Abstract
Replacement of nitrite with hop components in cooked beef-sausage (CBS) was studied. For this purpose, lupulon–xanthohumol loaded nanoliposome (L–X-NL) was produced using sonication at optimized condition (time = 10.8 min, power = 72.7 W, lecithin concentration = 140 mg/mL). The release of lupulon and xanthohumol to liquid meat extract followed the Rigter–Peppus model. Samples of CBS (60% meat) supplemented by different ratios of nitrite/L–X-NL were produced. Microbial analysis and lipid oxidation measurement were carried out to evaluate the safety of CBS samples. The formulation consisted 30 ppm of nitrite and 150 ppm of L–X-NL remained microbiologically safe during 30-d storage at 4 °C. It was observed that L–X-NL could postpone the oxidation. Addition of L–X-NL has not impaired the sensory properties of final product, while the presence of nitrite for inducing the demanding color of CBS was important. Considering the results, partial removal of nitrite in formulation of CBS (up to 50%) and replacing with L–X-NL as a new promising preservative is recommended.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04299-4) contains supplementary material, which is available to authorized users.
Keywords: Sonication, Encapsulation, Sausage, Model, Nitrite
Introduction
Sausages are manufactured from different animal sources, e.g., pig, cow, sheep, buffalo and chicken (Raju et al. 2003; Sallam et al. 2004; Sachindra et al. 2005). Several ingredients such as wheat flour, soybean flour, starch, oil, salt, other spices and preservatives are added to meat for production of sausages. Preservatives are usually used to improve the quality, shelf life and safety of the final product (Sultana et al. 2014). Cooked beef-sausage (CBS) is the most common sausage consumed in some countries of the Middle East, e.g., Iran. Similar with many other meat products, sausages are prone to biochemical destroying interactions such as oxidation, formation of off-flavors, and also contamination by pathogens and spore forming bacteria. To avoid deterioration, food additives such as salts, sugars, and other chemicals such as nitrite and nitrate have been supplemented in the formulation of meat products (Sebranek and Bacus 2007).
Among regular additives used in meat products, sodium nitrate is basically preferred as the antimicrobial agent, in particular against Clostridium botulinum by prevention of toxin formation (Huang et al. 1996). Additionally, sodium nitrate postpones the oxidative reactions in meat products, thereby it is considered as a key component for manifesting the desired pink color of sausage (Sebranek and Bacus 2007). To reach these aims, addition of 120 ppm sodium nitrate in the meat derived products formulation has been suggested (Epley et al. 1992). Nevertheless, using high concentration of nitrate in food products has been forbidden, due to the risk of the formation of ‘nitrosamine’ during the cooking step or storage (Honikel 2008). This hazardous metabolite of nitrite is known as a possible carcinogen (Oostindjer et al. 2014). Sodium nitrate may lower the content of vitamin A in human liver and interfere with the normal functions of thyroid gland. Given these disadvantageous, alternative natural preservatives have been highly requested (Banon et al. 2007). Recently, a number research projects have been carried out to find alternatives for sodium nitrate in meat products. The alternatives should possess antimicrobial and antioxidant activity and also retain the present organoleptic characteristics of the product. Some examples include the extract of grape and rosemary plant and chitosan from fish (Banon et al. 2007; Balentine et al. 2006; Corpet 2011; Oostindjer et al. 2014; Mourouti et al. 2015). Despite many advances, this chemical is still considered as the main additive to keep the safety of sausage.
Humulus lupulus L. (Hop) is a perennial and climbing plant from the family of Cannabaceae, mostly produced in humid-tropical regions (Zanoli et al. 2005). Breweries are the main customers of hop producers due to the special flavor of ingredients of hops (Siragusa et al. 2008). Antioxidant, antimicrobial and antitumor activity along with number of health inducing-applications have been approved in the case of hop components (Proestos et al. 2013). Bitter resins including α- and β-acids are the main components of hop extract (Tanaka et al. 2014). While α-acids are broadly used in beer to induce appropriate flavor, β-groups are less bitter and possess antimicrobial activity (Villalobos-Delgado et al. 2015). Lupulon is the main component of β-acids with the chemical formula C26H38O4 (Siragusa et al. 2008). Another interesting component of hop is xanthohumol C21H22O5, a chalcone polyphenol with high antioxidant activity (Tanaka et al. 2014). These natural components have been recommended as substitutes for chemical additives in food products (Villalobos-Delgado et al. 2015). Nevertheless, a new technological approach should be employed to prevent the loss of the biological activity of these components during heat treatment and post-processing, and to mask possible bitter taste which may adversely affect the organoleptic properties.
Encapsulation is an emerging technology to maintain the biological activity of bioactive compounds during the manufacturing of food and pharmaceutical ingredients and for the delivery of those to the cells (Zanetti et al. 2018). High dispersibility uniform shape of particles, high specific surface area, improved solubility, thermodynamic stability and controlled release are some of the techno-functional advantageous which are induced by proper encapsulation process (Zanetti et al. 2018; Khalesi et al. 2014). Bioactive compounds inside the encapsulating materials may be retained for longer time during processing and post-processing, and less prone to chemical deterioration phenomena and physical shocks. The release rate of bioactive ingredients and the stability of encapsulating materials determine the applications of the encapsulation system. Liposomes are promising encapsulation vehicles with a hydrophilic inner core covered by phospholipids, which composed of mixtures of positively or negatively charged head tail (Nkanga et al. 2017).
In this study, lupulon and xanthohumol were co-encapsulated in nanoliposome fabricated with optimized sonication method. The release of active ingredients was analysed by different models. The nanoliposomes were supplemented to CBS formulation in different amounts to (partially) replace sodium nitrite. The products were assessed in regards of microbiological safety, chemical stability (oxidation) and organoleptic properties during storage.
Materials and methods
Materials
Egg yolk lecithin was purchased from Sigma Aldrich (St. Louis, MO). Lupulon and xanthohumol were supplied by Hopsteiner (Mainburg, Germany). Beef was purchased from local market, and the materials needed for the sausage formulation was purchased from Dastpaz knowledge-based company (North Khorasan Razavi, Iran). Microbial culture media were purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade.
Nanoliposome production and optimization
Manufacturing of nanoliposomes was performed following the procedures described previously with some modifications (Fatouros and Antimisiaris 2001; Paini et al. 2015). A pulse sonicator (HD3200 Bandelin electronic GmbH & Co., Berlin, Germany) (2 s on/2 s off) was employed for the formation of nanoliposomes. Optimization was performed considering the response surface methodology (RSM). The variables for optimization were considered to be the concentration of lecithin (20–180 g/mL), the time of sonication (up to 21 min), and the power of device (30–150 W). The yield of encapsulation of lupulon and xanthohumol were evaluated to obtain the optimized condition (Khatib et al. 2019).
Modelling the release of lupulon and xanthohumol
The kinetic release of lupulon and xanthohumol from nanoliposome was studied. For this purpose, a liquid medium with same characteristics as meat was prepared as a model food system (Corbo et al. 2016). This model food consisted liquid meat extract (LMEx) (5 g/L), peptone (10 g/L), triptone (5 g/L), potato starch (12 g/L), nitrite and nitrate (120 ppm), lactose (0.4%), salt (5%) and black pepper (0.1%). LMEx was prepared by addition of meat sample (5 g) mixed with 40 mL of Na2HPO4 (15.6 mM)/KH2PO4 (3.5 mM) (1:1, pH = 7.5) for 1 min using an Ultra-Turrax homogenizer (IKA T25 basic, IKA-Werke GmbH & Co., Staufen, Germany). After that, the mixture was centrifuged (4000 g, 20 min, 4 °C). Transparent supernatant was collected and considered as the enriched sarcoplasmic protein sample. The residue was mixed with KCl (0.45 M), Na2HPO4 (15.6 mM) and KH2PO4 (3.5 mM) (pH = 7.5) and homogenized for 1 min. The sample was centrifuged again (4000 g, 20 min, 4 °C). The obtained supernatant was considered as the myofibril protein. A mixture of sarcoplasmic and myofibril samples was considered as the LMEx. Nanoliposomes were added to this model food and the release of active ingredients was investigated.
To measure the concentration of xanthohumol and lupulon released to the model food, the samples were subjected to Amicon Ultra centrifugal filter units (MW cut off 10 kDa, Millipore Ltd., MA, USA). The filtrate was injected to HPLC (Knauer, Germany) equipped with C18 column (Sphere-Image ODS2, BISCHOFF, Germany) and Smart-line UV Detector 2600 (Knauer, Germany). Mobile phases were considered to be water containing (0.025%, v/v) formic acid and methanol containing formic acid (0.025%, v/v). The absorbance at wavelengths of 314 nm (for β-acids) and 370 nm (for xanthohumol) were recorded (De Keukeleire et al. 2003). The concentrations were obtained by the comparison of the peak areas of the samples detected at certain elution time with those of calibration curve achieved after injection of references within the concentration ranges of 1–100 ppm.
The release kinetics of lupulon and xanthohumol was fitted with zero-order (C = Kt), first-order (C =[1 − exp(−Kt)]×100), Higuchi (C = Kt0.5) and Rigter–Peppus (C = Ktn) models (Dash et al. 2010; Fathi et al. 2013). In these models, C is the concentration of released components at the time of t, K represents kinetic constant, and n describes the release exponent which governs the release model mechanism. The n value lower than 0.45 describes the release model based on the Fician diffusion mechanism, while dissolution mechanism dominants for n value greater than 0.89. Values in between correspond to the combination mechanism.
Production of cooked beef-sausage
The ingredients used to produce CBS in pilot plant located in the Department of Food Science and Technology (Shiraz University, Iran) included minced beef (60 g/100 g), ice water (18.51 g/100 g), acidified potato starch (2.8 g/100 g), wheat gluten (1.7 g/100 g), soybean oil (12.5 g/100 g), sodium phosphate (0.4 g/100 g), ascorbic acid (0.05 g/100 g), sausage spice (0.9 g/100 g), salt (1.5 g/100 g), milk powder (1.5 g/100 g), carrageenan (0.2 g/100 g), and nitrite/L–X-NL (0.012–0.020 g/100 g).
First of all, beef was grinded using a meat grinder machine (Philips, HR2743, Amsterdam, Netherlands). Next, all ingredients were mixed together and different batches were produced. The mixture of sodium nitrate/L–X-NL was added at different mixing amounts: CBSC (0 ppm:0 ppm), CBS1 (120 ppm:0 ppm), CBS2 (90 ppm:50 ppm), CBS3 (60 ppm:100 ppm), CBS4 (30 ppm:150 ppm), and CBS5 (0 ppm:200 ppm).
When the preservative was only nitrite, we added 120 ppm, and when it was only L–X-NL, we added 200 ppm. The amount of nanoliposome was considered on the basis of the data obtained for antioxidant and antimicrobial activity of loaded nanoliposome (Khatib et al. 2019). The lupulon/xanthohumol ratio was considered to be 4:1.
The products were packaged in polyamide plastics and cooked at 75 °C for 60 min. After that, they were cooled down and stored at 4 °C for further experiments. The duration of storage was considered to be 30 days. Sensory evaluation, microbial analysis, lipid oxidation, colorimetery and pH determination were carried out during storage in the days of 0, 8, 15, 22 and 30.
Proximate analysis of cooked beef-sausage
Protein, carbohydrate and lipid contents of CBS were assessed based on the standard methods of Association of Official Analytical Chemists (AOAC). Moisture content (on the wet basis) was obtained by calculation of the difference between the sausage weight (5.0 g) before and after dying (105°, 4 h). The pH of samples (10.0 g CBS mixed with 90 mL distilled water) was measured using a pH meter (room temperature, n = 3).
Lipid oxidation measurement
To measure lipid oxidation during storage, tiobarbiotic acid reactive substances (TBARS) were analysed using the method described by Pfalzgraf et al. (1995). Briefly, TCA (20 g) was dissolved in 100 mL H3PO4 (2 M). A sample of minced meat sausage was mixed with 20 mL of TCA solution for 2 min. Mixture was diluted with 50 mL of distilled water and filtrated by paper filter. This extract (5.0 mL) was added to the tube containing thiobarbituric acid (TBA) solution (0.01 M, 5.0 mL) and maintained in water bath (90 °C) for 60 min. The absorbance was measured at λ = 532 nm. The amount of malon dialdehyde (MDA) in the samples was calculated using Eq. (1):
| 1 |
Colorimetery
To evaluate the color of the samples, digital imaging and Photoshop software (Adobe Systems Inc., San Jose, California, USA) were used according to Afshari-Jouybari and Farahnaky (2011). A box with dimension of 50 × 50 × 60 cm with natural daylight source was prepared and a digital camera (Canon Powershot A540 with 6 Mega Pixels) positioned vertically at 25 cm distance from the samples. The images were taken at resolution of 2816 × 2112 pixels and corresponding value of each region was measured by filter/blur/average command in Photoshop software (Yam and Papadakis 2004). Here, Li (black–white indicator), ai (red–green indicator) and bi (yellow–blue indicator) were considered as color indicators for treated samples.
Microbial analysis
Microbial analyses (including total counts, coliforms, mold and yeast, Staphylococcus aureus, Salmonella and Chlostridium prefrenges) were carried out. To perform theses analyses, the outside of the package was disinfected by ethanol 70%. The polyamide package was cut by a sterile cutter. Sausage sample (25 g) was added to sterile normal saline solution (225 g) to obtain a dilution of 10−1. The sample was homogenized for 2 min at sterile condition. From this, dilutions of 10−2 to 10−9 were prepared. Total count was performed using plate count agar (PCA) media, at 30 °C for 72 h following the standard method. Coliforms were counted on violet red bile agar (VRBA). The media of sulfite polymyxin sulfadiazine agar (SSA) was used to measure the number of Chlostridium prefrenges (incubation at 37 °C for 24 h). For Salmonella, Rappaport–Vassiliadis Salmonella enrichment broth (RV broth), tetrathionate broth base (TTB) and brilliant green agar (BGA) were used. For counting Staphylococcus aureus, Baird Parker agar (BPA) (incubation at 37 °C for 48 h), and for molds and yeasts, Sabouraud dextrose agar (SDA) (incubation at 25 °C for 5 days) were used (Filimon et al. 2010; Šojić et al. 2015; Tayel 2016).
Sensory evaluation
After microbial analysis, sensory evaluation of prepared sausages was carried out by nine skilled assessors (including 6 females and 3 males with age range 20–35 years) on the safe samples based on the oral and non-oral attributes. The samples were uncovered and kept at room temperature for 15 min prior to the analysis. A piece of sausage with a thickness of 1 cm was provided for each panelist under the natural light. Evaluation was performed in hedonic scale (5 points) (Lim 2011; Kilcast and Clegg 2002; Siripatrawan and Noipha 2012; Soukoulis et al. 2010). The evaluated parameters in questioning forms included taste, color, texture and total acceptance. Distilled water and rice cake were provided for panelists to drain their mouth within analysis of different samples. Analysis of results was based on the method of Qin et al. (2014) with slight modifications.
Statistical analysis
Design expert DX11 was used to determine the optimization condition of manufacturing nanoliposomes based on the RSM. For other data, all experiments were performed in triplicates, and mean values ± standard deviation (SD) were reported. Analysis of variance was performed on the data, and the means were compared using “Duncan” test (5% significant level).
Results and discussion
Evaluation of lupulon–xanthohumol nanoliposome: experimental and model study
Based on the results of RSM, the optimized condition for ultrasonication device was 10.8 min, 72.7 W, and lecithin concentration of 140 mg/mL. The correlation between these factors and the efficiency of encapsulation (EE%) of lupulon and xanthohumol are shown in Supplementary Fig. 1.
The concentration of lupulon and xanthohumol released from the nanoliposome to the LMEx was determined using HPLC during 10 days. A typical chromatogram is shown in Supplementary Fig. 2. The elution time for xanthohumol was 18.8 min; while the fraction of lupulon was split into two regions (i.e., 23.3 and 23.9 min).
Figure 1 shows the release (%) of lupulon and xanthohumol to the LMEx during experiment. A rapid release of lupulon and xanthohumol was observed in the beginning of experiment. During 5 days, around 50–60% of each component was released. The release of lupulon was stopped at this time; while xanthohumol releasing continued for two other days, which caused releasing up to 80%. The maximum release amount was observed to be occurred in the first day of experiments, equal to 20% and 40% for lupulon and xanthohumol, respectively. The difference in rate of releasing between lupulon and xanthohumol perhaps is attributed to the difference in position of these components in nanoliposome. It seems that higher rate of release in the case of hydrophobic xanthohumol corresponds to the release from the phospholipid bilayer comparing with the release from the hydrophilic core of the system (which likely lupulon is located). After 8 days, no significant changes were recorded. Considering the ratio of lupulon:xanthohumol (i.e. 4:1) in nanoliposomes, it can be concluded that in seven-day retention of nanoliposomes in LMEx, around 67% of total active ingredients were released.
Fig. 1.

The percentage of lupulon and xanthohumol released from lecithin-nanoliposome to the model food system during 10 days
It was previously reported that the maximum release rate of lupulon and xanthohumol from nanochitosan occurred in the first 4 h of the experiment (Leonida et al. 2018). The authors observed eight-fold higher release for lupulon than xanthohumol, corresponded to the difference in hydrophobicity of those compounds. The difference between the structure of encapsulant (which induce different interactions and stability) and the difference in the location of core materials in the nanoliposome are the main reasons for the difference between those results and the current study.
Stability of encapsulant and releasing condition determine the efficacy of nanoliposome. A release model of loaded components is associated to the concentration of target materials (in this case lupulon and xanthohumol) in the model food as a function of a certain time. Among a number of mathematical models proposed for prediction of loaded components released to the liquid media, we evaluated zero-order, first-order, Higuchi, and Rigter–Peppus models. Table 1 lists the kinetic constant (K), correlation coefficients (R) and release exponent (n) (where applicable) for lupulon and xanthohumol releasing obtained from different assessed models.
Table 1.
Model parameters of lupulon and xanthohumol release
| Model | Zero order | First order | Higuchi | Rigter–Peppas | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | K | R | RMSE | K | R | RMSE | K | R | RMSE | K | N | R | RMSE |
| Lupulon | 7.753 | 0.664 | 11.770 | 0.124 | 0.880 | 7.021 | 20.99 | 0.928 | 5.426 | 21.55 | 0.486 | 0.929 | 5.605 |
| Xanthohumol | 10.17 | 0.372 | 18.090 | 0.214 | 0.828 | 9.473 | 27.83 | 0.904 | 7.047 | 36.12 | 0.358 | 0.946 | |
It seems that Higuchi and Rigter–Peppus are fitted for prediction of hop components’ release. The R value obtained from the model of Rigter–Peppus was the highest among all examined models, in particular for xanthohumol. The n value obtained for lupulon and xanthohumol were 0.486 and 0.358, respectively. This indicates that the release model in the case of lupulon followed a combination of Fician diffusion and dissolution mechanism, while in the case of xanthohumol followed Fician diffusion. The zero-model showed the lowest K and R values for both components.
Proximate analysis of the cooked beef-sausage
The results of compositional analysis of different CBS samples showed that the percentages of fat, proteins, carbohydrates, moisture and ash are equal to 16.73 ± 0.47, 15.24 ± 0.92, 9.40, 56.12 ± 0.87, and 2.51 ± 0.06, respectively. Changes in the pH of different CBS samples during 30-day storage (4 °C) are given in Table 2. As shown, pH was slightly reduced during 8 days of storage. After this period, a slight increase was observed. Except for the control sample without preservative (CBSC), this trend continued until the day 15, and then remained almost constant. As expected, pH of CBSC was continually increased, perhaps due to the metabolites formed from high microbial contamination and other chemical reactions such as oxidation.
Table 2.
Changes in pH and growth of microorganisms during 30-day storage (4 °C) of cooked beef sausage (CBS) treated by preservations
| Measured factor | Sample | 0 day | 8 days | 15 days | 22 days | 30 days |
|---|---|---|---|---|---|---|
| pH | CBSC | 6.08 ± 0.07 | 6.12 ± 0.12 | 6.41 ± 0.09 | 6.79 ± 0.09 | 7.01 ± 0.12 |
| CBS1 | 6.12 ± 0.11 | 5.92 ± 0.06 | 5.90 ± 0.14 | 6.12 ± 0.11 | 6.17 ± 0.08 | |
| CBS2 | 6.14 ± 0.13 | 6.10 ± 0.21 | 6.08 ± 0.11 | 6.12 ± 0.12 | 6.18 ± 0.14 | |
| CBS3 | 6.03 ± 0.10 | 5.86 ± 0.13 | 5.91 ± 0.12 | 5.98 ± 0.09 | 6.11 ± 0.11 | |
| CBS4 | 6.14 ± 0.08 | 5.98 ± 0.13 | 6.12 ± 0.15 | 6.17 ± 0.12 | 6.19 ± 0.16 | |
| CBS5 | 6.18 ± 0.12 | 6.07 ± 0.08 | 6.14 ± 0.12 | 6.20 ± 0.10 | 6.27 ± 0.11 | |
| Clostridium perfringens (logcfu/g) | CBSC | < 1.7* | 4.34 ± 0.42 | 4.3 ± 0.71 | 5.63 ± 1.31 | 7.61 ± 0.74 |
| CBS1 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | 3.81 ± 0.61 | |
| CBS2 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | |
| CBS3 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | 1.83 ± 0.96 | |
| CBS4 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | |
| CBS5 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | < 1.7 | |
| Coliform (logcfu/g) | CBSC | < 1 | 2.42 ± 2.11 | 3.87 ± 0.71 | 6.87 ± 1.09 | 12.39 ± 2.87 |
| CBS1 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| CBS2 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| CBS3 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| CBS4 | < 1 | < 1 | < 1 | < 1 | 1.8 ± 0.78 | |
| CBS5 | < 1 | < 1 | 2.41 ± 0.82 | 2.62 ± 0.29 | 3.01 ± 0.73 | |
| Total count | CBSC | 3.4 ± 0.1 | 4.2 ± 0.0 | 5.2 ± 0.0 | 7.3 ± 0.0 | 6.2 ± 0.0 |
| CBS1 | 3.9 ± 0.1 | 4.6 ± 0.0 | 5.2 ± 0.0 | 6.3 ± 0.1 | 5.9 ± 0.1 | |
| CBS2 | 2.5 ± 0.1 | 3.6 ± 0.0 | 4.7 ± 0.1 | 5.9 ± 0.1 | 5.1 ± 0.1 | |
| CBS3 | 2.8 ± 0.1 | 3.7 ± 0.1 | 4.2 ± 0.1 | 5.1 ± 0.0 | 4.8 ± 0.1 | |
| CBS4 | 3.1 ± 0.1 | 5.1 ± 0.1 | 5.7 ± 0.1 | 6.2 ± 0.0 | 6.1 ± 0.1 | |
| CBS5 | 4.0 ± 0.1 | 3.9 ± 0.2 | 5.1 ± 0.1 | 6.5 ± 0.1 | 5.6 ± 0.0 | |
| Mold and yeast | CBSC | 0 | 2.4 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.1 | 4.3 ± 0.0 |
| CBS1 | 0 | 0.7 ± 0 | 1.5 ± 0.1 | 2.2 ± 0.0 | 2.8 ± 0.0 | |
| CBS2 | 0 | 0 | 0 | 0 | 0 | |
| CBS3 | 0 | 0 | 1.2 ± 0.1 | 1.9 ± 0.1 | 2.1 ± 0.0 | |
| CBS4 | 0 | 1.0 ± 0.0 | 1.3 ± 0.0 | 1.7 ± 0.0 | 2.6 ± 0.1 | |
| CBS5 | 0 | 1.7 ± 0.1 | 2.7 ± 0.1 | 3.1 ± 0.0 | 3.8 ± 0.1 |
CBSC, CBS1, CBS2, CBS3, CBS4 and CBS5 represent CBS without preservation, treated by 120 ppm nitrite, 90 ppm nitrite and 50 ppm lupulon–xanthohumol nanoliposome (L–X-NL), 60 ppm nitrite and 100 ppm L–X-NL, 30 ppm nitrite and 150 ppm L–X-NL, and 200 ppm L–X-NL, respectively
*The maximum acceptable CFU/g for C. perfringens and coliform is 1.7 and 1, respectively
The results demonstrated that removal of nitrite and replacement with L–X-NL did not significantly impact on pH. A slight difference might be due to the presence of weak nitric acid generated during conformation of nitrite to nitrate.
Microbial analysis of cooked beef-sausage with different ratio of preservations
Analysis of total count was carried out (Table 2). The maximum acceptable amount of C. perfringens in meat product has been reported to be 50 CFU/g while the maximum acceptable amount of coliforms in meat product has been reported to be 10 CFU/g. In the Table 2, the maximum log CFU/g has been considered for the limitation of the microbial counts. Accordingly, the maximum acceptable CFU/g for C. perfringens and coliform was found to be 1.7 and 1, respectively. The growth of microorganisms during the storage period was observed in all samples. While the highest growth corresponded to the CBSC, samples CBS3 and CBS2 showed the least contamination. This demonstrates the synergistic effect of nitrite and L–X-NL to postpone microbial contamination.
Table 2 shows the results of the growth of C. perfringens and coliforms. As it is indicated, C. perfringens was inhibited effectively by addition of L–X-NL and/or nitrite. Only for the sample CBSC and sample treated by 50 ppm L–X-NL, growth of C. perfringens was high. Spores of C. perfringens may tolerate high concentration of salt in sausage (Hugo and Hugo 2015). They can also reactivate within the processing steps such as wet cooking (which may increase the water activity). Inactivation of these spores using synthetic or natural additives is highly important. In a study, the authors recommended using natural additive derived from Annatto to 60% replacement of nitrite (Zarringhalami et al. 2009). Lupulon has been also previously proposed as an effective plant based additive to inactivate C. perfringens (Siragusa et al. 2008). In that in vivo study, the authors showed that after 22-day feeding of chickens with or without supplemented lupulon in their diets, the growth of C. perfringens in control chickens was significantly greater. Accordingly, authors proposed lupulon as a promising alternative for antibiotics. In addition, effectiveness of lupulon against spore forming bacteria during heat processing has been reported (Siragusa et al. 2008). Positive effect of hop β-acids to inactivate C. perfringens and C. difficile with concentration of 1 ppm has been indicated as well (Examiner and Saucier 2001).
Our results showed that samples of CBSC and CBS5 contaminated by high levels of coliform. Moreover, sample CBS4 contained high amount of coliform in the last day of experiment (day 30). In better words, addition of L–X-NL in concentration of 200 ppm to CBS was insufficient to prevent the growth of coliforms, suggesting use of the blend of this complex with an effective antimicrobial agent with the capability to inhibit coliforms. Presence of phospholipid in the formulation of nanoliposome may be a reason for poor efficacy of L–X-NL against coliforms. Gram negative bacteria are probably more resistant against lupulon than gram positives. The lowest count of coliforms corresponded to samples with higher amounts of nitrite, which confirms the active role of nitrite (as expected) against these contaminants.
While all samples were contaminated by yeasts and molds, CBSC and CBS5 were more prone to these contaminants than the samples with higher ratio of nitrite (Table 2). Effect of nitrite on the yeasts and molds was observed to be critical. Several parameters such as quality of raw meat, processing conditions, period and temperature of storage, and the packaging may alter the count of yeasts and molds.
Except for control sample (CBSC), other samples were not contaminated by S. aureus. This contaminant is mostly associated to the meat quality and the storage conditions, rather than the processing steps. No contamination was detected by Salmonalla.
Previously, acidification of medium was proposed to improve the microbial quality of some products such as milk, Cottage cheese and coleslaw, via enhancing the antimicrobial impact of hop extract against some microorganisms, e.g. Listeria. Probably, ascorbic acid in the formula and nitrite as preservative may play synergistic role to improve the efficiency of antimicrobial activity of hop compounds in meat products.
In summary, microbial analysis of the samples treated by L–X-NL and nitrite indicated that in all treatments (except for the CBSC), the microbial growth was insignificant in 30 days of storage showing the efficiency of preservatives (especially in combination), high quality of meat, adequacy of manufacturing process, and proper condition of storage. This means that the shelf life of the sausage containing L–X-NL (100 ppm) in combination with nitrite (60 ppm) could be similar as the same product containing nitrite (120 ppm) as the only preservative.
Lupulon–xanthohumol nanoliposome prevents lipid oxidation
The shelf-life of CBS is not only related to microbial safety, but also to physicochemical reactions. Lipid oxidation is a deteriorative reaction occurred as a result of exposing lipid contained products to oxygen. Lipid oxidation was indicated by measuring the amount of TBARS. The acceptable amount of TBA in food products has been reported to be lower than 1 mg MDA/kg. We recently obtained IC50 value of antioxidant activity possessed by L–X-NL equal to 60.38 µg/mL (Khatib et al. 2019), which is comparable with some synthetic antioxidants.
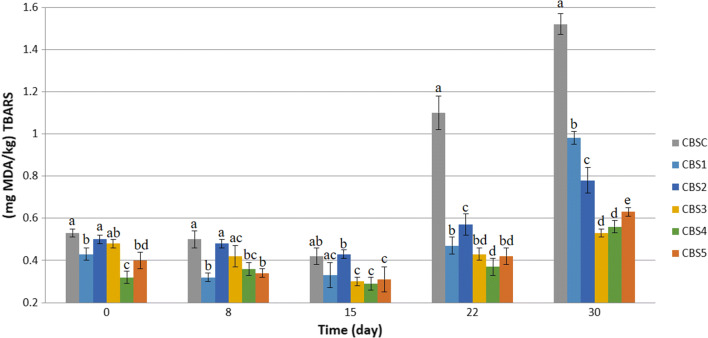
Figure 2 shows the trend of lipid oxidation in CBS samples supplemented by different preservation ratios. As expected, the highest amount of TBARS was related to sample CBSC. The trend shows that oxidative reaction has been promoted in the middle of storage period. Addition of 100 ppm and 150 ppm L–X-NL in combination with nitrite successfully prevented the development of lipid oxidation in the samples. The amount of TBARS in CBS3 and CBS4 was equal to 0.53 and 0.56 mg MDA/kg, respectively.
Fig. 2.
Lipid oxidation during 30-day storage (4 °C) of cooked beef sausage (CBS) treated by preservations. CBSC, CBS1, CBS2, CBS3, CBS4 and CBS5 represent CBS without preservation, treated by 120 ppm nitrite, 90 ppm nitrite and 50 ppm lupulon–xanthohumol nanoliposome (L–X-NL), 60 ppm nitrite and 100 ppm L–X-NL, 30 ppm nitrite and 150 ppm L–X-NL, and 200 ppm L–X-NL, respectively. Different letters on columns for each day indicate significant differences (p < 0.05)
Previous reports showed that nitrite in cured meat products postponed oxidation via scavenging iron, which is the catalyzer of oxidation reaction (Devatkal et al. 2010).
Small reduction of MDA observed in some samples at the beginning of storage could be due to degrading effect of some flora in the product. Indeed, conversion of MDA to other metabolites such as acids and alcohol is occurred. Interaction of MDA with other components in CBS, especially carbohydrates and proteins may reduce MDA as well. Georgantelis et al. (2007) reported a reduction of MDA in pork stored at 4 °C for 15 days.
Colorimetery
Table 3 represents the differences between the color factors (L, a, b) obtained by digital imaging for samples. Sample CBS1 contained only nitrite showed significant difference with other samples in regards of L, a, and b parameters. Maximum value of “a” factor was related to CBS1 showing that the redness of samples mainly corresponded to the nitrite. Low lightness (L factor) and high yellowness (b factor) might be due to biological and physicochemical modifications such as microbial generation and lipid oxidation. These effects were significant for the control sample. While sample CBS1 showed the highest ‘a” and “L” levels, sample CBS5 showed the highest “b” level in accordance with minimum oxidation reaction. In similar study, Marazzeq et al. (2015) replaced nitrite with olive leaves extract to preserve sausage and observed reducing lightness after replacement (Marazzeq et al. 2015). Sample CBS5 (with only L–X-NL as preservative) showed slight lower “a” factor due to lack of nitrite. However, partial replacement with nitrite (30 ppm) resorted the redness. In better words, only 30 ppm nitrite was sufficient amount for inducing proper redness and the rest could be likely replaced with potential safe, natural preservatives such as hop components. Horsch et al. (2014) reported that the yellowness of sausage was not changed as a function of time. Some authors also observed a correlation between the lightness of meat products to the amount and type of carotenoids, moisture content and water holding capacity of the products (Rodríguez-Sánchez et al. 2009).
Table 3.
Color changes during 30-day storage (4 °C) of cooked beef sausage (CBS) treated by preservations
| Sample | Factor | Time (day) | ||||
|---|---|---|---|---|---|---|
| 0 | 8 | 15 | 22 | 30 | ||
| CBSC | L | 57.42 ± 2.20 | 56.15 ± 3.12 | 54.41 ± 3.32 | 52.63 ± 4.12 | 51.62 ± 3.02 |
| a | 19.97 ± 1.09 | 19.73 ± 2.63 | 19.19 ± 1.93 | 18.82 ± 2.23 | 16.23 ± 1.11 | |
| b | 18.34 ± 1.77 | 18.78 ± 3.15 | 21.17 ± 1.12 | 22.41 ± 2.61 | 22.06 ± 2.24 | |
| CBS1 | L | 56.97 ± 2.91 | 56.82 ± 3.98 | 56.02 ± 4.18 | 55.14 ± 5.42 | 55.03 ± 3.41 |
| a | 21.43 ± 2.02 | 21.70 ± 1.71 | 20.51 ± 2.21 | 20.01 ± 1.42 | 19.62 ± 1.92 | |
| b | 19.64 ± 2.52 | 19.22 ± 1.41 | 20.16 ± 1.11 | 20.94 ± 2.28 | 22.15 ± 1.64 | |
| CBS2 | L | 56.89 ± 3.61 | 56.24 ± 5.23 | 55.12 ± 4.15 | 54.77 ± 3.12 | 53.96 ± 5.70 |
| a | 21.62 ± 2.21 | 21.14 ± 1.12 | 20.71 ± 0.93 | 19.79 ± 1.29 | 19.42 ± 1.62 | |
| b | 18.98 ± 2.36 | 19.37 ± 2.02 | 20.15 ± 1.14 | 21.04 ± 2.41 | 22.24 ± 1.48 | |
| CBS3 | L | 57.14 ± 3.16 | 56.90 ± 4.89 | 56.21 ± 2.15 | 55.43 ± 3.93 | 54.91 ± 2.52 |
| a | 20.82 ± 1.97 | 20.53 ± 2.14 | 20.14 ± 1.71 | 19.61 ± 2.12 | 18.98 ± 2.01 | |
| b | 18.14 ± 1.92 | 18.90 ± 1.61 | 19.46 ± 2.04 | 20.11 ± 1.24 | 21.38 ± 1.32 | |
| CBS4 | L | 57.12 ± 3.14 | 56.32 ± 3.98 | 55.06 ± 2.11 | 54.43 ± 4.12 | 53.21 ± 3.02 |
| a | 21.47 ± 2.31 | 21.19 ± 1.52 | 20.62 ± 2.12 | 20.23 ± 1.37 | 19.56 ± 1.14 | |
| b | 18.11 ± 1.61 | 19.09 ± 0.82 | 19.56 ± 1.43 | 20.64 ± 2.14 | 20.93 ± 1.82 | |
| CBS5 | L | 57.06 ± 3.08 | 56.23 ± 3.12 | 55.40 ± 3.83 | 53.08 ± 4.51 | 51.38 ± 2.22 |
| a | 20.18 ± 1.15 | 19.22 ± 2.43 | 18.47 ± 1.14 | 17.24 ± 1.15 | 15.83 ± 2.12 | |
| b | 18.52 ± 1.26 | 18.93 ± 2.17 | 19.74 ± 2.18 | 20.64 ± 1.12 | 21.92 ± 0.87 | |
CBSC, CBS1, CBS2, CBS3, CBS4 and CBS5 represent CBS without preservation, treated by 120 ppm nitrite, 90 ppm nitrite and 50 ppm lupulon–xanthohumol nanoliposome (L–X-NL), 60 ppm nitrite and 100 ppm L–X-NL, 30 ppm nitrite and 150 ppm L–X-NL, and 200 ppm L–X-NL, respectively
Effects of lupulon–xanthohumol nanoliposome on sensory evaluation of cooked beef-sausage
In order to investigate the capability of L–X-NL as alternative preservative of nitrite, the samples CBS1–CBS5 verified as safe products and were selected for sensory evaluation. The analysis was performed after storage (4 °C) of products for 20 days.
Table 4 presents the mean value of the grades given by assessors. Regarding the taste, odor and texture, no statistically significant difference was reported by the assessors. The least grade of the color of samples was related to CBS5 (which did not contain nitrite). This may associate to positive impact of nitrite on visual property of the products. However, this criterion did not influence on the final acceptance of the products, except for sample CBS5 which the mean grade acceptance was significantly lower. Moreover, the mean grade acceptance of sample CBS1 was slightly higher than other samples.
Table 4.
Sensory evaluation of cooked beef sausage (CBS) treated by preservations
| Sample | Taste | Odor | Texture | Color | Customer acceptance |
|---|---|---|---|---|---|
| CBS1 | 4.17 ± 0.35a | 4.23 ± 0.42a | 4.42 ± 0.43a | 4.77 ± 0.48a | 4.38 ± 0.53a |
| CBS2 | 3.90 ± 0.20a | 4.12 ± 0.42a | 4.16 ± 0.51a | 4.63 ± 0.46a | 4.26 ± 0.42a |
| CBS3 | 4.25 ± 0.44a | 4.30 ± 0.29a | 4.61 ± 0.56a | 4.75 ± 0.32a | 4.56 ± 0.61a |
| CBS4 | 4.29 ± 0.47a | 3.92 ± 0.57a | 4.78 ± 0.38a | 4.02 ± 0.52a | 4.52 ± 0.51a |
| CBS5 | 3.74 ± 0.48a | 4.17 ± 0.47a | 4.60 ± 0.39a | 3.11 − 0.36b | 3.44 ± 0.35b |
CBS1, CBS2, CBS3, CBS4 and CBS5 represent CBS treated by 120 ppm nitrite, 90 ppm nitrite and 50 ppm lupulon–xanthohumol nanoliposome (L–X-NL), 60 ppm nitrite and 100 ppm L–X-NL, 30 ppm nitrite and 150 ppm L–X-NL, and 200 ppm L–X-NL, respectively
Mean values in the same column with different letters are significantly (p < 0.05) different, as determined by Duncan’s multiple range test
Previously, Villalobos-Delgado et al. (2015) reported that addition of hop extract and hop powder to the Patty lamb with concentration of 2000 ppm could stabilize the color of the product. However, sensory evaluation showed that the taste and the odor of products treated by hop extract were not agreeable for assessors. Total acceptance of these products was also rejected. Indeed, high concentration of utilized hop in free state was the reason of that negative effect of hop extract in food ingredients.
Conclusion
Replacement of nitrite due to the potential risk of nitrosamine generation with natural preservatives has been explored in a number of studies. Hop plant, popular for brewers due to its particular odor and taste, contains a wide range of components with biological functions. Among those, lupulon and xanthohumol were selected for use as alternative of nitrite in cooked beef-sausage (CBS). These two components were encapsulated in a lecithin layer using ultrasonication device. We showed that the release of lupulon–xanthohumol loaded nanoliposome fits with the Rigter–Peppus model suggesting a combination of Fician diffusion and dissolution mechanism. After releasing, these components could possess their antimicrobial and antioxidant activity. Some microorganisms such as coliform were less sensitive to hop components. Therefore, it is necessary to combine these natural preservatives with nitrite. The presence of 60 ppm nitrite beside 100 ppm lupulon–xanthohumol loaded nanoliposome effectively prevented microbial growth and lipid oxidation, while retained the proper sensory properties of CBS. This preservative is recommended for supplementation in other meat products to lower the consumption of hazard nitro-compounds.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank to the staff of the Center for Nanotechnology in Drug Delivery at Shiraz University of Medical Sciences for supporting the analysis of nanoliposomes. We also appreciate Hopsteiner (Mainburg, Germany) for supplying the hop components.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afshari-Jouybari H, Farahnaky A. Evaluation of Photoshop software potential for food colorimetry. J Food Eng. 2011;106:170–175. doi: 10.1016/j.jfoodeng.2011.02.034. [DOI] [Google Scholar]
- Balentine CW, Crandall PG, O’Bryan CA, Duong DQ, Pohlman FW. The pre- and post-grinding application of rosemary and its effects on lipid oxidation and colour during storage of ground beef. Meat Sci. 2006;73:413–421. doi: 10.1016/j.meatsci.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Banon S, Diaz P, Rodrıguez M, Garrido MD, Price A. Ascorbate, green tea and grape seed extracts increase the shelf-life of low sulphite beef patties. Meat Sci. 2007;77:626–633. doi: 10.1016/j.meatsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Corbo MR, Bevilacqua A, Speranza B, Di Maggio B, Gallo M, Sinigaglia M. Use of alginate beads as carriers for lactic acid bacteria in a structured system and preliminary validation in a meat product. Meat Sci. 2016;111:198–203. doi: 10.1016/j.meatsci.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Corpet DE. Red meat and colon cancer: should we become vegetarians, or can we make meat safer? Meat Sci. 2011;89(3):310–316. doi: 10.1016/j.meatsci.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm Drug Res. 2010;67(3):217–223. [PubMed] [Google Scholar]
- De Keukeleire J, Ooms G, Heyerick A, Roldan-Ruiz I, Bockstaele E Van, De Keukeleire D. Formation and accumulation of alpha-acids; beta-acids;desmethylxanthohumol; and xanthohumol during flowering of hops (Humulus lupulus L.) J Agric Food Chem. 2003;51(15):4436–4441. doi: 10.1016/j.ecoleng.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85(1):155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Epley RJ, Addis PB, Warthesen JJ (1992) Nitrite in meat. Minnesota Extension Service, pp 2–3
- Examiner P, Saucier SE (2001) (12) United States Patent FIG, A FG, B, vol 1, no 12
- Fathi M, Varshosaz J, Mohebbi M, Shahidi F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: preparation, characterization, and modeling. Food Bioprocess Technol. 2013;6(6):1464–1475. doi: 10.1007/s11947-012-0845-2. [DOI] [Google Scholar]
- Fatouros DG, Antimisiaris SG. Physicochemical properties of liposomes incorporating hydrochlorothiazide and chlorothiazide. J Drug Target. 2001;9(1):61–74. doi: 10.3109/10611860108995633. [DOI] [PubMed] [Google Scholar]
- Filimon MN, Borozan A, Bordean D, Radu F, Popescu R. Microorganisms, qualitative indicators for meat products. Anim Sci Biotechnol. 2010;43(2):346–349. [Google Scholar]
- Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA. Effect of rosemary extract, chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci. 2007;76(1):172–181. doi: 10.1016/j.meatsci.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Honikel KO. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78(1–2):68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Horsch AM, Sebranek JG, Dickson JS, Niebuhr SE, Larson EM, Lavieri NA, Ruther BL, Wilson LA. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014;96(1):400–407. doi: 10.1016/j.meatsci.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Huang YG, Ji JD, Hou QN. A study on carcinogenesis of endogenous nitrite and nitrosamine, and prevention of cancer. Mutat Res Fundam Mol Mech Mutagen. 1996;358(1):7–14. doi: 10.1016/0027-5107(96)00087-5. [DOI] [PubMed] [Google Scholar]
- Hugo CJ, Hugo A. Current trends in natural preservatives for fresh sausage products. Trends Food Sci Technol. 2015;45(1):12–23. doi: 10.1016/j.tifs.2015.05.003. [DOI] [Google Scholar]
- Khalesi M, Mandelings N, Herrera-Malaver B, Riveros-Galan D, Gebruers K, Derdelinckx G. Improvement of the retention of ocimene in water phase using Class II hydrophobin HFBII. Flavour Fragr J. 2014;30:451–458. doi: 10.1002/ffj.3260. [DOI] [Google Scholar]
- Khatib N, Varidi MJ, Mohebbi M, Varidi M, Hosseini SMH. Co-encapsulation of lupulon and xanthohumol in lecithin-based nanoliposomes developed by sonication method. J Food Process Preserv. 2019;43:e14075. doi: 10.1111/jfpp.14075. [DOI] [Google Scholar]
- Kilcast D, Clegg S. Sensory perception of creaminess and its relationship with food structure. Food Qual Prefer. 2002;13(7–8):609–623. doi: 10.1016/S0950-3293(02)00074-5. [DOI] [Google Scholar]
- Leonida MD, Belbekhouche S, Benzecry A, Peddineni M, Suria A, Carbonnier B. Antibacterial hop extracts encapsulated in nanochitosan matrices. Int J Biol Macromol. 2018;120:1335–1343. doi: 10.1016/j.ijbiomac.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Lim J. Hedonic scaling: a review of methods and theory. Food Qual Prefer. 2011;22(8):733–747. doi: 10.1016/j.foodqual.2011.05.008. [DOI] [Google Scholar]
- Marazzeq K Al, Haddadin M, Abdullah B Al, Angor M. Effect of nitrite substitution with olive leaves extract on color and sensory properties of beef mortadella. J Agric Sci. 2015;7(12):120. doi: 10.5539/jas.v7n12p120. [DOI] [Google Scholar]
- Mourouti N, Kontogianni MD, Papavagelis C, Plytzanopoulou P, Vassilakou T, Psaltopoulou T, Malamos N, Linos A, Panagiotakos DB. Meat consumption and breast cancer: a case-control study in women. Meat Sci. 2015;100:195–201. doi: 10.1016/j.meatsci.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Nkanga CI, Krause RW, Noundou XS, Walker RB. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int J Pharm. 2017;526(1–2):466–473. doi: 10.1016/j.ijpharm.2017.04.074. [DOI] [PubMed] [Google Scholar]
- Oostindjer M, Alexander J, Amdam GV, Andersen G, Bryan NS, Chen D, Corpet DE, De Smet S, Dragsted LO, Haug A, Karlsson AH, Egelandsdal B. The role of red and processed meat in colorectal cancer development: a perspective. Meat Sci. 2014;97(4):583–596. doi: 10.1016/j.meatsci.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Paini M, Daly SR, Aliakbarian B, Fathi A, Tehrany EA, Perego P, Dehghani F, Valtchev P. An efficient liposome based method for antioxidants encapsulation. Colloids Surf, B. 2015;136:1067–1072. doi: 10.1016/j.colsurfb.2015.10.038. [DOI] [PubMed] [Google Scholar]
- Pfalzgraf A, Frigg M, Steinhart H. α-Tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J Agric Food Chem. 1995;43(5):1339–1342. doi: 10.1021/jf00053a039. [DOI] [Google Scholar]
- Proestos C, Lytoudi K, Mavromelanidou O, Zoumpoulakis P, Sinanoglou V. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants. 2013;2(1):11–22. doi: 10.3390/antiox2010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y-Y, Wu Y, Zhang Z-H, Li B, Liang X-B, Cao J-X. Effect of an active film from chitosan and pomegranate rind powder extract on shelf-life extension of pork meat patties. Mod Food Sci Technol. 2014;30(4):181–188. [Google Scholar]
- Raju CV, Shamasundar BA, Udupa KS. The use of nisin as a preservative in fish sausage stored at ambient (28 ± 2 °C) and refrigerated (6 ± 2 °C) temperatures. Int J Food Sci Technol. 2003;38:171–185. doi: 10.1046/j.1365-2621.2003.00663.x. [DOI] [Google Scholar]
- Rodríguez-Sánchez JA, Ripoll G, Calvo S, Ariño L, Latorre MA. The effect of seasonality of the growing-finishing period on carcass, meat and fat characteristics of heavy barrows and gilts. Meat Sci. 2009;83(3):571–576. doi: 10.1016/j.meatsci.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Sachindra N, Sakhare P, Yashoda K, Rao DN. Microbial profile of buffalo sausage during processing and storage. Food Control. 2005;16:31–35. doi: 10.1016/j.foodcont.2003.11.002. [DOI] [Google Scholar]
- Sallam KI, Ishioroshi M, Samejima K. Antioxidant and antimicrobial effects of garlic in chicken sausage. LWT-Food Sci Technol. 2004;37(8):849–855. doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebranek JG, Bacus JN. Cured meat products without direct addition of nitrate or nitrite: what are the issues? Meat Sci. 2007;77(1 SPEC. ISS.):136–147. doi: 10.1016/j.meatsci.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Siragusa GR, Haas GJ, Matthews PD, Smith RJ, Buhr RJ, Dale NM, Wise MG. Antimicrobial activity of lupulone against Clostridium perfringens in the chicken intestinal tract jejunum and caecum. J Antimicrob Chemother. 2008;61(4):853–858. doi: 10.1093/jac/dkn024. [DOI] [PubMed] [Google Scholar]
- Siripatrawan U, Noipha S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocoll. 2012;27(1):102–108. doi: 10.1016/j.foodhyd.2011.08.011. [DOI] [Google Scholar]
- Šojić B, Tomović V, Kocić-Tanackov S, Škaljac S, Ikonić P, Džinić N, Živković N, Jokanović M, Tasić T, Kravić S. Effect of nutmeg (Myristica fragrans) essential oil on the oxidative and microbial stability of cooked sausage during refrigerated storage. Food Control. 2015;54:282–286. doi: 10.1016/j.foodcont.2015.02.007. [DOI] [Google Scholar]
- Soukoulis C, Lyroni E, Tzia C. Sensory profiling and hedonic judgement of probiotic ice cream as a function of hydrocolloids, yogurt and milk fat content. LWT—Food Sci Technol. 2010;43(9):1351–1358. doi: 10.1016/j.lwt.2010.05.006. [DOI] [Google Scholar]
- Sultana T, Rana J, Chakraborty SR, Das KK, Rahman T, Noor R. Microbiological analysis of common preservatives used in food items and demonstration of their in vitro anti-bacterial activity. Asian Pac J Trop Dis. 2014;4:452e456. doi: 10.1016/S2222-1808(14)60605-8. [DOI] [Google Scholar]
- Tanaka Y, Yanagida A, Komeya S, Kawana M, Honma D, Tagashira M, Kanda T, Shibusawa Y. Comprehensive separation and structural analyses of polyphenols and related compounds from bracts of hops (Humulus lupulus L.) J Agric Food Chem. 2014;62(10):2198–2206. doi: 10.1021/jf405544n. [DOI] [PubMed] [Google Scholar]
- Tayel AA. Microbial chitosan as a biopreservative for fish sausages. Int J Biol Macromol. 2016;93:41–46. doi: 10.1016/j.ijbiomac.2016.08.061. [DOI] [PubMed] [Google Scholar]
- Villalobos-Delgado LH, Caro I, Blanco C, Bodas R, Andrés S, Giráldez FJ, Mateo J. Effect of the addition of hop (infusion or powder) on the oxidative stability of lean lamb patties during storage. Small Rumin Res. 2015;125:73–80. doi: 10.1016/j.smallrumres.2015.02.008. [DOI] [Google Scholar]
- Yam KL, Papadakis SE. A simple digital imaging method for measuring and analyzing color of food surfaces. J Food Eng. 2004;61(1 SPEC.):137–142. doi: 10.1016/S0260-8774(03)00195-X. [DOI] [Google Scholar]
- Zanetti M, Carniel TK, Dalcanton F, dos Anjos RS, Gracher Riella H, de Araújo PHH, de Oliveira D, Antônio Fiori M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: a brief review. Trends Food Sci Technol. 2018;81:51–60. doi: 10.1016/J.TIFS.2018.09.003. [DOI] [Google Scholar]
- Zanoli P, Rivasi M, Zavatti M, Brusiani F, Baraldi M. New insight in the neuropharmacological activity of Humulus lupulus L. J Ethnopharmacol. 2005;102(1):102–106. doi: 10.1016/j.jep.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Zarringhalami S, Sahari MA, Hamidi-Esfehani Z. Partial replacement of nitrite by annatto as a colour additive in sausage. Meat Sci. 2009;81(1):281–284. doi: 10.1016/j.meatsci.2008.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.