Abstract
Purpose
Glucose dysregulation is one of the distinctive features of type 2 diabetes that is associated with an increased risk of cognitive impairment and dementia. The low concentrations of brain-derived neurotrophic factor (BDNF) are reported in people with insulin resistance, metabolic syndrome, and type 2 diabetes. BDNF can be increased by an adjustment in lifestyle including caloric restriction and exercise training. Studies have reported controversial findings about physical activity and its association with BDNF, but there is no comprehensive conclusions on this issue. The aim of this study was to systematically review the effects of exercise training on BDNF levels in patients with type 2 diabetes.
Methods
The electronic databases of Embase, Pedro, PubMed, Medline, Cochrane Library, as well as the Google Scholar search engine were used to obtain the related data about the role of exercise training on BDNF levels in patients with type 2 diabetes. The search period was set from inception to August 2019. Keywords of “exercise”, “training”, “physical activity”, “brain-derived neurotrophic factor”, “type 2 diabetes”, and “randomized clinical trials”, were used in persian and English. The PEDro scale was used to evaluate the quality of the included articles. Results. Finally, 11 articles (four human and seven animal articles) with medium to high quality were included in the study which 5 articles reported elevation (one human and four animal articles), 4 articles reported a reduction (one human and three animal articles), and 2 articles reported no changes (both of them in human articles) in BDNF level following the exercise training.
Conclusion
Decreased energy intake and increased energy expenditure through exercise training may modulate BDNF levels in patients with type 2 diabetes.
Keywords: Exercise, Training, Brain-derived Neurotrophic factor, Type 2 diabetes, Systematic review
Introduction
The prevalence of diabetes and obesity in Western societies is rapidly increasing due to the consumption of high-calorie foods and lack of physical activity [1]. Studies have shown in 2013 that there were 382 million diabetics worldwide, which is expected to reach 592 million by 2035 [2]. Type 2 diabetes is a condition characterized by insulin resistance and insufficient compensation for insulin secretion resulting in impaired glucose regulation [3]. People with diabetes are more likely to have vascular and neurological damage than healthy individuals, which may be the reason for increased rates of cardiovascular disease and cognitive decline in diabetic patients [4–7]. Diabetes mellitus leads to a wide range of problems including peripheral nerve disorders [8]. Atrophy and even neuron death can be due to decreased growth factors in diabetic neuropathy [9]. Studies have also shown that diabetes threatens the central nervous system and is associated with an increased risk of developing neurodegenerative diseases [10, 11]. A variety of neurotransmitter abnormalities have been described in association with diabetes that acetylcholine, believed to be important in mediating the cognitive effects of AD. Amongst other blood brain barrier abnormalities reported in association with diabetes is a reduced rate of choline transport which has been described in chronically diabetic rats. Mitochondrial abnormalities have been proposed as possibly mediating cerebral dysfunction in Type 2 DM independently of glycaemic control and neurotoxic processes may be involved similar to those proposed for mediating peripheral neuropathy via the aldose reductase pathway. Advanced glycation end products (AGEPs) have been identified in the matrix of neurofibrillary tangles and senile plaques in post-mortem samples of AD brains. This raises the possibility of glycation as a mechanism contributing to amyloidogenesis and the assembling of tau protein molecules into neurofibrillary tangles in AD. Recently, in vitro studies revealed AGEP-specific binding activity to apolipoprotein E (apo E). It is therefore possible that AGEPs mediate crosslinking of apo E to the insoluble fibrils and thereby participate in plaque formation in AD. On the other hand, there is in vivo evidence for glycation of tau extracted from AD brain. Perturbations in vascular homeostasis that occur in diabetes may be due to toxicity mediated through engagement of AGEPs by the RAGE receptor. Expression of RAGE also occurs in the central nervous system and it has been recently described that it’s binding by b-amyloid, also an oxidizing agent (as are AGEPs), triggers a neurotoxic cascade that may lead to neuronal loss in AD. The signaling pathway triggered by IGF or insulin appears to be important both in amyloid plaque formation and neuronal loss in AD and in amylin-induced toxicity in diabetes. Insulin receptors are known to be dense in the hippocampus, an area particularly affected in early AD and increased levels of insulin have been shown to inhibit hippocampal synaptic activity in vitro. In addition insulin has been shown to reduce choline acetyl transferase activity which could potentially lead to adverse cognitive effects secondary to depleted acetylcholine levels. An association between diabetes and AD may exist due to similar biochemical pathways controlled by IGF-I or insulin [11].
Neurotrophins are known as regulators of neuronal processes mainly through TrK receptor tyrosine kinases. Also, there is some suggestion that trophic factors are critical modifiers of the structure and function of neuromuscular networks [12]. Mammalian neurotrophins include nerve growth factors (NGF), neurotrophin-3 (NT-3), neurotrophin-4/5 (NT-4/5), and brain-derived neurotrophic factor (BDNF) levels [13, 14]. NGF was shown to be internalized by receptor-dependent mechanisms and to be transported for vast distances along axons in small membrane vesicles by an energy and microtubule-dependent mechanism with eventual degradation of NGF in lysosomes. NGF is also synthesized in mast cells and is released following mast cell activation. NT-3 expression is observed in muscle spindles and the ventral spinal cord, both targets of proprioceptive Ia afferents, consistent with NT-3 functioning as a target-derived trophic factor. These neurons are lost almost immediately after neurogenesis, however, which suggests that they depend on NT-3 provided initially by intermediate targets. BDNF and NT-4 are specific for TrkB [13]. BDNF as a most essential neurotrophic factor in the brain is thought to be the critical mechanism in the learning process, behaviors, movements, memory and a wide range of stress responses [15]. It has been suggested that BDNF can regulate and promote neurogenesis, neuroplasticity, and cell survival in the central nervous system [16]. In addition, BDNF has been reported to stimulate the production of Bcl-2 (B cell lymphoma 2), antioxidant enzymes, and proteins involved in calcium regulation that prevent the death of cultured neurons in the laboratory [17]. According to new findings, BDNF appears to be a myokine that by the autocrine or paracrine method, has strong effects on peripheral metabolism including lipid oxidation and subsequently on fat mass [18, 19]. In general, BDNF is produced and distributed by platelets in peripheral and central nervous systems, endotheliocytes, smooth muscle, immunocytes, and skeletal muscle [20]. Low levels of BDNF are associated with cognitive/learning dysfunction, depression, neurodegenerative conditions, and mortality [21–24]. The effects of diabetes on the hippocampal neuronal structure are qualitatively similar to the effects of BDNF signaling restriction. So that, the increase in trkB truncated receptor expression in combination with the reduced neuronal expression of BDNF protein in hippocampus and temporal cortex may contribute to the progressive atrophy of basal forebrain cholinergic neurons associated with Alzheimer’s disease as well as to the loss of hippocampal neuronal populations [21].
Studies have shown that serum and plasma levels of BDNF in patients with type 2 diabetic are lower than in non-diabetic individuals and high levels of cognitive impairment in diabetic individuals may be the result of low levels of BDNF [25, 26]. Similar findings have been reported in non-diabetic individuals, such that low levels of the serum BDNF are associated with insulin resistance and fat mass [27]. Conversely, other studies have found an inverse relationship between serum levels of the BDNF and these factors in type 2 diabetic patients [28].
Nutrition and exercise are the first lines of intervention to slow down the progression of metabolic disorders associated with pre-diabetes and type 2 diabetes [29]. so that, by modifying the level of circulating insulin required to maintain glucose homeostasis in insulin-resistant individuals, variations in diet composition have the ability to either accentuate, or attenuate, the manifestations of the insulin resistance [1]. Physical activity has a potential therapeutic effect on glucose regulation and cardiovascular health, while either of these is at risk may threaten cognitive integrity [30–33]. Studies have shown that increased physical activity improves cognitive function, peripheral nerve function, and vascular function through vascular remodeling, angiogenesis, and neurogenesis [34–38].In human and animal studies, growth factors with angiogenic and neurotrophic features (IGF-1, VEGF, and BDNF) are involved in vascular and neurological repair [39–41]. The results of animal studies have shown that imbalance and impairment of the response of these factors are likely the causes of vascular disorder and neuropathy in elderly and diabetic patients [42, 43]. BDNF can increase via intervention in lifestyle through the combination of caloric restriction and exercise training [44, 45]. Voluntary wheel running and caloric restriction increase the BDNF levels in the hippocampus and improve peripheral metabolism [46–49].Many studies suggest that the improvement of peripheral metabolism is caused by changes in central metabolic markers with consequences on neuronal function [50, 51]. It has been found that rats with high levels of voluntary wheel running, indicate an improved peripheral metabolism, and greater exercise-induced up regulation in the hippocampal BDNF [52]. The results of studies on animals also indicate increasing BDNF in hippocampus after activity and improvement of memory and learning processes [53]. This metabotropic hypothesis for the effects of exercise and caloric restriction on the structure and biochemistry of the hippocampus is potentially relevant to the treatment and prevention of neurodegenerative diseases. The results of previous studies on the effect of exercise training on BDNF levels are controversial not clear; some studies have shown elevated serum and plasma levels of BDNF after aerobic training, while other studies have not shown a significant change in BDNF levels after both aerobic and resistance training [37, 54–58]. Despite the importance of the subject, there is no systematic review in this area, so the present study aimed to systematically review the articles published to examine the effects of exercise training on BDNF levels in patients (human and animal) with Type 2 diabetes.
Materials and Methods
Search Strategy
The present study was a systematic review. All steps of the research, including searching, reviewing articles, and extracting the required information were performed independently by two researchers and if there were disagreements, it was solved by discussion. The electronic databases of Embase, Pedro, PubMed, Medline, Cochrane Library, as well as the Google Scholar search engine were searched to find related articles about the role of exercise training in the BDNF levels in patients with type 2 diabetes. The search period was set from the inception to August 2019. Keywords of Exercise, training, physical activity, brain-derived neurotrophic factor, type 2 diabetes, and Randomized clinical trials, were used in Persian and English.
Inclusion and Exclusion Criteria
Inclusion criteria included randomized controlled trials, having or induction of type 2 diabetes in subjects, a variety of exercise interventions, assessment of gene/protein expression of BDNF, and having no other disease. Studies without control group and also Review Articles excluded from the review. Also, search was not restricted to either human or animal articles.
Data Extraction
Final included articles reviewed and pre-prepared by a checklist and the required data was extracted. The extracted data included authors’ names, publication year and quality score, subject characteristics (sample size, mean of the age, gender, and clinical conditions), independent variables (training type, time of each session, training frequency and volume), controls group attribute, dependent variables (glucose and insulin) and the results of study.
Evaluating the Quality of Articles
The PEDro scale was used to evaluate the quality of the articles [59]. The PEDro is a qualitative scale for rating of randomized controlled trials in which the studies rate from 0 to 10; and includes 11 following criteria: specification of eligibility criteria for subjects; random and concealed allocation of subjects to groups; baseline similarity of the groups regarding the most important prognostic indicators; blinding of subjects, therapists, and assessors; measuring at least one key outcome from more than 85% of the subjects initially allocated to groups; reporting the results of between-group statistical comparisons for at least one key outcome; providing both point estimate and measures of variability for at least one key outcome.
Results
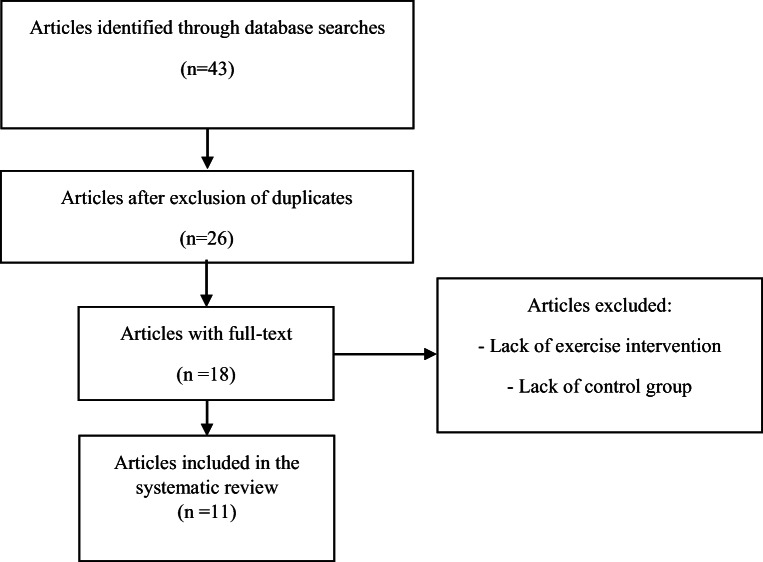
Forty-three articles found in this study based on searching of related keywords. Seventeen articles that were duplicated, were eliminated from the list; so, 26 articles remained, of which 18 had full-text. Besides, 7 articles excluded due to lack of exercise training intervention and lack of control group. Finally, 11 articles (4 human studies [45, 60–62], 6 rat studies [63–68] and 1 mouse study [69]) included in the review that conducted between 2009 and 2017 (Fig. 1). The total included subjects in this systematic review were 230 human subjects (120 females and 110 males), 200 male Wistar rats and 48 male mice. The summary of extracted data of human and animal studies are presented in Table 1 and Table 2, respectively. In addition, brain-derived neurotrophic factor (BDNF) was the primary and key outcome, and some variables such as glucose (6 studies [60, 61, 65, 66, 69]), and insulin (4 studies [60, 61, 68, 69]) were the secondary outcomes.
Fig. 1.
Process of exclusion and inclusion of articles in the systematic review
Table 1.
Summary of studies evaluating the effect of exercise training on BDNF levels and related metabolic indicators in patients with type 2 diabetes (human studies)
| Author-Date/PEDro Quality | Participants | Experimental Group(Independent Variable) | Control Group | Dependent Variable | Results |
|---|---|---|---|---|---|
| Baker et al. (2010)/5 [60] | 28 men (57–83 years old) suffering from glucose intolerance Groups: Training and Control. | Aerobic training (elliptical machine, ergometer cycle or treadmill) during 6 months, 4 sessions per week, each session 45–60 min, with intensity 75% -85% of heart rate reserve. | balance and stretching training, 6 months, 4 sessions per week, each session 45–60 min, with intensity ≤50% of the heart rate reserve. | BDNF Glucose Insulin | Insignificant changes in all variables |
| Lee et al. (2014)/5 [45] | 26 adolescents (13–19 years old); 17 boys and 9 girls; healthy, obese, and with type 2 diabetes. Groups: Diabetes-Training, Obese-Training, and Healthy inactive. | Diabetic and obese groups. Aerobic training (walking and running), 12 weeks, 3 sessions per week, with 50% - 60% VO2max, each session 40–60 min. | Age matching of participants of the control group based on other groups | BDNF | Significant increase of BDNF in the obese group |
| Swift et al. (2012)/5 [62] |
150 men and women (30–75 years old), 86 women and 64 men, suffering from type 2 diabetes Groups: aerobic training, resistance training, concurrent training, and without training. |
Aerobic training: 50%–80% VO2max. Resistance training: 3 sessions per week, each session consisting of 2 sets of 4 exercises for the upper trunk, 3 sets of 3 exercises for the lower trunk, and 2 sets of 2 exercises for core (each set with 10–12 repetitions). Concurrent training: 2 sessions per week, each session consisting of 1 set of 9 exercises. | Recommended weekly stretching and relaxation exercises and maintaining daily activity for 9 months | BDNF | Insignificant changes in serum BDNF levels after aerobic, resistance and concurrent training compared to the control group. |
| Stomby et al. (2017)/5 [61] | 30 participants (men: 30–75 years old, and women: up to 75 years old), obese and overweight with type 2 diabetes. Groups: Paleolithic diet, Paleolithic diet-training, and training. | 12 weeks, 3 sessions per week, each session 60 min of resistance and aerobic training. | Consumption of pure meat, fish, eggs, fruits, berries, vegetables and nuts. | BDNF Glucose Insulin | BDNF elevation in both diet and training-diet groups. Significant reduction in glucose and insulin in both diet and training-diet groups. |
Table 2.
Summary of studies evaluating the effect of exercise training on BDNF levels and related metabolic indicators in patients with type 2 diabetes (animal studies)
| Author-Date/PEDro Quality | Animals | Experimental Group (Independent Variable) | Control Group | Dependent Variable | Results |
|---|---|---|---|---|---|
| Eslami et al. (2016)/8 [63] | 28 male Wistar rats, 10 months old. Diabetes Induction by intraperitoneal injection of streptozotocin. Groups: Healthy-Training, Diabetes-Training, Healthy-Inactive, and Diabetes-Inactive. | Treadmill running with moderate-intensity (50% -55% VO2max), 6 weeks, 5 session per week, 30 min per session, speed of 10 m in the minute of 24, for 10 min at speeds of 18–17 m per minute. | water and food feeding without training | BDNF Glucose | Significant compensation of BDNF reduction-induced by diabetes mellitus in the Diabetic-Training Group compared to Diabetic-Control Group at the sensory and motor roots of the lumbar nerves (absolute values were still lower than in the Healthy-Control Group). Significant reduction of glucose in the Diabetic-Training Group compared to Diabetic-Control Group. |
| Salehi et al. (2010)/8 [67] | 48 male Wistar rats, 12-week-old. Diabetes Induction by intraperitoneal injection of streptozotocin. Groups: Healthy-Training, Diabetes-Training, Healthy-Inactive, and Diabetes-Inactive. | Swimming, 8 weeks, 6 Session per week, one hour per session. | Keeping in the lab and in the same conditions of animals of the training group, without swimming. | BDNF | Increased BDNF mRNA and protein gene expression in response to diabetes induction. Significant decrease in BDNF gene content and expression in diabetic rats in adaptation to training compared to the control group. |
| Kim et al. (2015)/7 [65] | 18 Male Rats, Genetic Model of Type 2 Diabetes, 6 weeks. Groups: Active and inactive obese male rats, inactive lean male rats. | Progressive resistance training using a ladder and a weight attached to the tail of the rat, 8 weeks, 3 days per week (rats should reach the top of the ladder at 50% of their body weight and rest after 2 min, increasing by 20 g each time, this process repeated 10 times). | Without training |
BDNF Glucose |
Significant decrease in BDNF levels in the active obese group compared to the inactive obese group. Significant decrease in glucose level of active obese group compared to inactive obese group. |
| Stranahan et al. (2009)/6 [69] | Two groups, 24 male mice (one month old), ethnic background C57Bl/6, db/db mutant, and wild type. Groups: Training-Caloric restriction, Training-fed ad libitum, Inactive-Caloric restriction, Inactive-fed ad libitum (in mutated and wild-type groups) | Nutrition restriction: 60% of fed animals and once daily, The daily record of mice’s activities with software. The daily record of exercise activity (number of pedaling per day) on a wheel running with the software. 12 weeks. | Keeping in cage without wheel running | BDNF Glucose Insulin | Significant increase in BDNF levels with caloric restriction, training, or their combination in the db/db group, the elevation was higher in the wild group. Significant reduction in glucose between groups with combined running and caloric restriction. Significant decrease in insulin in the inactive-caloric restriction group. |
| Rashidi Molaei, Kazemi, & Rahmati (2015)/6 [66] | 16 male Wistar rats (two weeks old). Diabetes induction by streptozotocin injection. Groups: diabetes-control, diabetes-training, healthy-control, and healthy-training. | Endurance training, 6 weeks, Moderate intensity, (speed of 10 m/min at first week, 17–18 m/min for 30 min). | Without training | BDNF Glucose | Significant decrease in BDNF expression and blood glucose level in the diabetes-training group |
| Hajizadeh Moghaddam et al. (2012)/6 [64] | 42 male rats (eight weeks old). Diabetes induction by alloxan monohydrate injection. Groups: control, training, diabetes-training, diabetes-control, diabetes-alum, diabetes-alum-training. | 6 weeks of voluntary wheel running. | Without training with alum feeding | BDNF | Increased hippocampal BDNF in voluntary wheel running with alum paradoxoxum. |
| Salehi & Hoseini (2017)/6 [68] | 48 male rats, diabetes induction by streptozotocin. Groups: diabetic rats sacrificed at first-week, diabetic rats sacrificed at last week, diabetic rats doing moderate-intensity endurance training, diabetic rats doing High-intensity endurance training, healthy rats sacrificed at first week, healthy rats sacrificed at last week. | Eight weeks treadmill running, three sessions per week, 60 min per session, at speed of 10–17 m/min and 17–28 m/min. | Without training | BDNF Insulin | 8 weeks of moderate to high intensity endurance training significantly increased BDNF levels. But did not affect insulin levels in diabetic rats. |
The quality of the animal studies was at least 6 [64, 66, 68, 69] and at most 8 [63, 67] according to the PEDro scale. The quality of all human studies was 5, and the major reasons for this score were: low sample size; non-random allocation of subjects to groups; the high age range of subjects; and the lack of blinding of all subjects, all therapists who administered the therapy, and also all assessors who measured at least one key outcome.
Descriptive of Included Studies
In a study, Baker et al. (2010) found that 6 months of aerobic training (treadmill, stationary bicycle, or elliptical trainer) with 75%–85% of heart rate reserve decreased serum BDNF levels in men with glucose intolerance. Besides, the body fat percentage, body mass index, glucose and insulin in the aerobic training group were not different from the control group [60].On the other hand, Stomby et al. (2017) showed that serum BDNF levels increased following combined exercise (50% aerobic and 50% resistance training) with and without the paleolithic diet in obese and overweight individuals with type 2 diabetes. Also, there was a significant decrease in glucose, insulin, body fat percentage and body mass index in both exercise and diet groups [61]. In two studies by Lee et al. (2014) and Swift et al. (2012), there was no significant change in BDNF levels following aerobic, resistance and combined training in type 2 diabetic subjects, respectively [45, 62]. Also, in the Lee et al. (2014) study, body weight, body mass index, and body fat percentage decreased significantly in the obese group, whereas those in the diabetic group increased significantly [45]. Additionally, Eslami et al. (2016) found that aerobic exercise increased BDNF gene expression in the sensory and motor nerve roots of number 4, 5 and 6 of lumbar vertebrae in diabetic rats. In addition, aerobic exercise significantly suppressed diabetes-induced weight loss in the diabetic-exercise group compared to the diabetic-control group, but did not change in the healthy control group. Also, aerobic exercise significantly reduced the glucose level of the diabetic-exercise group compared to the diabetic-control group [63].Also, Salehi et al. (2010) found that swimming exercise reduced the BDNF gene and protein expression of the hippocampus of diabetic rats [67]. In addition, Kim et al. (2015) reported that resistance training reduced BDNF protein expression of the soleus muscle of type 2 diabetes rats. Weight and glucose levels of the diabetic-trained group were significantly lower than the control-diabetic group [65]. Rashidi Molaei, Kazemi, & Rahmati (2016) showed that untrained diabetics found a significant decrease in BDNF gene expression and blood glucose levels [66]. The results of Hajizadeh Moghaddam et al. (2012) further showed that six weeks of voluntary wheel running and consumption of Allium paradoxum can increase BDNF, which may be useful in counteracting the deleterious effects of diabetes and its associated oxidative stress [64]. Salehi & Hoseini (2017) also found that eight weeks of moderate- and high-intensity endurance training significantly increased BDNF levels but had no effect on insulin levels in rats [68]. Moreover, Stranahan et al. (2009) showed that aerobic exercise and caloric restriction could increase hippocampal BDNF levels in diabetic rats. Also, the combination of running and caloric restriction reduced glucose levels. Insulin levels were the lowest in the diet-inactive group. In addition, caloric restriction and its combination with running resulted in significant weight loss [69].
In conclusion, endurance training in 4 studies with quality score of 8 and 6, had incremental effect [63, 64, 68, 69], in 3 studies with quality score of 5, 6 and 8, had reduction effect [60, 66, 67] and in 2 studies with quality score of 5 had a neutral effect [45, 62] on BDNF levels of diabetic subjects. Also, resistance training in two studies with a quality score of 7 had a reduction [65] and neutral effect [62] on subjects’ BDNF levels. Combined exercise also had a neutral [62] and an increasing effect [61] in two studies with a quality score of 5 on BDNF levels. In addition, the decreasing effect of exercise activity on glucose and insulin levels was observed in type 2 diabetic subjects, in 5 studies with a quality score of 5 to 8, and 2 studies with a quality score of 5, respectively.
Discussion and Conclusion
The purpose of this systematic review was to investigate the effect of exercise training on BDNF levels in type 2 diabetes patients. Reported results of 5(one human and four animal articles), 4(one human and three animal articles) and 2 studies (both of them in human articles) showed an increasing, decreasing and neutral effects of exercise training, respectively, on BDNF levels. The results of the 6 studies (two human and four animal articles) showed a decreased level of glucose. Besides, the results of 2 studies (both of them in human articles) also reported a decreasing effect of exercise training on insulin levels.
Obesity is linked to thousands of devastating health consequences, such as cognitive dysfunction in childhood, increased risk of Alzheimer’s disease and dementia during the late-life period, possibly due to an increase in inflammatory cytokines that have systemic effects associated with the glycemic disorder and the onset of type 2 diabetes pathology. BDNF has neurologic and metabotropic effects, including regulation of whole-body energy homeostasis and nutritional behavior, skeletal muscle fat oxidation, beta-cell function, and hepatic glycemic control. Low levels of circulating BDNF are associated with both neurological and metabolic status such as severe depressive disorder, Alzheimer’s disease, obesity, and type II diabetes. BDNF is widely expressed in the brain (mainly in the hippocampal, cortex cerebri, hypothalamic and cerebral areas) of growing and adult subjects. Central BDNF can pass from the blood-brain barrier and be stored in other peripheral tissues. However, peripheral tissues such as skeletal muscle and adipose tissue are able to express BDNF, which does not enter circulation. Through activation of the tropomyosin kinase B receptor, BDNF plays an important role in many aspects of adult brain development and plasticity such as proliferation, differentiation, neuronal survival, neurogenesis, synaptic plasticity, dendritic growth, long-term neuronal amplification and cognitive function [70, 71].In fact, high levels of BDNF are associated with spatial, episodic, cognitive, and verbal memory as well as hippocampal function. In addition, decreased BDNF levels, especially in the elderly, have been associated with hippocampal atrophy, which may lead to memory impairment [70]. Findings have also shown that BDNF is involved in central metabolic pathways and mediates energy metabolism in the peripheral organs. Numerous studies have shown that BDNF has specific effects on the central pathways involved in the regulation of appetite and energy expenditure. BDNF also probably regulates glucose metabolism. Serum BDNF has a direct relationship with metabolic syndrome risk factors such as body mass index, total cholesterol, and triglycerides. Plasma BDNF has also been inversely correlated with insulin resistance [72].
Recent studies on obese and diabetic animal models have shown that administration with BDNF significantly inhibits blood glucose, food intake, and body weight gain associated with food intake, while it improved energy expenditure, glucose and lipid metabolism, and the resistance of the sympathetic nervous system [18, 19, 71, 72] . New findings suggest that the BDNF signaling pathway in the hypothalamus has the potential to regulate energy homeostasis, controlling body weight and nutritional behavior. In addition, BDNF has been identified as a protein derived from contraction-induced muscle cells that can increase lipid oxidation in skeletal muscle through the AMPK signaling pathway. It has also been proven that factors such as age, sex, and weight affect BDNF circulation [71]. In fact, adults with obesity and adults with type 2 diabetes have significantly lower circulating BDNF levels than lean and nondiabetic adults, possibly due to hyperglycemic-induced plasma BDNF reduction. Recently, it has been suggested that BDNF may have an etiological role in obesity and metabolic syndrome through evolutionary programming in the uterus of phenotypes with obesity and type 2 diabetes. In addition, overexpression of hypothalamic BDNF facilitates conversion of white to brown adipose tissue through sympathetic nerve activity, increases the energy expenditure and decreases the plasma glucose levels.
Recent findings have shown that exercise training can be considered as a potential strategy for BDNF activity induction to improve cognitive function and mental status. Muscle contraction over-regulates BDNF production in cultured muscle cells, and voluntary endurance exercise express both protein content of BDNF in skeletal muscle of rodents and gene mRNA in multiple brain areas such as the hippocampus and spinal cord. Similarly, endurance exercise increases BDNF expression and protein content and acutely increases systemic BDNF concentrations in humans. The brain has also been shown to be the main source of systemic BDNF in response to endurance training. Taken together, studies have shown that endurance training can over-regulate tissue BDNF expression and transiently elevate systemic BDNF concentrations, which may subsequently induce metabolic and neurobiological adaptations in peripheral and central tissues. Resistance training is also a likely stimulus for the release of different neuroendocrine and growth factors from skeletal muscle and other tissues. However, resistance exercise may acutely stimulate BDNF elevation due to partially transient hormonal responses to exercise similar to observations during endurance exercise and intensive short-term aerobic exercise [71].In a meta-analysis examining the effect of 3 exercise patterns on BDNF levels of non-diabetic subjects, it was found that peripheral concentrations of BDNF had a significant but transient elevation in response to acute aerobic exercise. Besides, regular aerobic training showed a sustained increase in BDNF levels. But, the acute and chronic effects of resistance training showed no significant change on BDNF levels [70].
According to the present systematic review, the results of the Eslami et al. (2016), Hajizadeh Moghaddam et al. (2012), Salehi & Hoseini (2017), Stranahan et al. (2009) and Stomby et al. (2017) studies showed the increasingly effects of exercise on BDNF levels in type 2 diabetic subjects [61, 63, 64, 68, 69]. These results are inconsistent with the findings of Baker et al. (2010), Salehi et al. (2010), Kim et al. (2010) and Rashidi Molaei, Kazemi, &Rahmati (2016) [60, 65–67]. Studies by Lee et al. (2014) and Swift et al. (2012) also reported no significant change in BDNF levels in type 2 diabetic subjects following exercise training [45, 62].The results showed that weight loss-induced by exercise training associated with improvement of insulin sensitivity and glycemic status in type 2 diabetes mellitus and modulation of BDNF levels in these patients. The inverse relationship between exercise training and BDNF concentration is due to the mechanism of BDNF absorption by the brain because BDNF can pass two-way from the blood-brain barrier. In addition, the peripheral BDNF has a positive relationship with cardiovascular risk factors such as body mass index, visceral fat, triglyceride, total cholesterol and fasting blood glucose. In addition, administration with BDNF reduced food intake and body weight and improved hepatic insulin sensitivity in diabetic animals. These results suggest that BDNF primarily regulates food intake, and then consequently regulates body weight and insulin sensitivity. Previous studies have found that exercise training improves the glycemic and insulin status of patients with type 2 diabetes [67]. According to this systematic review, Baker et al. (2010) and Stomby et al. (2017) reported insignificant changes and significant decreases in insulin and glucose levels following exercise in type 2 diabetic subjects, respectively [60, 61]. Also, Eslami et al. (2016), Stranahan et al. (2009) and Rashidi Molaei, Kazemi, &Rahmati (2016) observed a significant decrease in glucose following exercise training in subjects with type 2 diabetes [63, 66, 69].
Since a limited number of type 2 diabetic patients do not exercise training, therefore, it is recommended to patients to do exercise safely and effectively [73]. The recommended frequency for these individuals is at least three sessions per week (in three non-consecutive days) [74, 75]. Recent studies also suggest five sessions per week [76–78].Aerobic exercise should be done at a moderate intensity with approximately 40–60% of maximal aerobic capacity, which greater benefits likely to be achievable at >60% of maximal aerobic capacity. Studies suggest that exercise intensity is more effective than exercise volume for the improvement of type 2 diabetic individuals. People with type 2 diabetes need at least 150 min per week of moderate to high-intensity exercise. Exercise training should be continuous for at least 10 min and the total time should be distributed throughout the week. Aerobic exercise can be any form of activity that utilizes large muscle groups and increases one’s heart rate. The best suggestion for controlling and maintaining the health of these patients is a combination of exercise, nutrition, and behavioral programs.
It is suggested that resistance training should be at least two sessions per week (three sessions per week is the best) in non-consecutive days as part of an exercise training program in combination with aerobic training. The intensity of exercise should be moderate (50% of one-repetition maximum) or intensive (75–80% of one-repetition maximum).Each training session should be included at least 5–10 exercises of the major muscle groups (lower and upper trunk, and core) with (about 10–15) repetitions until fatigue threshold in each set at the beginning of the workout program. The minimum recommended set is 1 set, but up to 4 sets are available. Individuals can increase the workout load when they are able to repeat the moves above the specified number; so, they should increase the number of sets and then the training frequency. The desired target probability is a 6-month progression with 3 sessions per week, three sets with 8–10 repetitions at 75–80% of one-repetition maximum.
Combination of aerobic and resistance training is better than separate training. Flexibility training can be a part of the exercise training program that cannot be substituted with any other type of training. It is recommended to elderly people with type 2 diabetes who are at high risk of falling to perform flexibility exercises to maintain and also improve their balance ability. Flexibility training along with resistance training can increase the range of motion of these patients and facilitate their daily activities [73].
The limitations of this systematic review were the inclusion of only English and persian articles in the study, the lack of sufficient number of included articles and consequently the entry of articles with medium quality based on the PEDro scale. Besides, since the included studies were not similar based on the type of interventions and their training intensity, volume, and frequency, it was not possible to conduct a meta-analysis. The strengths of this study were using the Cochrane Library and the PRISMA Guideline [79] for running this systematic review, and no time limitation for searching the databases.
According to literature, it seems necessary to evaluate the concomitant effects of antioxidants supplementation such as omega-3 polyunsaturated fatty acid and Alpha tocopherol [14] long to exercise training in patients with type 2 diabetes which has a reducing effect on oxidative stress and consequently can modulate BDNF levels and improve cognitive function. Also, considering the prevalence of depression in type 2 diabetic patients and also the increased rate of the mortality of depressed diabetic patients compared to non-depressed patients, it seems important to investigate the effects of exercise on cognitive factors related to depression, in addition to neurotrophic factors in these patients.
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shahnaz Shahrbanian, Email: sh.shahrbanian@modares.ac.ir.
Seyed Morteza Tayebi, Email: tayebism@atu.ac.ir, Email: tayebism@gmail.com.
References
- 1.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 4.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4(2):363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 6.Languren G, Montiel T, Julio-Amilpas A, Massieu L. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int. 2013;63(4):331–343. doi: 10.1016/j.neuint.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Moreira RO, Soldera AL, Cury B, Meireles C, Kupfer R. Is cognitive impairment associated with the presence and severity of peripheral neuropathy in patients with type 2 diabetes mellitus? Diabetology & metabolic syndrome. 2015;7:51. doi: 10.1186/s13098-015-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carsten RE, Whalen LR, Ishii DN. Impairment of spinal cord conduction velocity in diabetic rats. Diabetes. 1989;38(6):730–736. doi: 10.2337/diab.38.6.730. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69(4):229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 10.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabetic medicine : a journal of the British Diabetic Association. 1999;16(2):93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 12.Eslami R, Gharakhanlou R, Parnow A-H. The Response of Skeletal Muscle-Expressed Neurotrophins to Acute Resistance Exercise in Male Wistar Rats. Ann Appl Sport Sci. 2018;6(2):45–53. doi: 10.29252/aassjournal.6.2.45. [DOI] [Google Scholar]
- 13.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahmati-Ahmadabad S, Azarbayjani M, Nasehi M. The Effects of High-Intensity Interval Training with Supplementation of Flaxseed Oil on BDNF mRNA Expression and Pain Feeling in Male Rats. Ann Appl Sport Sci. 2017;5(4):1–12. doi: 10.29252/aassjournal.5.4.1. [DOI] [Google Scholar]
- 15.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250(4978):290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- 17.Fleischer A, Ghadiri A, Dessauge F, Duhamel M, Rebollo MP, Alvarez-Franco F, Rebollo A. Modulating apoptosis as a target for effective therapy. Mol Immunol. 2006;43(8):1065–1079. doi: 10.1016/j.molimm.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94(12):1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- 19.Rashidlamir A, Hoseinzadeh M, Zeiaddini DL. The Effects of Resistance and Endurance Training on the Liver Tissue FNDC5 mRNA Gene Expression in Male Rats. Ann Appl Sport Sci. 2017;5(2):51–60. doi: 10.18869/acadpub.aassjournal.5.2.51. [DOI] [Google Scholar]
- 20.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59(1):201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49(1–2):71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 22.Krabbe KS, Mortensen EL, Avlund K, Pedersen AN, Pedersen BK, Jorgensen T, et al. Brain-derived neurotrophic factor predicts mortality risk in older women. J Am Geriatr Soc. 2009;57(8):1447–1452. doi: 10.1111/j.1532-5415.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2007;101(1–3):239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 25.Fujinami A, Ohta K, Obayashi H, Fukui M, Hasegawa G, Nakamura N, Kozai H, Imai S, Ohta M. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008;41(10–11):812–817. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AMW, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 27.Karczewska-Kupczewska M, Straczkowski M, Adamska A, Nikolajuk A, Otziomek E, Gorska M, et al. Decreased serum brain-derived neurotrophic factor concentration in young nonobese subjects with low insulin sensitivity. Clin Biochem. 2011;44(10–11):817–820. doi: 10.1016/j.clinbiochem.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Suwa M, Kishimoto H, Nofuji Y, Nakano H, Sasaki H, Radak Z, Kumagai S. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55(7):852–857. doi: 10.1016/j.metabol.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4(2):147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 31.Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer's disease. Trends Neurosci. 2003;26(8):404–406. doi: 10.1016/s0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 32.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66(3):343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M. Association between features of the insulin resistance syndrome and Alzheimer's disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ (Clinical research ed) 1997;315(7115):1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complicat. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Cohen ND, Dunstan DW, Robinson C, Vulikh E, Zimmet PZ, Shaw JE. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract. 2008;79(3):405–411. doi: 10.1016/j.diabres.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, et al. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534(Pt 1):287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, Martin SA, Pence BD, Lin M, Parasuraman R, Greenwood PM, Fryxell KJ, Woods JA, McAuley E, Kramer AF, Erickson KI. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR, Crystal RG, Rafii S, Hempstead BL. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115(3):653–663. doi: 10.1172/jci22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23(20):7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messi ML, Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J Neurosci. 2003;23(4):1351–1359. doi: 10.1523/JNEUROSCI.23-04-01351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ola MS, Nawaz MI, El-Asrar AA, Abouammoh M, Alhomida AS. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell Mol Neurobiol. 2013;33(3):359–367. doi: 10.1007/s10571-012-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishi T, Sunagawa K. Exercise training plus calorie restriction causes synergistic protection against cognitive decline via up-regulation of BDNF in hippocampus of stroke-prone hypertensive rats. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2012;2012:6764–6767. doi: 10.1109/embc.2012.6347547. [DOI] [PubMed] [Google Scholar]
- 45.Lee SS, Yoo JH, Kang S, Woo JH, Shin KO, Kim KB, Cho SY, Roh HT, Kim YI. The effects of 12 weeks regular aerobic exercise on brain-derived Neurotrophic factor and inflammatory factors in juvenile obesity and type 2 diabetes mellitus. J Phys Ther Sci. 2014;26(8):1199–1204. doi: 10.1589/jpts.26.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 47.Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004;1(1):111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3(4):445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1–2):49–56. doi: 10.1016/0006-8993(96)00273-9. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28(11):2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139(4):1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 52.Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121(1):1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- 53.Rezaei M, Salarpor Kamarzard T, Najafian RM. The Effects of Neurofeedback, Yoga Interventions on Memory and Cognitive Activity in Children with Attention Deficit/Hyperactivity Disorder: A Randomized Controlled Trial. Ann Appl Sport Sci. 2018;6(4):17–27. doi: 10.29252/aassjournal.6.4.17. [DOI] [Google Scholar]
- 54.Castellano V, White LJ. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J Neurol Sci. 2008;269(1–2):85–91. doi: 10.1016/j.jns.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 55.Goekint M, Roelands B, De Pauw K, Knaepen K, Bos I, Meeusen R. Does a period of detraining cause a decrease in serum brain-derived neurotrophic factor? Neurosci Lett. 2010;486(3):146–149. doi: 10.1016/j.neulet.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 56.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104(5):934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Schiffer T, Schulte S, Hollmann W, Bloch W, Struder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2009;41(3):250–4. doi:10.1055/s-0028-1093322. [DOI] [PubMed]
- 58.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2008;59(Suppl 7):119–132. [PubMed] [Google Scholar]
- 59.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 60.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2010;22(2):569–579. doi: 10.3233/jad-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stomby A, Otten J, Ryberg M, Nyberg L, Olsson T, Boraxbekk CJ. A Paleolithic diet with and without combined aerobic and resistance exercise increases functional brain responses and hippocampal volume in subjects with type 2 diabetes. Front Aging Neurosci. 2017;9:391. doi: 10.3389/fnagi.2017.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, et al. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS One. 2012;7(8):e42785. doi: 10.1371/journal.pone.0042785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eslami R, Gharakhanlou R, Kazemi A, Dakhili AB, Sorkhkamanzadeh G, Sheikhy A. Does Endurance Training Compensate for Neurotrophin Deficiency Following Diabetic Neuropathy? Iran Red Crescent Med J. 2016;18(10):e37757-e. doi: 10.5812/ircmj.37757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajizadeh Moghaddam A, Fallah Mohammadi Z, Sheikh P, Mirzaei S. The effect of voluntary jogging training on rotundum and allium paradoxoxium extracts on brain-derived neurotrophic factor levels in the hippocampus of alloxan-induced diabetic rats. Iranian Journal of Diabetes and Metabolism. 2012;11(4):350–357. [Google Scholar]
- 65.Kim H-J, So B, Son JS, Song HS, Oh SL, Seong JK, Lee H, Song W. Resistance training inhibits the elevation of skeletal muscle derived-BDNF level concomitant with improvement of muscle strength in zucker diabetic rat. J Exerc Nutrition Biochem. 2015;19(4):281–288. doi: 10.5717/jenb.2015.15112402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rashidi Molaei R, Kazemi A, Rahmati M. The effect of a 6-week endurance training on BDNF and TrKB gene expression in the soleus of rats with diabetic neuropathy. J Kerman Univ Med Sci. 2016;23(6):741–753. [Google Scholar]
- 67.Salehi I, Farajnia S, Mohammadi M, Sabouri Ghannad M. The Pattern of Brain-Derived Neurotrophic Factor Gene Expression in the Hippocampus of Diabetic Rats. Iranian Journal of Basic Medical Sciences. 2010;13(3):146–153. doi:10.22038/ijbms.2010.5104.
- 68.Salehi OR, Hoseini A. The Effects of Endurance Trainings on Serum BDNF and Insulin Levels in Streptozotocin-Induced Diabetic Rats. The Neuroscience Journal of Shefaye-Khatam. 2017;5(2):52–61. doi: 10.18869/acadpub.shefa.5.2.52. [DOI] [Google Scholar]
- 69.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF) Neurosci Lett. 2010;479(2):161–165. doi: 10.1016/j.neulet.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 72.Karczewska-Kupczewska M, Kowalska I, Nikolajuk A, Adamska A, Zielinska M, Kaminska N, Otziomek E, Gorska M, Strczkowski M. Circulating brain-derived neurotrophic factor concentration is downregulated by intralipid/heparin infusion or high-fat meal in young healthy male subjects. Diabetes Care. 2012;35(2):358–362. doi: 10.2337/dc11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B, American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boule NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Effects of exercise training on glucose homeostasis: the HERITAGE family study. Diabetes Care. 2005;28(1):108–114. doi: 10.2337/diacare.28.1.108. [DOI] [PubMed] [Google Scholar]
- 75.King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. Journal of applied physiology (Bethesda, Md : 1985). 1995;78(1):17–22. doi:10.1152/jappl.1995.78.1.17. [DOI] [PubMed]
- 76.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 77.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 78.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutrition reviews. 2009;67(2):114–20. doi:10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed]
- 79.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]