Dear Editor,
Epilepsy is a common neurological disease. About 30% of epileptic patients develop resistance to antiepileptic drugs (AEDs), and are considered to have refractory or drug-resistant epilepsy [1]. Based on the time course over which drug-resistance develops, refractory epilepsy can be further divided into an early-onset type (drug-resistance more likely innate and correlated with gene mutations) and a delayed-onset type (drug-resistance emerges after a period of medication) [2]. The latter usually displays a more complex constellation of pathological findings, often associated with mesial temporal sclerosis [3]. Longitudinal neuroimaging studies have shown that the degree of brain atrophy is more extensive in patients with the delayed-onset type than in those with an early onset [4]. After repeated trials with unsuccessful drug treatments, uncontrolled seizures, along with other complications including impaired memory, often put patients’ health at risk. At that time, even epilepsy surgery adds limited value to the treatment of these patients. Thus, an alternative therapeutic strategy is urgently needed for patients with delayed-onset refractory epilepsy.
Deep brain stimulation (DBS), with either a low or a high frequency, is a promising treatment for refractory epilepsy. In particular, low-frequency stimulation (LFS), with mild and relatively safe characteristics, is more suitable for epileptic patients than high-frequency stimulation [5]. The therapeutic effect of LFS largely depends on the stimulus parameters and brain targets. The subiculum is a crucial output region of the hippocampus, and has been shown to be hyperexcitable in both patients and animals with temporal lobe epilepsy (TLE) [6]. More importantly, specific 1-Hz interictal spikes originate in the subiculum of patients with refractory TLE [7]. We previously reported that 1-Hz LFS at the subiculum significantly retards kindling acquisition and inhibits recurrent seizures in rats [8]. Further study has verified the anti-convulsant efficacy of LFS in animal models with early-onset refractory TLE. Moreover, drug resistance can be reversed by LFS treatment [9]. However, whether LFS at the subiculum is a promising treatment for delayed-onset refractory TLE is still unclear.
The lamotrigine (LTG)-resistant kindled rodent is one of the most widely-used animal models that resemble delayed-onset refractory epilepsy [10]. Kindled rats or mice exposed to a sub-therapeutic dose of LTG during the kindling process subsequently show LTG resistance. Thus, in this study, we established this classic hippocampal kindled LTG-resistant TLE model and evaluated the therapeutic efficacy of LFS at the subiculum in this model.
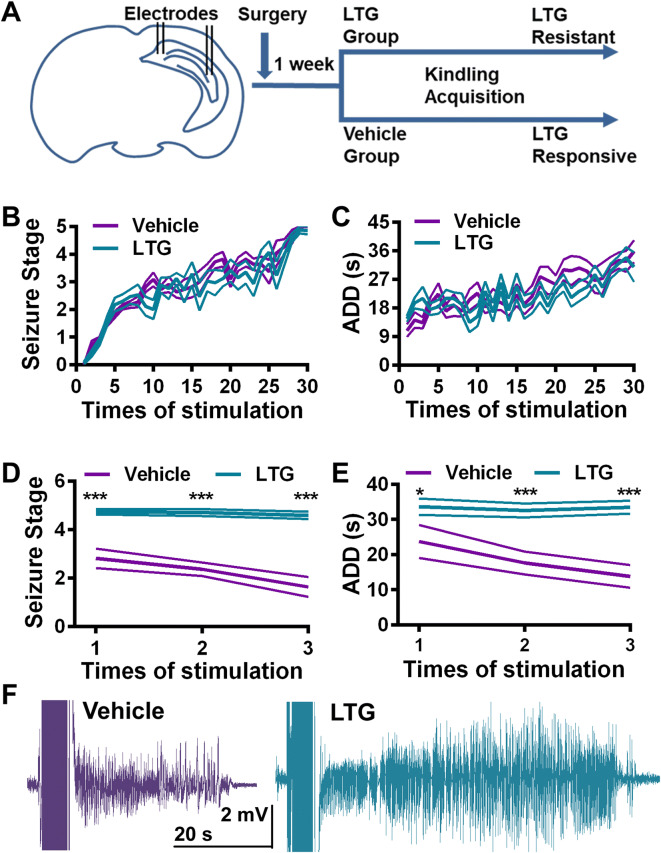
The LTG-resistant TLE model was established with rapid hippocampal kindling in mice[11]. The mice were divided into two groups based on their afterdischarge threshold. The LTG group received a sub-therapeutic dose of LTG while the vehicle group received vehicle, along with kindling (Fig. 1). The kindling acquisition process was not altered by LTG: both the progression of behavioral seizure stage and mean afterdischarge duration (ADD) showed no difference between the two groups (Fig. 1B, C). Once mice were fully kindled (three consecutive stage 5 seizures), the anti-convulsant effect of LTG was determined 3 times. LTG significantly alleviated the seizure severity in the vehicle group compared with the LTG group. Mice in the vehicle group only showed focal seizures (less than stage 3), while mice in the LTG group had generalized seizures (GSs, stages 4 and 5) after LTG treatment (Fig. 1D). The ADD was also shorter in the vehicle group (Fig. 1E). Thus, mice in the LTG group were considered to be LTG-resistant.
Fig. 1.
Establishment of a mouse model of TLE with delayed-onset lamotrigine (LTG) resistance. A Schematic of the establishment of the LTG-resistant TLE model. Electrodes were implanted into the CA3 and subiculum of C57 mice, which were then divided into two groups, one receiving a sub-therapeutic dose of LTG and the other receiving vehicle along with kindling acquisition. B, C The progression of seizure stage (B) and afterdischarge duration (ADD) (C) showed no differences between vehicle and LTG groups. D, E LTG at a therapeutic dose had an anti-convulsant effect in the vehicle but not in the LTG group (n = 11 for vehicle, n = 18 for LTG). LTG treatment significantly lowered the seizure stage (D, ***P < 0.001, unpaired t test) and shortened the ADD (E, *P = 0.047, ***P < 0.001, unpaired t test) in kindled mice of the vehicle group compared with the LTG group. F Representative seizure EEGs under LTG treatment from the vehicle and LTG groups.
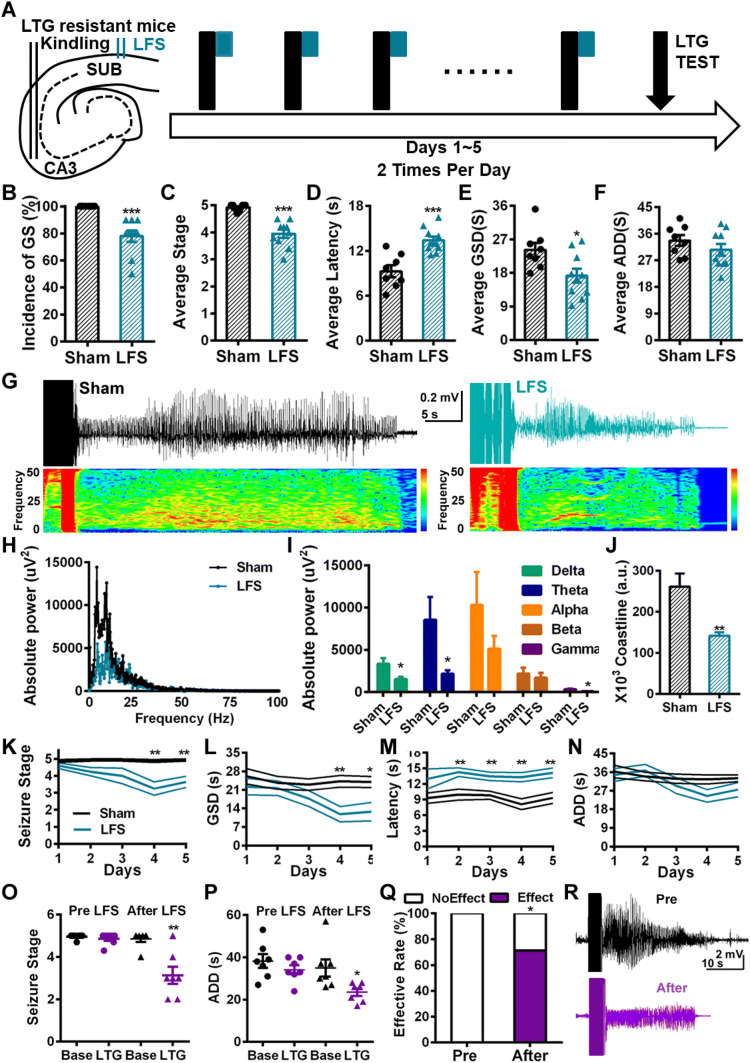
Then we delivered LFS 10 times (twice daily for 5 days) at the subiculum immediately after each CA3 kindling (Fig. 2A) and found that the seizure severity was alleviated in the LTG group. The incidence of GSs was significantly decreased (Fig. 2B), along with a lower average behavioral seizure stage (Fig. 2C). LFS also extended the average latency to GSs (Fig. 2D) and shortened the average GS duration (Fig. 2E). The average ADD did not change significantly (Fig. 2F). Power spectrum analysis showed that the absolute power of the seizure EEG in the LFS group was lower than that in the sham group (Fig. 2G, H). Further statistical analysis revealed that the absolute power in the delta, theta, and gamma bands was lower in the LFS group than in the sham group (Fig. 2I). We also measured the coastline index, which indicates the amplitude of epileptic EEG spikes, and found that it was significantly lower in the LFS group than in the sham group (Fig. 2J). These results suggested that LFS at the subiculum suppresses the severity of seizure EEGs, indicating that it has anti-convulsant efficacy. To define the time-window of the therapeutic effect of LFS, we analyzed its anti-convulsive action on a daily basis. LFS on the first day had no significant anti-convulsant effect (Fig. 2K–N), but the effect became prominent with time. On day 2, LFS increased the latency to GS (Fig. 2M), while on day 4, LFS reduced the seizure stage and the GS duration (Fig. 2K, L). These results suggested that LFS has a time-dependent cumulative effect, the anti-convulsant action being manifested only in the later period of treatment.
Fig. 2.
The anti-convulsant and anti-drug-resistant effects of LFS at the subiculum. A Schematic of LFS treatments. LFS at the subiculum (green) was delivered immediately after kindling stimulation (black) (LTG, lamotrigine). B–F LFS at the subiculum significantly alleviated the seizure severity in LTG-resistant kindled mice (n = 8 for sham, n = 10 for LFS). LFS lowered the incidence of GS (B) and average seizure stage (C), extended the average latency to GS (D), and shortened the average GS duration (E), but had no effect on the average ADD (F). G Representative seizure EEGs and spectra of the sham and LFS groups. H Representative seizure EEG power spectra of the sham and LFS groups. I LFS significantly reduced the absolute power in the delta, theta, and gamma bands (n = 8 for sham, n = 10 for LFS). J LFS treatment decreased the coastline index of seizure EEG (n = 8 for sham, n = 10 for LFS). K LFS lowered the seizure stage on day 4 of treatment (n = 8 for sham, n = 10 for LFS). L LFS shortened the GS duration on day 4 of treatment (n = 8 for sham, n = 10 for LFS). M LFS extended the latency to GS on day 2 of treatment (n = 8 for sham, n = 10 for LFS). N LFS had no effect on ADDs throughout treatment. O, P After 10 LFS sessions, seizure stage (O, n = 7) was lower, and ADD (P, n = 7) was shorter in LTG-resistant kindled mice (*P < 0.05, **P < 0.01 compared with baseline, paired t-test). Q The effectiveness of LTG was significantly raised by LFS (*P < 0.05, χ2 test). R Representative seizure EEG at baseline and under LTG treatment after LFS. *P < 0.05, **P < 0.01, ***P < 0.001 compared with sham, unpaired t test.
Our previous study showed that LFS at the subiculum reverses early-onset phenytoin resistance [9]. Therefore, in this study we aimed to determine whether long-term LFS also reverses LTG resistance. We tested the effect of LTG after 10 sessions of LFS at the subiculum on LTG-resistant kindled mice. We found that LTG significantly inhibited seizures in terms of the stage (Fig. 2O) and ADD (Fig. 2P). Also, the effectiveness of LTG after LFS was significantly greater than that before LFS (Fig. 2Q). These results suggest that LTG resistance is reversed by LFS at the subiculum.
Delayed-onset drug-resistance in epilepsy has been recognized for decades. In such patients, the brain lesion is often more extensive and the pathological findings more complicated than in patients with early-onset refractoriness [2, 4]. These features further illustrate the difficulty in treating such patients, even with epilepsy surgery. LFS, as an alternative to epilepsy surgery, provides a promising option. Clinical trials testing its effects on patients with refractory epilepsy have been carried out in different centers, though with contradictory results, possibly due to the different targets for stimulation. Furthermore, accumulating evidence suggests that the subiculum plays a crucial role in epilepsy [7, 12]. In this study, we showed for the first time that LFS at the subiculum had an anti-convulsant effect on TLE mice with delayed-onset LTG resistance. The EEG power in a broad range of frequency bands was decreased by LFS, which might be due to the lower amplitude of synchronized epileptic spikes. This result was further corroborated by coastline analysis. Interestingly, the ADD was not affected, perhaps because the subiculum mediates the propagation rather than the initiation of GSs. Together, these results suggest that LFS at the subiculum might also induce neural desynchronization. Our previous study also showed that only 1-Hz LFS at the subiculum has therapeutic effects on both kindling acquisition and early-onset refractoriness in TLE rats and has a relatively wide time window [8, 9]. Therefore, we consider the subiculum to be an optimal target of LFS for refractory epilepsy.
Delayed-onset drug-resistance is characterized by the diminishing efficacy of AEDs. In the later period of using the medication, patients become completely drug-resistant. Interestingly, we found that, unlike AEDs, LFS lowered the seizure stage and shortened the GS duration in the later period of treatment. In addition, LFS had an earlier effect of extending the latency to GS, probably associated with a delay in seizure propagation, given the role of the subiculum as the main output region of the hippocampus [6]. The distinct features of the therapeutic effects of LFS and AEDs might be due to the differences in their mechanisms of action. Almost all AEDs act on ion channels and postsynaptic receptors to directly reduce the epileptic hyperexcitability, while LFS might have a long-term de-potentiation effect via the neural circuits between the subiculum and seizure foci [13], resulting in seizure control. In this way, LFS at the subiculum had a cumulative anti-convulsant effect on delayed-onset drug-resistant TLE.
Our previous study reported that LFS at the subiculum reverses early-onset phenytoin resistance [9]. In line with this, here we further showed that LFS reversed delayed-onset LTG resistance. However, the mechanism underlying this type of resistance remains elusive. An alternative hypothesis proposes the induction of multidrug transporter expression by recurrent seizures, resulting in later drug-resistance [14]. Besides, epilepsy, especially TLE with hippocampal sclerosis, is a progressive condition. Changes in neural circuitry may conceivably lead to delayed-onset drug-resistance over time [15]. Our results indicated that, besides the drug transport hypothesis, the subiculum might be involved in the altered neural circuits resulting in drug-resistance, since it is reported to be more susceptible to hyperexcitability during the epileptic process [6]. Further circuitry studies combined with multifaceted techniques are needed to uncover the subiculum-mediated mechanisms of delayed-onset drug-resistance.
In conclusion, this study revealed that LFS at the subiculum has an anti-convulsant and anti-drug-resistant effects on delayed-onset LTG resistance in a TLE model. These findings highlight LFS at the subiculum as an effective therapeutic strategy for refractory epilepsy.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81703480 and 81603084) and the Fundamental Research Funds for Central Universities, China (2019FZA7016 and 2019QNA5001).
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Yeping Ruan and Cenglin Xu have contributed equally to this work.
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–190. doi: 10.1212/01.WNL.0000031792.89992.EC. [DOI] [PubMed] [Google Scholar]
- 3.Berg AT. The natural history of mesial temporal lobe epilepsy. Curr Opin Neurol. 2008;21:173–178. doi: 10.1097/WCO.0b013e3282f36ccd. [DOI] [PubMed] [Google Scholar]
- 4.Bilevicius E, Yasuda CL, Silva MS, Guerreiro CA, Lopes-Cendes I, Cendes F. Antiepileptic drug response in temporal lobe epilepsy: a clinical and MRI morphometry study. Neurology. 2010;75:1695–1701. doi: 10.1212/WNL.0b013e3181fc29dd. [DOI] [PubMed] [Google Scholar]
- 5.Burbaud P, Vital A, Rougier A, Bouillot S, Guehl D, Cuny E, et al. Minimal tissue damage after stimulation of the motor thalamus in a case of chorea-acanthocytosis. Neurology. 2002;59:1982–1984. doi: 10.1212/01.WNL.0000038389.30437.1E. [DOI] [PubMed] [Google Scholar]
- 6.Stafstrom CE. The role of the subiculum in epilepsy and epileptogenesis. Epilepsy Curr. 2005;5:121–129. doi: 10.1111/j.1535-7511.2005.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 8.Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, et al. Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis. 2012;48:20–26. doi: 10.1016/j.nbd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, Wang Y, Zhang S, Nao J, Liu Y, Wang Y, et al. Subicular pyramidal neurons gate drug resistance in temporal lobe epilepsy. Ann Neurol. 2019;86:626–640. doi: 10.1002/ana.25554. [DOI] [PubMed] [Google Scholar]
- 10.Postma T, Krupp E, Li XL, Post RM, Weiss SRB. Lamotrigine treatment during amygdala-kindled seizure development fails to inhibit seizures and diminishes subsequent anticonvulsant efficacy. Epilepsia. 2000;41:1514–1521. doi: 10.1111/j.1499-1654.2000.001514.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wang Y, Xu C, Wang S, Tan N, Chen C, et al. Direct septum-hippocampal cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol Psychiatry. 2019 doi: 10.1016/j.biopsych.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 12.de Guzman P, Inaba Y, Biagini G, Baldelli E, Mollinari C, Merlo D, et al. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16:843–860. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- 13.Berretta N, Cherubini E. A novel form of long-term depression in the CA1 area of the adult rat hippocampus independent of glutamate receptors activation. Eur J Neurosci. 1998;10:2957–2963. doi: 10.1111/j.1460-9568.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 14.Rizzi M, Caccia S, Guiso G, Richichi C, Gorter JA, Aronica E, et al. Limbic seizures induce P-glycoprotein in rodent brain: functional implications for pharmacoresistance. J Neurosci. 2002;22:5833–5839. doi: 10.1523/JNEUROSCI.22-14-05833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.