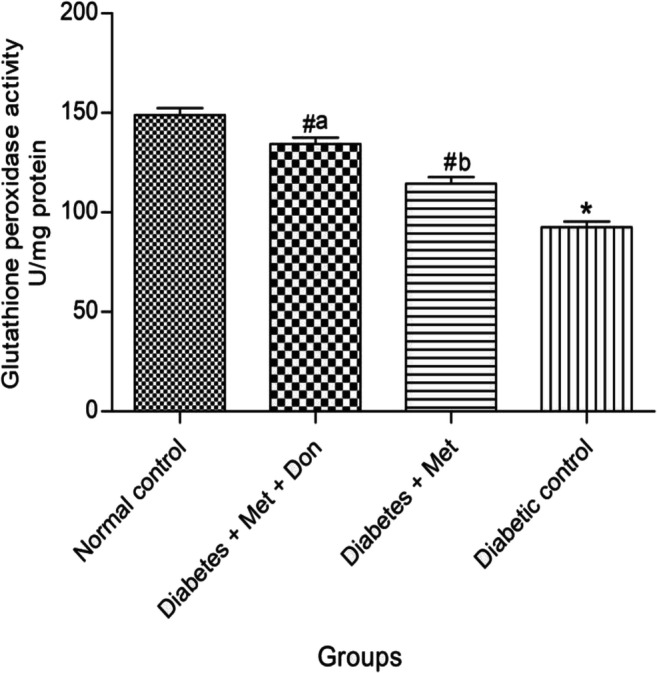
Effect of metformin/donepezil combination and metformin only on activity of GPx in the brain of diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
As presented in Fig. 8, MDA level in the brain of rats in diabetic control group was significantly (p < 0.05) higher than those observed in other groups in the study. In addition, brain of diabetic rats treated with metformin/donepezil combination had a significantly (p < 0.05) reduced MDA levels when compared with those treated with metformin only.
Fig. 8.
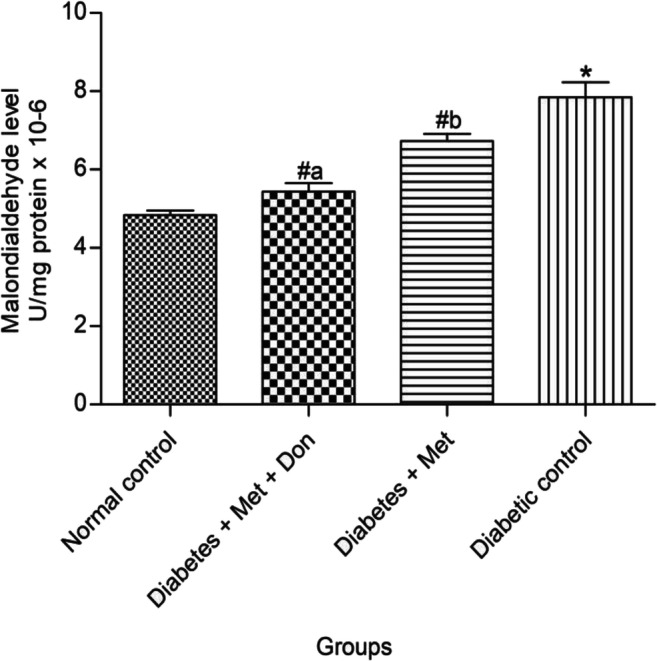
Effect of metformin/donepezil combination and metformin only on level of MDA in the brain of diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
As shown in Fig. 9, there was no significant (p < 0.05) difference between in TNF-α levels in the brain of diabetic rats treated with combination of metformin and donepezil as well as with metformin only. However, both treatments caused a significant (p < 0.05) reduction in brain TNF-α levels when compared with diabetic control.
Fig. 9.
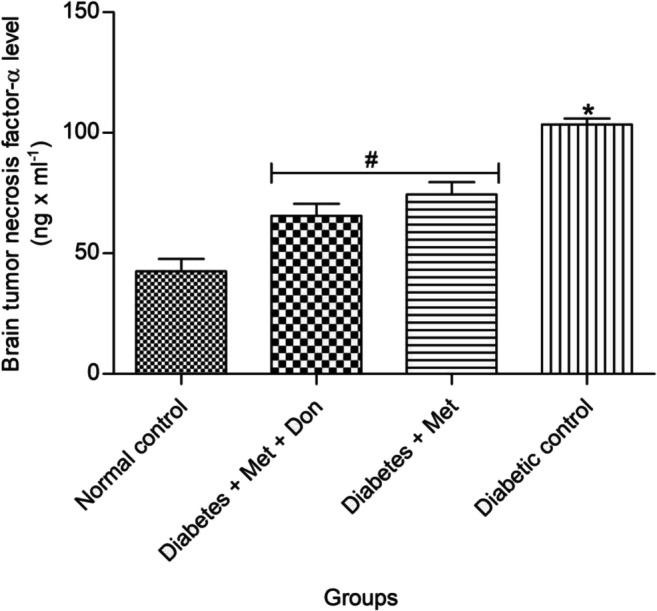
Effect of metformin/donepezil combination and metformin only on level of TNF-α in the brain of diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Met = Metformin, Don = Donepezil
It was observed in Fig. 10 that level of IL-6 in the brain of diabetic rats treated with combination of metformin and donepezil was significantly (p < 0.05) lower than those treated with metformin only. Both treatments however reduced brain IL-6 level in diabetic rats when compared with the diabetic control group.
Fig. 10.
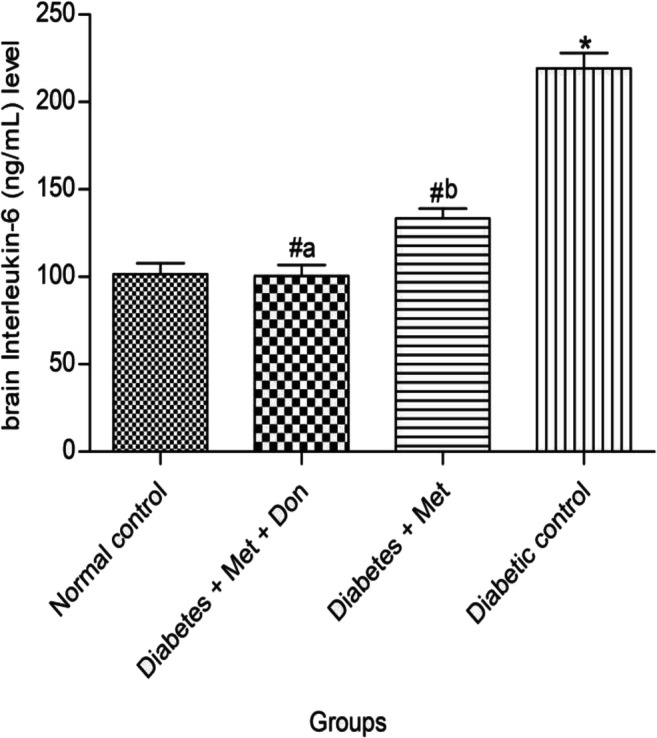
Effect of metformin/donepezil combination and metformin only on level of IL-6 in the brain of diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
Figure 11 shows that level of GRP78 mRNA in hippocampus of diabetic control rats was significantly (p < 0.05) higher when compared with other groups in the study. In addition, hippocampal GRP78 mRNA of diabetic rats treated with combination of metformin and donepezil was significantly (p < 0.05) lower when compared with those treated with metformin only.
Fig. 11.
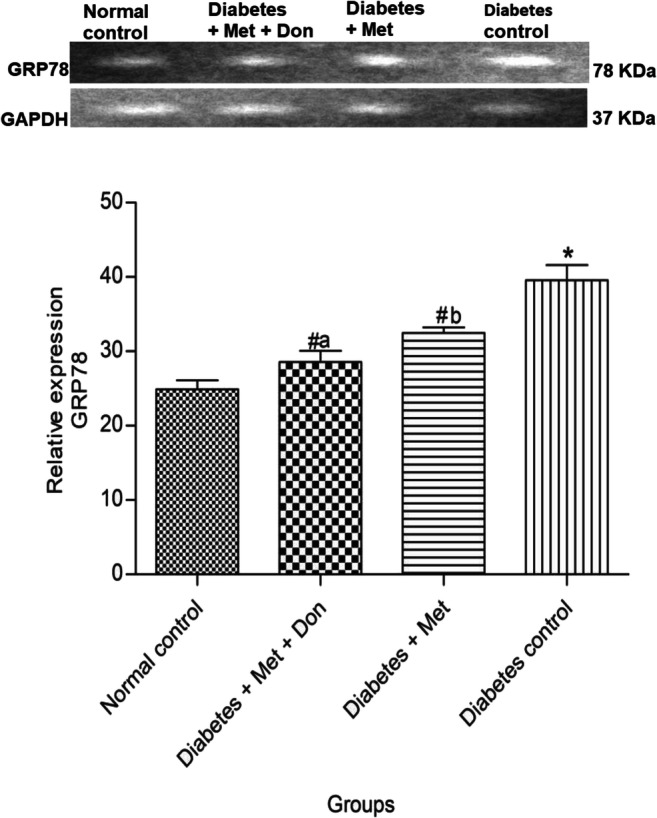
Effect of metformin/donepezil combination and metformin only on hippocampal GRP78 expression in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
It was shown in Fig. 12 that both metformin only as well as combination of metformin and donepezil significantly (p < 0.05) reduced the level of ATF4 mRNA in the hippocampus of diabetic rats when compared with diabetic control. However, ATF4 mRNA level was significantly (p < 0.05) higher in hippocampus of diabetic rats treated with metformin only when compared with those treated with combination therapy metformin and donepezil.
Fig. 12.
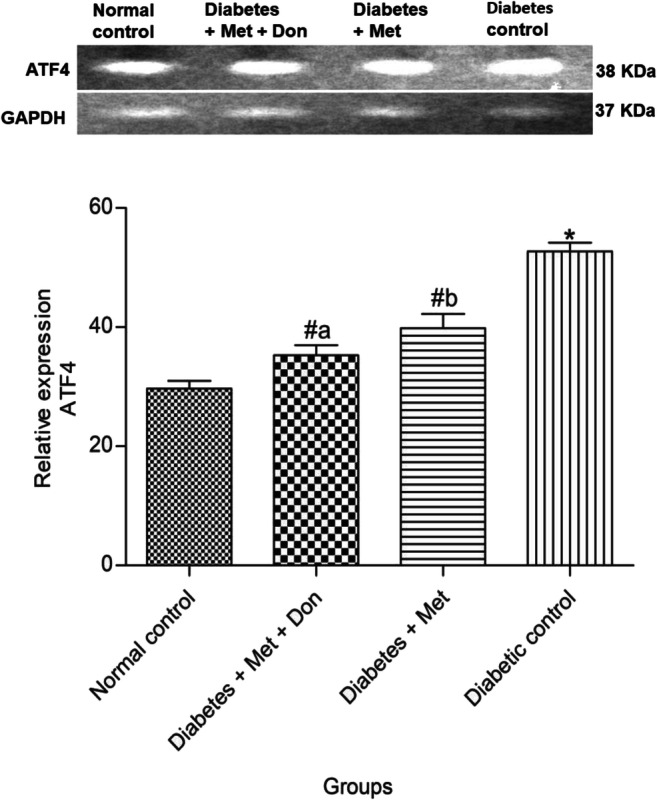
Effect of metformin/donepezil combination and metformin only on hippocampal expression of ATF4 in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
Figure 13 shows that treatment of diabetic rats with metformin/donepezil combination significantly (p < 0.05) repressed the expression of CHOP gene in the hippocampus of diabetic rats when compared with treatment with metformin only. Metformin only also significantly (p < 0.05) repressed the expression of CHOP gene in the hippocampus of diabetic rats when compared with diabetic control group.
Fig. 13.
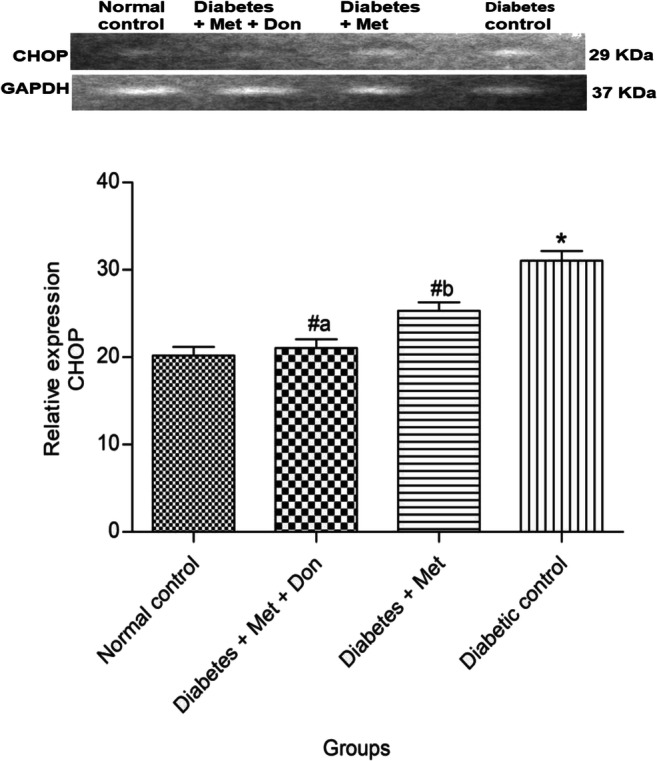
Effect of metformin/donepezil combination and metformin only on hippocampal expression of CHOP in diabetic rats. Values are expressed as mean ± standard deviation of five determinations. *p < 0.05 vs. normal control, #p < 0.05 vs. diabetic control. Bars with different alphabets are significantly different. Met = Metformin, Don = Donepezil
Discussion
Combination therapy has several benefits when compared to monotherapy as it produces improved efficacies and also reduces the possibility of adverse effects [41]. As a result of this, it has been employed in treating multiple complex diseases [42]. The present study therefore explored the benefit of combination therapy with metformin and donepezil in comparison with monotherapy with metformin only in type 2 diabetes-associated dementia with a focus relevant biochemical processes such as oxidative stress, endoplasmic stress, activities of cholinesterases and inflammation, that have been implicated in type 2 diabetes-associated dementia.
Alteration in cognition associated with T2DM patients who are yet to be diagnosed of dementia has been earlier reported [43]. Moreover, T2DM patients have a higher likelihood of progressing from slight impairment to cognition to dementia, and to AD [44]. In such patients, the level and period of hyperglycemia are related to extent of dementia. Actually, faster decline in cognition is associated with both increased glycated hemoglobin and duration of diabetes, with reduced cognition observed for every 1% rise in glycated hemoglobin [45]. Cerebral hemorrhage, aggregation of amyloid-β as well as neuronal damage in terms of structure and function are some of the mechanisms through which hyperglycemia worsens cognition [46]. In this study, a significant reduction in serum glucose level was observed in diabetic rats treated with metformin only and its combination with donepezil with no significant difference between the antihyperglycemic effect of both treatments.
It has been suggested that treatment of T2DM with cholinesterase inhibitors might delay the onset of AD [47]. This could be due to ability of cholinesterase inhibitors to prevent depletion of acetylcholine which is the principal substrate of the Ache and BuChe, and thereby inhibit eventual cholinergic dysfunction that usually characterizes AD [48]. In our study, we observed that both metformin/donepezil combination and metformin only significantly reduced activity of Ache and BuChe in the brain of diabetic rats. A similar trend was observed in earlier studies on AChe with metformin [49] and combination of metformin and donepezil [50]. However, in diabetic rats treated with metformin/donepezil combination, AChe activity was significantly lower than in metformin only group, an indication of possible synergy between both metformin and donepezil. A similar trend was earlier observed by Markowicz-Piasecka et al., 2018 [50]. Our study did not show a significant difference in inhibition of BuChe.
Oxidative stress and subsequent increase in the level of its markers are known to be involved in the pathological processes leading to AD [51]. The brain consumes in excess of 20% of the total oxygen available to the organism, 98% of which passes through the mitochondrial electron transport chain with attendant production of reactive oxygen species (ROS). Coupled with this is high level of polyunsaturated fatty acids (PUFA), low activity of brain antioxidant enzymes, and high level of pro-oxidant metal ions in the brain, which makes it particularly susceptible to oxidative stress [52, 53]. This is especially relevant in the settings of AD as research has revealed that oxidative stress is the most consistent risk factors in AD [54]. It has been abundantly proven that exposure of β-cells to chronic hyperglycemia increases ROS production and decreases both insulin content and glucose-stimulated insulin release [55]. Like the brain, β-cells have low levels of antioxidant enzymes and this also makes them susceptible to oxidative stress [56]. Thus, oxidative stress indicators such as reduced levels of antioxidant enzymes and their activities, as well as increased lipid peroxidation products are known to be present in diabetic patients [10]. This study showed that metformin/donepezil combination significantly improved brain antioxidant status in diabetic rats. Even though a previous study established the fact that metformin ameliorates brain oxidative stress arising from diabetes [57], our study showed that metformin/donepezil combination had a better ameliorative effect on brain antioxidant status when compared with metformin only as observed in the activities of catalase and glutathione peroxidase as well as in the level of MDA.
Inflammation is associated with the pathological processes leading to both AD and T2DM [58]. Higher level of TNF-α was linked with AD [59]. Similarly, earlier research demonstrated that increase in the level of inflammatory markers (e.g. IL-6) correlates with development of T2DM [60]. Besides, inflammation correlates with decline in cognition [61]. Anti-inflammatory effect of metformin and cholinesterase inhibitors like donepezil has earlier been reported. Metformin is known to reduce the expression of NF-kB, the transcription factor involved in inflammation as well as NF-kB–dependent genes [62, 63]. In the same vein, inhibition of acetylcholine hydrolysis by cholinesterase inhibitors sustains the anti-inflammatory effect of acetylcholine, which it exerts by suppressing generation of pro-inflammatory cytokines [64]. In the present study, it was discovered that both metformin/donepezil combination and metformin only significantly reduced inflammation in the brain of diabetic rats. Nevertheless, a significantly lower level of IL-6 was observed in the brain of diabetic animals treated with metformin/donepezil combination when compared with those treated with metformin only, while no significant difference was observed in the level of TNF-α between the two treatment groups. Both drugs could have exerted their anti-inflammatory effects via the mechanisms stated earlier, and the significant anti-inflammatory effect of both drugs especially as observed in the level of IL-6 in the brain could be as a result of synergy between both drugs.
Apart from its expression in peripheral tissues, GLUT4 is also expressed in various parts of the brain such as the hippocampus and cerebellum [65, 66]. Its role as blood glucose sensor as well as in the uptake and utilization of glucose in the brain has been reported [67]. Improvement in cognition as a result of insulin stimulated glucose uptake by GLUT4 has made its hippocampal overexpression a promising drug target [68]. In this study, treatment of diabetic rats with metformin/donepezil combination and metformin only resulted in significantly higher level of GLUT4 in the brain of diabetic rats when compared with diabetic control. It is noteworthy that GLUT4 levels in the brain of diabetic rats treated with metformin/donepezil combination was significantly higher than those treated with metformin only. Metformin has earlier been reported to markedly improve GLUT4 expression in muscle of streptozotocin induced diabetic rats [69]. In our study, we observed an identical effect in the brain of diabetic rats, which was apparently complemented by donepezil.
ER stress has been associated with cognitive impairment in experimental diabetic animals [70, 71]. GRP78 is critical in protecting cell death due to several stresses on the ER [72]. Relatedly, ATF4 is a transcription factor and a downstream mediator in the PERK arm of ER stress pathway that regulates ER redox homeostasis, protein folding and cell fate through its induction of CHOP which in turn induces apoptosis by activating proapoptotic factors like p53 upregulated modulator of apoptosis (PUMA) [73] and suppression of pro-survival components of B-cell lymphoma 2 (Bcl-2) family of proteins [74].
Increase in the number of CHOP positive neurons was reported in the hippocampus of diabetic rats [75]. Besides, in Alzheimer’s disease brain post mortem, upregulation of GRP78 was observed in both hippocampus and cerebral cortex [76] while brain protein level of ATF4 was also significantly upregulated in murine models of Alzheimer’s disease [77]. In the present study, expression of GRP78, ATF4 and CHOP was upregulated in the hippocampus of diabetic rats while treatment with metformin/donepezil combination and metformin only significantly attenuated the expression of the three ER stress markers. Furthermore, treatment of diabetic rats with combination of metformin and donepezil showed better ER stress ameliorating effect when compared with metformin only by attenuating the expression of the three ER stress markers evaluated in this study. The significantly higher expression of CHOP in the diabetic control group suggests that ER stress in this group persisted such that the ATF4-induced CHOP-mediated apoptotic pathway could be prompted, as expression of CHOP is under the influence of ATF4 [78]. This is particularly significant as loss of neuronal cells in different parts of the brain is characteristic of both neurodegenerative diseases [79] and diabetic rats [80]. The hippocampus is a brain region with very important functions in learning and memory [81]. Earlier studies confirmed that ER stress in the hippocampus is associated with cognitive impairment in diabetes. Thus, inhibiting hippocampal ER stress could be a means of improving cognition in diabetes [75]. It therefore follows that both treatment modalities in this study could improve cognition in diabetic rats.
Conclusion
This study revealed that there is a synergistic effect between metformin and donepezil, and that combination of both drugs had an edge over metformin only in modulating antioxidant status, inflammation, endoplasmic reticulum stress and activity of cholinesterases in the brain of type 2 diabetic rats. This implies that combination of both drugs could be considered in managing type 2 diabetes-associated dementia-cum-Alzheimer’s disease. Further research on dose variation of both drugs in order to determine the optimum doses of both drugs as well as the effect of a combination of both drugs on behavioral studies associated with dementia is recommended.
Author contributions
Obafemi T. O. and Olasehinde O. R designed the study; Olaoye O. A, Jaiyesimi K. F and Adewumi D.F. did most of the bench work; Afolabi B. A prepared the first draft of the manuscript; Adewale O. B proof-read and edited the manuscript.
Funding information
Authors did not receive any funding from institutions or individuals for this research.
Data availability
Available on request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
An ethical approval with number ABUAD-SCI19/03/102 was obtained from Animal Care Committee of the Afe Babalola University Research Directorate, Ado-Ekiti, Nigeria.
Consent to participate
Not applicable.
Consent for publication
All authors approved the submission of the manuscript for publication
Code availability
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Z, Fang P, Shia M, Zhu Y, Bo P. Elevated galanin may predict the risk of type 2 diabetes mellitus for development of Alzheimer’s disease. Mech Ageing Dev. 2015;150:20–6. doi: 10.1016/j.mad.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Ascher-Svanum H, Chen YF, Hake A, Kahle-Wrobleski K, Schuster D, Kendall D, et al. Cognitive and functional decline in patients with mild Alzheimer dementia with or without comorbid diabetes. Clin Ther. 2015;37(6):1195–205. doi: 10.1016/j.clinthera.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen R, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;14:301–8. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–14. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 5.Takeda S, Sato N, Uchio-Yamada K. Diabetes accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–41. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das UN. Acetylcholinesterase and butyrylcholinesterase as markers of low-grade systemic inflammation. Ann Hepatol. 2012;11(3):409–11. [PubMed] [Google Scholar]
- 7.Mushtaq G, Greig NH, Khan JA, Kamal MA. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol Disord Drug Targets. 2014;13(8):1432–9. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–78. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Sato N, Rakugi H, Morishita R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: Beta-amyloid peptide, insulin signaling, and neuronal function. Mol Biosyst. 2011;7:1822–7. doi: 10.1039/c0mb00302f. [DOI] [PubMed] [Google Scholar]
- 10.Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22–38. doi: 10.1016/j.nbd.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Talbot K, Wang HY, Kazi H, Han L, Bakshi KP, Stucky A. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedict C, Grillo CA. Insulin resistance as a therapeutic target in the treatment of Alzheimer’s disease: a state-of-the-art review. Front Neurosci. 2018;12:215. doi: 10.3389/fnins.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallberg-Henriksson H, Zierath JR. GLUT4: a key player regulating glucose homeostasis? Insights from transgenic and knockout mice (review) Mol Membr Biol. 2001;18:205–11. doi: 10.1080/09687680110072131. [DOI] [PubMed] [Google Scholar]
- 14.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. Glut4 glucose transporter expression in rodent brain: Effect of diabetes. Brain Res. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 15.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 16.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 17.Kind KR, Ball KK, Cruz NF, Dienel GA. The unfolded protein response to endoplasmic reticulum stress in cultured astrocytes and rat brain during experimental diabetes. Neurochem Int. 2013;62(5):784–95. doi: 10.1016/j.neuint.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–94. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheper W, Hoozemans JJ. Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy. Curr Med Chem. 2009;16:615–26. doi: 10.2174/092986709787458506. [DOI] [PubMed] [Google Scholar]
- 21.Salminen A, Kauppinen A, Suuronen T, kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duran-Aniotz C, Cornejo VH, Espinoza S, Ardiles AO, Medinas DB, Salazar C, Foley A, Gajardo I, Thielen P, Iwawaki T, Scheper W, Soto C, Palacios AG, Hoozemans JJM, Hetz C. IRE1 signaling exacerbates Alzheimer’s disease pathogenesis. Acta Neuropathol. 2017:1e18. [DOI] [PubMed]
- 23.Hamos JE, Oblas B, Pulaski-Salo D, Welch WJ, Bole DG, Drachman DA. Expression of heat shock proteins in Alzheimer’s disease. Neurology. 1991;41:345e350. doi: 10.1212/wnl.41.3.345. [DOI] [PubMed] [Google Scholar]
- 24.Hoozemans JJM, van Haastert ES, Nijholt DAT, Rozemuller AJM, Scheper W. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2012;10:212e215. doi: 10.1159/000334536. [DOI] [PubMed] [Google Scholar]
- 25.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic b cells by an endoplasmic reticulum- resident protein kinase IRE1. Cell Metab. 2006;4:245–54. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Kuca K, Soukup O, Maresova P, Korabecny J, Nepovimova E, Kilmova B. Current approaches against Alzheimer’s disease in clinical trials. J Braz Chem Soc. 2016;27:641–9. [Google Scholar]
- 27.Kumar K, Kumar A, Keegan RM, Deshmukh R. Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed Pharmacother. 2018;98:297–307. doi: 10.1016/j.biopha.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 28.Cacabelos R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007;3:303–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci. 2012;1;22:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotermund C, Machetanz G, Fitzgerald JC. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama H, Ogawa M, Honjo J, Okizaki S, Yamada D, Shudo R, et al. Risk factors associated with abnormal cognition in Japanese outpatients with diabetes, hypertension or dyslipidemia. Diabetol Int. 2015;6:268–74. [Google Scholar]
- 32.Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged >/=65 years with diabetes. Neurology. 2017;89:1877–85. doi: 10.1212/WNL.0000000000004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong L, Zhou Q, Zhang Z, Zhu Y, Duan T, Feng Y. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. J Obstet Gynaecol Res. 2012;38:1077–85. doi: 10.1111/j.1447-0756.2011.01839.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep. 2012;64:129–39. doi: 10.1016/s1734-1140(12)70739-9. [DOI] [PubMed] [Google Scholar]
- 35.Tsakiris S, Schulpis KH, Marinou K, Behrakis P. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+K+–ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res. 2004;49:475–9. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Guide for the care and use of laboratory animals. 8th ed. The National Academic Press, Washington, DC 20001; 2011.
- 37.Varshney R, Kale RK. Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–43. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 38.Misra HP, Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 39.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 40.Rotruck JT, Pope AL, Ganther HE, Swason AB. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588e90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 41.Ali MA, Rizvi S, Syed BA. Trends in the market for antihypertensive drugs. Nat Rev Drug Discov. 2017;16:309–10. doi: 10.1038/nrd.2016.262. [DOI] [PubMed] [Google Scholar]
- 42.Cheng F, Kovacs IA, Barabasi A. Network-based prediction of drug combinations. Nat Commun. 2019:1–11. 10.1038/s41467-019-09186-x. [DOI] [PMC free article] [PubMed]
- 43.Vincent C, Hall PA. Executive function in adults with type 2 diabetes: a meta-analytic review. Psychosom Med. 2015;77(6):631–42. doi: 10.1097/PSY.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 44.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:323–34. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 45.Gomez AM, Umpierrez GE. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol. 2014;8:930–6. doi: 10.1177/1932296814546025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diehl T, Mullins R, Kapogiannis D. Insulin resistance in Alzheimer’s disease. Transl Res. 2017;183:26–40. doi: 10.1016/j.trsl.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamal MA, Greig NH, Reale M. Anti-inflammatory properties of acetylcholinesterase inhibitors administered in Alzheimer’s disease. Anti Inflamm Anti-Allergy Agents Med Chem. 2009;8(1):85–100. [Google Scholar]
- 48.Nordberg A, Ballard C, Bullock R, Darreh-Shori T, Somogyi M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim Care Companion CNS Disord. 2013;15(2):PCC.12r01412. doi: 10.4088/PCC.12r01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saliua JA, Oboh G, Omojokun OS, Rochad J, Schetinger MR, Guterries J, et al. Effect of dietary supplementation of Padauk (Pterocarpus soyauxii) leaf on high fat diet/streptozotocin induced diabetes in rats’ brain and platelets. Biomed Pharmacother. 2016;84:1194–201. doi: 10.1016/j.biopha.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 50.Markowicz-Piasecka M, Huttunen KM, Sikora J. Metformin and its sulphonamide derivative simultaneously potentiateanti-cholinesterase activity of donepezil and inhibit beta-amyloid aggregation. J Enzyme Inhib Med Chem. 2018;33(1):1309–22. doi: 10.1080/14756366.2018.1499627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22:223–32. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Maciejczyk M, Zebrowska E, Zalewska A, Chabowski A. Redox, balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet- induced insulin resistance. Oxid Med Cell Longev. 2018;2018:1–11. doi: 10.1155/2018/6940515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marksbery WR. The role of oxidative stress in Alzheimer’s disease. Arch Neurol. 1999;56:1449–52. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 55.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flekac M, Skrha J, Hilgertova J, Lacinova Z, Jarolimkova M. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC Med Genet. 2008;9:30–5. doi: 10.1186/1471-2350-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark GJ, Pandya K, Lau-Cam CA. The effect of metformin and taurine, alone and in combination, on the oxidative stress caused by diabetes in the rat brain. Adv Exp Med Biol. 2017;975(1):353–69. doi: 10.1007/978-94-024-1079-2_31. [DOI] [PubMed] [Google Scholar]
- 58.Chatterjee S, Mudher A. Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front Neurosci. 2018;12:383. doi: 10.3389/fnins.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Decourt B, Lahiri DK, Sabbagh MN. Targeting tumor necrosis factor alpha for alzheimer’s disease. Curr Alzheimer Res. 2017;14(4):412–25. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obafemi TO, Olaleye MT, Akinmoladun AC. Antidiabetic property of miracle fruit plant (Synsepalum dulcificum Shumach. & Thonn. Daniell) leaf extracts in fructose-fed streptozotocin-injected rats via anti- inflammatory activity and inhibition of carbohydrate metabolizing enzymes. J. Ethnopharmacol. 2019;244:112124. 10.1016/j.jep.2019.112124. [DOI] [PubMed]
- 61.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–72. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 62.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMPactivated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–8. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 63.Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, et al. Transgenic inhibition of astroglial NF-κB leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110:765–78. doi: 10.1111/j.1471-4159.2009.06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic infl ammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 65.Pearson-Leary J, McNay EC. Novel roles for the insulin-regulated glucose transporter- 4 in hippocampally dependent memory. J Neurosci. 2016;36:11851–64. 42. [DOI] [PMC free article] [PubMed]
- 66.Ashrafi G, Wu Z, Farrell RJ, Ryan TA. GLUT4 mobilization supports energetic demands of active synapses. Neuron. 2017;93:606–15. doi: 10.1016/j.neuron.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ngarmukos C, Baur EL, Kumagai AK. Co-localization of glut1 and glut4 in the blood- brain barrier of the rat ventromedial hypothalamus. Brain Res. 2001;900:1–8. doi: 10.1016/s0006-8993(01)02184-9. [DOI] [PubMed] [Google Scholar]
- 68.Mehta V, Parashar A, Sharma A, Singh TR, Udayabanu M. Quercetin ameliorates chronic unpredicted stress-mediated memory dysfunction in male Swiss albino mice by attenuating insulin resistance and elevating hippocampal GLUT4 levels independent of insulin receptor expression. Horm Behav. 2017;89:13–22. doi: 10.1016/j.yhbeh.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Cheng J, Huang C, Liu I, Tzeng T, Chang C. Novel mechanism for plasma glucose– lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes. 2006;55:819–25. doi: 10.2337/diabetes.55.03.06.db05-0934. [DOI] [PubMed] [Google Scholar]
- 70.Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Shan Q. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun. 2011;25:1658–67. doi: 10.1016/j.bbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Ye T, Meng X, Wang R, Zhang C, He S, Sun G, Sun X. Gastrodin alleviates cognitive dysfunction and depressive-like behaviors by inhibiting ER stress and NLRP3 inflammasome activation in db/db mice. Int J Mol Sci. 2018;19(12):3977. doi: 10.3390/ijms19123977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imaizumi K, Miyoshi K, Katayama T, Yoneda T, Taniguchi M, Kudo T, Tohyama M. The unfolded protein response and Alzheimer’s disease. Biochim Biophys Acta. 2001;1536:85–96. doi: 10.1016/s0925-4439(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 73.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–74. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, et al. Identification of novel stress-induced genes downstream of CHOP. EMBO J. 1998;17:3619–33. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Xu L, He D, Ling S. Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. Biomed Res Int. 2013:1–9. [DOI] [PMC free article] [PubMed]
- 76.Hoozemans JJM, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110(2):165–72. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 77.Ohno M. Roles of eIF2alpha kinases in the pathogenesis of Alzheimer’s disease. Front Mol Neurosci. 2014;7:22. doi: 10.3389/fnmol.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang H, Jing G, Wang JJ, Sheibani N, Zhang SX. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J Inflamm (Lond) 2015;12:31. doi: 10.1186/s12950-015-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chi H, Chang H, Sang T. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19:3082. doi: 10.3390/ijms19103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shruthi K, Reddy SS, Chitra PS, Reddy GB. Ubiquitin-proteasome system and ER stress in the brain of diabetic rats. J Cell Biochem. 2018:1–12. 10.1002/jcb.27884. [DOI] [PubMed]
- 81.Lagali PS, Corcoran CP, Picketts DJ. Hippocampus development and function: role of epigenetic factors and implications for cognitive disease. Clin Genet. 2010;78:321–33. doi: 10.1111/j.1399-0004.2010.01503.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on request.