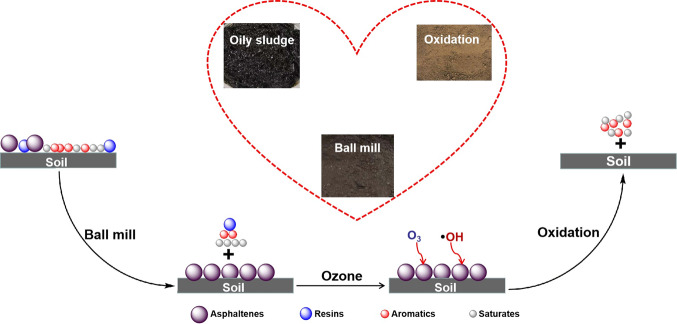
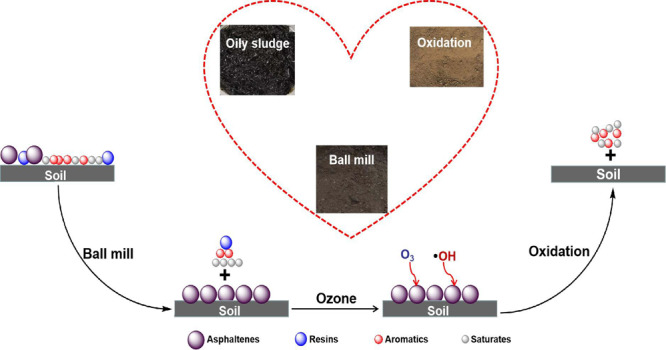
Abstract
Difficult separation of oil–solid phase and high fine content of the recovered oil were two problems in the treatment of oily sludge from the tank bottom by the hot water-based extraction process. To solve the problems, one technology with “ball milling + ozone-catalyzed oxidation” as the core was studied, and the process parameters of ball milling and ozone-catalyzed oxidation were respectively optimized. After ball milling treatment, the oil content of dry oily sludge decreased from 33.9 to 10.2%. Then, an ozone catalytic oxidation treatment technology with aluminum ore as the catalyst was developed to further treat this stubborn oily sludge. Under the optimal conditions, the oil content of oily sludge could be further reduced to 0.28%, which met the treatment and disposal requirements stipulated in GB4284-2018. For further research on the contribution of the catalyst to the ozone catalytic oxidation system, the reaction activation energy and reaction rates of ozone oxidation and ozone catalytic oxidation were compared from the perspective of kinetics. The results showed that, with the catalyst addition, the reaction rate constants increased about three times and the reaction activation energy reduced 82.26%, which showed the effectiveness of the catalyst on the kinetics quantitatively. The combined process with “ball milling + ozone-catalyzed oxidation” as the core can solve the two problems in the treatment of oily sludge from the tank bottom by hot water-based extraction and provides a reference for the harmless and resourceful treatment of oily sludge from the tank bottom.
1. Introduction
Oily sludge is produced during the process of mining, transportation, and storage in the petroleum industry.1 Generally, oily sludge is a complex water in oil (W/O) emulsion, typically including, 30–50% of water, 30–50% of oil, and 10–12% of solids by mass.2 Because of its combustible and toxic characteristics, oily sludge has been classified as one hazardous solid waste in many countries, and China has also listed it in the national hazardous waste catalog, belonging to the HW08 category.3 The hazard of oil sludge is mainly due to its polycyclic aromatic hydrocarbons, heavy metals, anthracene, pyrene, phenols, and other poisonous substances; in the case of long-term stacking without timely treatment, it will cause serious pollution to the surrounding soil, groundwater, and atmosphere, which will further affect the normal growth of plants, animals, and human health.4 Due to the complexity of oil sludge composition and its great harm to the environment, the treatment of oil sludge has become a worldwide problem, which has also attracted extensive attention of relevant experts and scholars.5 Now, the removal and recovery of oily sludge are two general approaches that can be considered in mitigating oily sludge hazards.6−10 There have been numerous methods designed and developed for oily sludge removal and recovery, such as froth flotation,11,12 solvent extraction,13,14 pyrolysis,15−17 thermochemical cleaning,18−20 ultrasound-assisted treatment,21−24 and microwave treatment.25,26 Among them, thermochemical cleaning, pyrolysis, and solvent extraction are the most studied currently.27 In comparison, pyrolysis has defects such as easy coking and high energy consumption, and the solid phase of the treated oily sludge loses soil vitality, leading to great resistance to its promotion and application.28 The effect of solvent extraction process is very significant, but there are some defects, such as large solvent loss, high operating cost, and easy production of secondary pollution during the process, which limit the scope of its application.29 Due to the advantages of mild operation conditions, moderate operation cost, and simple and safe operation, the thermochemical cleaning process is favored by more and more oil field owners and scientific research workers.30 However, difficult separation of the oil phase and solid phase and high water content and solid content of the recovered oil were two problems in the treatment of oily sludge from the tank bottom by thermochemical cleaning.31 To solve this problem, some scholars have tried to convert oily sludge into valuable materials, of which the preparation of activated carbon is the most widely studied due to the high carbon content in oily sludge. However, the reported activated carbon derived from oily sludge is still not good enough for industrial application, and there are certain environmental risks.32−34
In this paper, one technology with “ball mill + ozone-catalyzed oxidation” as the core was studied to treat the oily sludge from the tank bottom, and the process parameters of ball milling and ozone-catalyzed oxidation were respectively optimized. Under the optimal conditions, the oil content of oily sludge from the tank bottom could be reduced to 0.28%, which met the treatment and disposal requirements stipulated in GB4284-2018. The combined process with “ball mill + ozone-catalyzed oxidation” as the core can solve the two problems in the treatment of oily sludge from the tank bottom by the thermochemical cleaning process. Especially due to the fact that the main composition of aluminum ore used as a catalyst in this paper is aluminum, which is the most abundant metal element in the crust, it makes it easier to be acquired. In addition, during the fracturing process of oil well, proppant with aluminum ore as the main component is used in large quantities, there would inevitably be a relative amount of discarded proppant produced, which could be used as a catalyst of oily sludge treatment to achieve the purpose of using waste to treat waste. Overall, this study provides a reference for the harmless and resourceful treatment of oily sludge from the tank bottom.
2. Results and Discussion
2.1. Characterization of Oily Sludge
Oily sludge was supplied by Huabei Oilfield in China and was obtained during the clearing process of the oil tank, just as depicted in Figure 1. In order to find out the position distribution of water, oil, and solid in oil sludge visually and intuitively, an optical microscope test was done. Figure S1 shows that the oily sludge was water-in-oil (W/O) type emulsion, and the solid dispersed in the oil, absorbed on the W/O interface, or dispersed in a big water drop. The phase composition of the oily sludge and elemental analysis, SARA (saturates, aromatics, resins, and asphaltenes), density, and viscosity of oil from oily sludge by Soxhlet extraction were determined. The solid left from Soxhlet extraction was used for determining the particle size, morphology characterization, and heavy metal content. The characteristics of the oily sludge are summarized in Table 1. The oil content of dry oily sludge was 33.9 wt %, and the calorific value was 43,217 kJ·kg–1, which indicated that the oily sludge had a high recycling value, while the high content of resins (13.01 wt %) and asphaltenes (12.49 wt %) increased the difficulty of oil–solid separation.35,36
Figure 1.
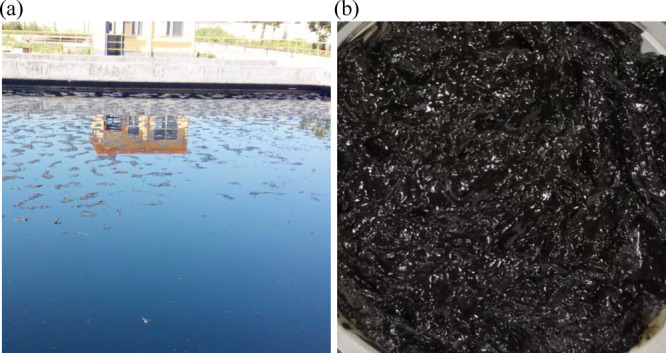
(a) Oily sludge storage pool. (b) Oily sludge.
Table 1. Property Analysis of Oily Sludge.
| items | content |
|---|---|
| water (wt %) | 31.2 |
| oil (wt %) | 23.3 |
| solid particles (wt %) | 45.5 |
| SARA analysis (wt %) | |
| saturates | 43.68 |
| aromatics | 30.82 |
| resins | 13.01 |
| asphaltenes | 12.49 |
| elemental content (wt %) | |
| C | 81.26 |
| H | 12.48 |
| O | 2.88 |
| N | 0.55 |
| S | 2.83 |
| density (g·cm–3) (20 °C) | 0.9705 |
| viscosity (mm2·s–1) (80 °C) | 168.6 |
| pH | 6.42 |
| calorific valuea (kJ·kg–1) | 43,217 |
Calorific value was calculated from elemental analysis data using the following equation:37 calorific value (H) = 339C + 1256H – 109(O – S), where C, H, O, and S are mass ratios of element carbon, hydrogen, oxygen and sulfur.
Besides the basic physical and chemical components of oil, the solid phase had also been studied. The heavy metal content is shown in Table S1; it could be noted the heavy metal content of the oily sludge in this paper was up to standard, and no treatment was required. The particle size distribution is shown in Table S2. Some previous work demonstrated that when the fine content (i.e., <45 μm fraction, as defined in the oil sand industry) was higher than 20% in weight, oil recovery could start to be affected.38 The result shows that the fine content of the sample was very high, which further increased the processing difficulty of oily sludge just like the high asphaltene content. This was also the significant reason that ball milling alone could not achieve efficient recovery of oil, and it was necessary to use ozone-catalyzed oxidation to further achieve oil–solid separation. For a clearer understanding, Figure 2 shows the scanning electron microscope images of the solid from ball milling and ball milling combined with ozone-catalyzed oxidation. It was noted that the solid from ball milling contained oil significantly; in contrast, no oil was observed in the group of ball milling combined with ozone-catalyzed oxidation.
Figure 2.
SEM images of the solid (a) from ball milling and (b) from ball milling combined with ozone-catalyzed oxidation.
2.2. Characteristics of Catalysts
As shown in Table S3, the surface area, pore volume, and pore diameter of CAO were 1.143 m2/g, 0.004 cm3/g, and 13.97 nm, respectively, whereas the corresponding values for NAO were 0.873 m2/g, 0.002 cm3/g, and 9.46 nm. The pHpzc values of NAO and CAO were 7.20 and 11.48, respectively. One possible reason for these changes was that calcination most probably caused the transformation of some carbonate in NAO into corresponding oxides. The chemical compositions of NAO, used NAO (UNAO), CAO, and used CAO (UCAO) catalysts showed that the dominant chemical constituents were SiO2, Al2O3, Fe2O3, and TiO2 (Figure 3). The sum values of SiO2, Al2O3, Fe2O3, and TiO2 were about 94.7, 84.4, 95.8, and 96.2 wt % for NAO, UNAO, CAO, and UCAO, respectively, among which the content values of Al2O3 were 50.6, 45.7, 57.5, and 56.9 wt %, respectively. The changes of the main constituents were insignificant for CAO and UCAO compared to those of NAO and UNAO. Na2O, CaO, K2O, and MgO were minor constituents of catalysts. Most of these metal oxides in catalysts have significant catalytic potential in ozone-catalyzed oxidation of various organics.39,40
Figure 3.
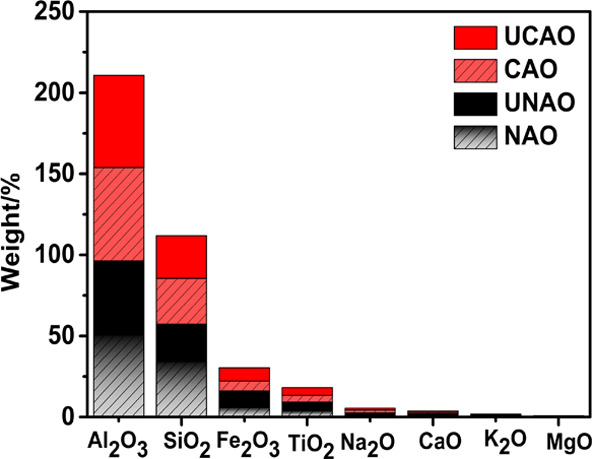
Chemical composition of NAO, UNAO, CAO, and UCAO catalysts.
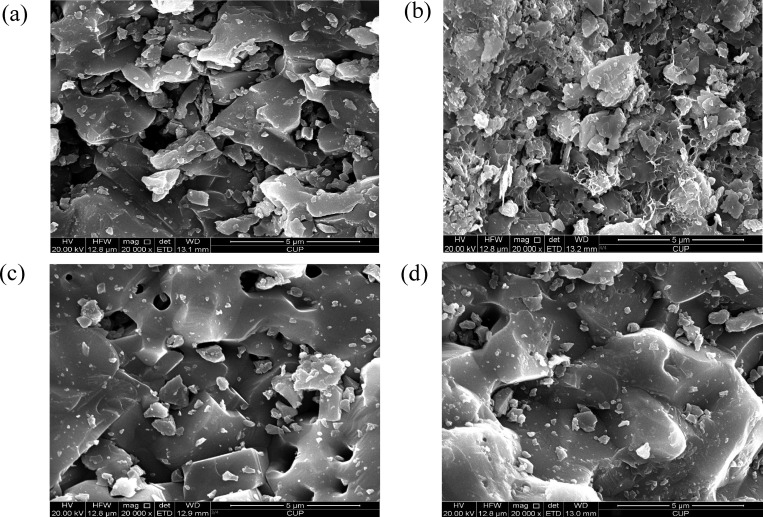
The SEM images (see Figure 4) confirmed that CAO showed smaller pore structures compared with NAO, which might be related to high-temperature calcination. Further, it could be found that the surface morphology of NAO changed greatly after being used, while CAO did not change significantly after being used, which indicates from another perspective that calcination was conducive to enhance the stability of the aluminum ore catalyst.
Figure 4.
SEM micrographs of catalysts (a) NAO, (b) UNAO, (c) CAO, and (d) UCAO.
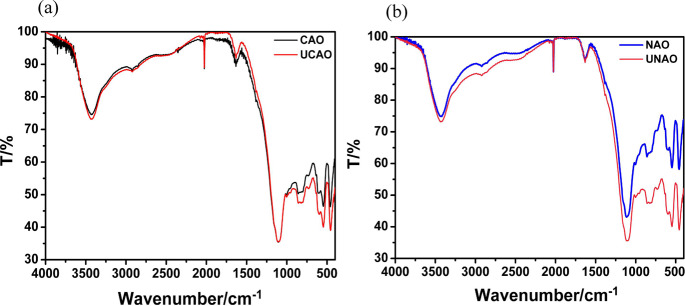
The FTIR spectra of the catalysts are shown in Figure 5, which confirmed that the functional groups at 350–800 cm–1 (mainly Al–O, Si–O, and Fe–O bonds41) on the catalyst surfaces showed smaller differences between CAO and used CAO compared with NAO and used NAO, suggesting higher stability of CAO than that of RAO.
Figure 5.
FTIR spectra of catalysts (a) CAO and UCAO and (b) NAO and UNAO.
2.3. Influence of Surfactant, Time, Liquid–Solid Ratio, and Surfactant Dose on the Oil Content of Oily Sludge by Ball Milling
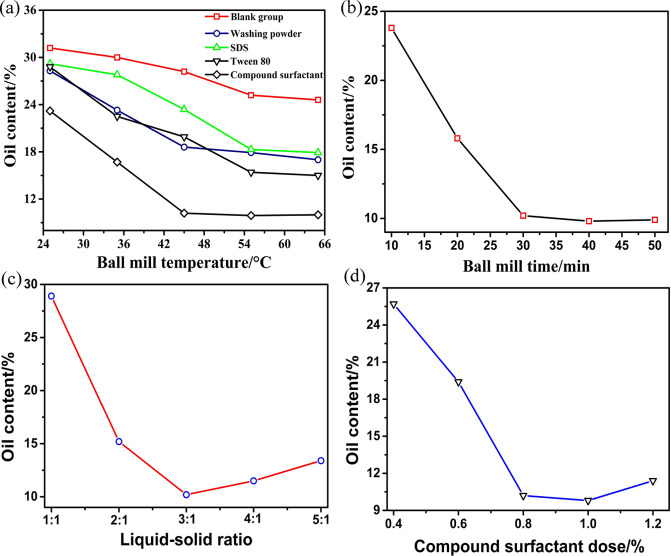
Under the condition that the mass ratio of water to sludge was 3:1 and the ball milling time was 30 min, the influence of compound surfactant, Tween 80, SDBS, and washing powder on the ball milling effect was studied. The dose of the four surfactants was 8 g/1000 g oily sludge, and the blank group was set. The effect of ball milling is shown in Figure 6a, which confirmed that the best effect of the blank group was only 25.5%, which was significantly lower than the other groups. The ball milling effect of the compound surfactant group was significantly better than the other three groups, and the optimum temperature was 45 °C, at which the oil content of the oily sludge could be reduced to 10.2%. Therefore, the compound surfactant is the preferred ball milling agent.
Figure 6.
Influence of (a) surfactant, (b) time, (c) liquid–solid ratio, and (d) compound surfactant dose on the oil content of oily sludge by ball milling.
Under the condition that the mass ratio of water to oil sludge was 3:1, the ball milling temperature was 45 °C, and the compound surfactant dose was 8 g/1000 g oily sludge; the influence of time on the ball milling effect was further studied as shown in Figure 6b. The oil content decreased with ball milling time and had no higher change when the time was more than 30 min.
Under the condition that the ball milling temperature was 45 °C, the ball milling time was 30 min, and the compound surfactant dose was 8 g/1000 g oily sludge; the influence of liquid–solid ratio on the ball milling effect was further studied as shown in Figure 6c. The ball milling effect increased with increasing liquid–solid ratio and reached the best effect at 3:1. Further increasing the liquid–solid ratio, the oil content of oily sludge unexpectedly appeared to increase; one possible reason for that was when the liquid–solid ratio was more than 3:1, the main restraining factor of the ball milling system converted from the liquid–solid ratio into the contact frequency and friction strength between the glass ball and oily sludge. Excessive water joining in the ball milling system reduced the contact frequency and friction intensity between the ball and oily sludge, thus causing the deterioration of the effect of ball milling.
Under the condition that the mass ratio of water to oily sludge was 3:1, the ball milling temperature was 45 °C, the ball milling time was 30 min, the influence of compound surfactant dose on the ball milling effect was studied as shown in Figure 6d, the optimum compound surfactant dose in the ball milling system was 0.8%, and the oil content of the oily sludge would rebound when the compound surfactant dose was beyond 1.0%. One possible reason was that the excessive compound surfactant would lead to the secondary emulsification of oil, water, and solid in the process of ball milling, during which the separated oil would recombine with the solid to form a stable emulsion slurry system, thus affecting the effect of ball milling.
2.4. Influence of Time, Temperature, Ozone Dose, Catalyst Dose, and pH on the Oil Content of Oily Sludge by Ozone-Catalyzed Oxidation
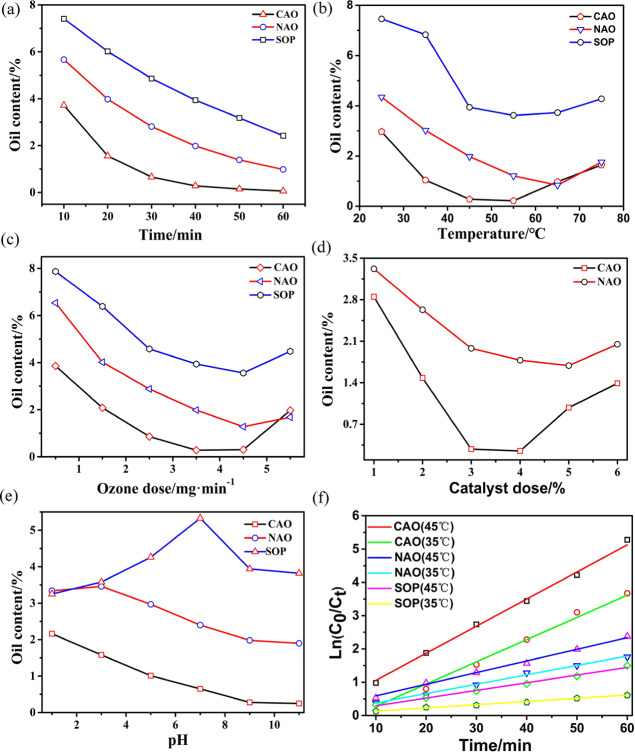
Under the conditions of temperature = 45 °C, pH 9, ozone dose = 3.5 mg/min, and catalyst dose = 3 g/100 g oily sludge (3%), the influence of time on single ozone oxidation (SOP), ozone-catalyzed oxidation using natural aluminum ore as the catalyst (NAO), and ozonation catalyzed using calcined aluminum ore (CAO) as the catalyst was studied. The results are shown in Figure 7a. It could be noted that the deoiling effect of NAO and CAO was better than SOP, which was due to the different reaction mechanisms between ozone-catalyzed oxidation and single ozone oxidation (as shown in Figure 12). During the catalyzed oxidation process, a lot of ·OH, which show no selectivity and high reactivity, were produced, improving the treatment effect.42 Compared with the NAO, the deoiling effect of CAO was more obvious because the specific surface area, pore diameter, and pore volume of the catalyst after calcination were all significantly improved (as shown in Table S2), making the active sites of the catalyst increase and the conversion rate of O3 to ·OH increase. At 40 min, the oil content of oily sludge during CAO was reduced to 0.28%. Further increasing the time, the decrease of oil content became gradual. With comprehensive consideration of treatment cost and standard requirement, the optimal time was 40 min.
Figure 7.
Influence of (a) time, (b) temperature, (c) ozone dose, (d) catalyst dose, and (e) pH on the oil content of oily sludge by ozone-catalyzed oxidation. (f) First-order kinetic model for oil removal of the oxidation process.
Figure 12.
Proposed possible pathways of oil removal.
Under the conditions of pH 9, catalyst dose = 3%, ozone dose = 3.5 mg/min, and time = 40 min, the influence of temperature on the deoiling effect was studied, and the results are shown in Figure 7b. Compared with the other groups, the CAO group had the best deoiling effect, and the oil content could be reduced to 0.28% at 45 °C, meeting the treatment requirements stipulated in the GB4284-2018 standard. Moreover, the lowest oil content was 0.22% at 55 °C. According to the explanation in the literature,43 increasing the reaction temperature of the ozone catalytic oxidation process was conducive to the decomposition of O3 to produce more ·OH, thus enhancing the effect of oil removal. However, when there are too many ·OH in the solution, quenching reactions would occur among them, and the solubility of ozone would gradually decrease with increasing temperature. These two factors led to the decrease of the deoiling effect when the reaction temperature exceeded 55 °C. Therefore, the optimum temperature was 45 °C.
Under the conditions of pH 9, catalyst dose = 3%, time = 40 min, and temperature = 45 °C, the effect of ozone dose on deoiling was studied. As shown in Figure 7c, during the process of ozone dose gradually increasing from 1.5 to 3.5 mg/min, the oil content of three groups was all reduced in a certain degree, and the CAO ozone catalytic oxidation was the most significant followed by NAO ozone catalytic oxidation, again for SOP. The reason was within the range of 1.5–3.5 mg/min, the O3 and ·OH both were not in a saturated state, and the contact probability between O3 or ·OH with oily sludge was strengthened with increasing ozone dose. However, as described above, the deoiling intensity of ·OH was much greater than O3, so the effect of catalyst groups was better than that of the SOP group. Further increasing the ozone dose, within the range of 3.5–5.5 mg/min, CAO and NAO all had a very obvious turning point, beyond which the deoiling effect would be decreased. One major reason was that when the ·OH concentration in solution exceeded the threshold, quenching reaction would happen between each other. As the oil content of oily sludge in the CAO group had been reduced to less than 0.3% under the ozone dose of 3.5 mg/min, with comprehensive consideration of treatment cost and standard requirement, the optimal ozone dose was set as 3.5 mg/min.
Under the conditions of pH 9, ozone dose = 3.5 mg/min, reaction temperature = 45 °C, and reaction time = 40 min, the influence of catalyst dose on deoiling was studied. The results are shown in Figure 7d. With increasing catalyst dose, the oil content of oily sludge in the ozone-catalyzed oxidation groups both showed a trend of first decreasing and then increasing. This was because when the catalyst dose increased to a certain degree, the amount of ·OH reached a supersaturation state relative to the oil content of oily sludge, after which quenching reaction would happen, affecting the reaction efficiency. The deoiling effect of CAO was better than that of NAO because the CAO catalyst had more active points, which could produce more ·OH acting with O3, which also caused the inflection point of its curve appearing earlier than that of NAO.
Under the conditions of ozone dose = 3.5 mg/min, catalyst dose = 3%, reaction temperature = 45 °C, and reaction time = 30 min, the effect of pH on deoiling was studied, and the results are shown in Figure 7e. Under different pH conditions, the oil content of the oily sludge treated by three different processes all had some change, and the change in trend was as follows: the oil content of SOP increased first and then decreased, while the oil content of catalyzed oxidation groups both decreased continuously after treatment. The influence of pH on the oil content was mainly due to the fact that OH– could induce O3 decomposition to produce ·OH.43 In addition, pH also determined the charge type of water solution or catalyst surface.44 When the pH of the solution was higher than the pHpzc of the catalyst, the catalyst surface would undergo deprotonation reaction; on the contrary, when the pH of the solution was lower than the pHpzc of the catalyst, the catalyst surface would undergo protonation reaction. In the lower pH range, the reaction was dominated by O3. With increasing pH , the effect of ·OH gradually strengthened, and the mutual inhibition of these two effects directly led to the deoiling effect of the NAO ozone-catalyzed oxidation group even lower than that of the SOP group at pH 3–5. Further increasing the pH, the advantage of ·OH was gradually reflected, and the deoiling effect of NAO was significantly increased. After pH > 6, its deoiling effect was gradually better than that of SOP. In the catalytic oxidation process with CAO as the catalyst, the deoiling effect was continuously better than that of SOP or NAO at pH 3–11, indicating that ·OH had been playing a dominant role and also indicating that the CAO catalyst could adapt to both acid and alkali environment. At pH 9, the oil content of the treated oily sludge could be reduced to 0.28%. With comprehensive consideration of treatment cost and standard requirement, the optimal pH was 9.
2.5. Deoiling Reaction Kinetics
As could be seen from Figure 7f, the reactions of SOP, NAO, and CAO all met the first-order reaction kinetics model.19 According to the Arrhenius formula (eq 1) and first-order reaction kinetics equation (eq 2),45 the reaction rate constant k and the activation energy Ea of the three groups at 308.15 and 318.15 K could be calculated, as shown in Table S4.
Compared with SOP, the reaction activation energy of the CAO catalytic group was significantly reduced from 69.062 to 10.884 kJ/mol and decreased by ∼82.26%. Moreover, the reaction rate constant k could be increased by ∼3 times, which further proved the good catalytic performance of the CAO catalyst in theory.
| 1 |
| 2 |
where k is the reaction rate constant (min–1), A is the predaceous factor (dimensionless), Ea is the reaction activation energy ( kJ·mol–1), Ct is the oil content of soil or sludge at time t (%),and T and t represents the reaction temperature (K) and reaction time (min), respectively.
2.6. Reusability and Stability of the CAO Catalyst
Under the optimal conditions of ozone dose = 3.5 mg/min, catalyst dose = 3%, temperature = 45 °C, time = 30 min, and pH 9, the deoiling effect of oily sludge in sequential 10 repeated uses of CAO and NAO was monitored. The results are shown in Figure 8. The oil content of the treated oily sludge was maintained in the range of 0.25–0.29% for CAO treatment and within 1.92–2.98% in NAO. The results suggested that CAO was more stable in comparison with NAO and could be repeatedly used, which was consistent with the characterization result of scanning electron microscopy (SEM) or X-ray fluorescence (XRF).
Figure 8.
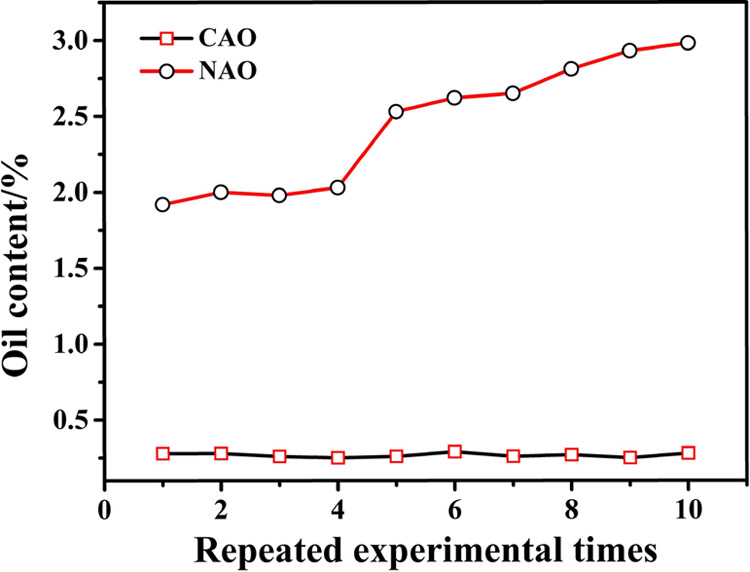
Oil content of treated oily sludge in repeated uses of CAO and NAO.
2.7. Analysis of Recovered Oil and Treatment Mechanism
To evaluate the quality and value of CO (crude oil), BO (recovered oil from ball milling), and BOO (recovered oil from ball milling combined with ozone-catalyzed oxidation), thermal gravimetric analysis was done for the three oils, and the results are shown in Figure S3. Their weights leveled off at approximately 500 °C, at which CO and BOO both had approximately 5–6% residue while BO had approximately 12% residue. The residues mainly were asphaltenes and fine clay solids. The asphaltene content of BO was not much, as shown in Figure 9, which clarified that ball milling alone could not effectively separate oil from fines.
Figure 9.
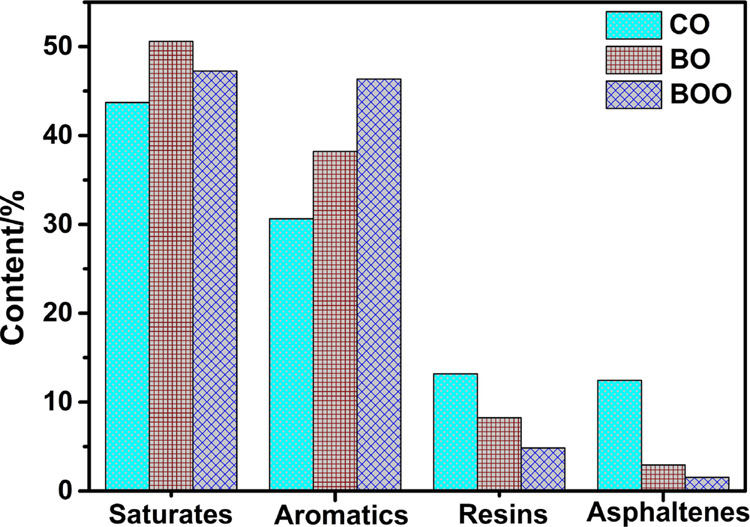
SARA comparison of CO, BO, and BOO.
Figure 9 shows the SARA comparison of CO, BO, and BOO. It could be seen that the asphaltene content of BO was less than CO, which indicated that most of the asphaltenes were still adsorbed on the solid surface combining with the oil content of the solid by ball milling mentioned earlier, was up to 10.2%. Some studies have reported that the saturates and aromatics in the oil-contaminated soil were mainly adsorbed on the surface of soil particles by physical action (such as van der Waals forces),46 while the large asphaltene molecules containing some heteroatoms (N, S, Zn, Cu, Pb, Ni, and other metals) had strong polarity and are usually combined with soil firmly by hydrogen or chemical bonds.47 Ball milling could remove saturates, aromatics, and most of the resins effectively but does nothing about the large asphaltene molecules in the soil. With further observation, the asphaltene content of BOO was also less than CO, and the oil content of solid by ball milling combined with ozone-catalyzed oxidation was less 0.3%, which indicated that asphaltenes were not present in the oil nor on the solid surface. Under the attack of O3 and ·OH, the asphaltenes were broken into small molecules, and the different pH values of the three oils in Table S5 further confirmed the inference.
To explore the reaction mechanisms between ozone-catalyzed oxidation and ozone oxidation, the oxidation mechanism of the three processes was analyzed by the EPR method using DMPO as the capturing agent, and the results are shown in Figure 10. When the peak is 1:1:1:1, it is the peak of OOH; when the peak is 1:2:2:1, it is the peak of ·OH; and when the peak is 1:1:1, it is 1O2.48 Combined with the judgment to observe Figure 10, it could be found that there was no free radical signal in the process of SOP, which indicated that the reaction process was O3 oxidation, while the NAO and CAO process both had a quad signal peak, and the peak shape accorded with 1:2:2:1, therefore, the two processes of NAO and CAO were mainly ·OH oxidation.
Figure 10.
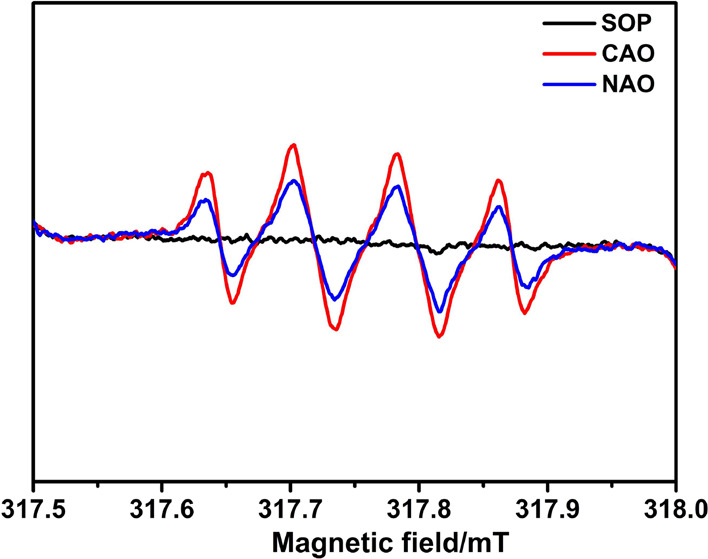
EPR spectra of SOP, CAO, and NAO.
Previous studies reported that hydrogen or chemical bonds (mainly the O–H···O) between asphaltenes and soil were the essence of the strong interaction that vigorously hampered asphaltene desorption and increased the handling difficulty of oily sludge.46 The experimental results show that the separation of asphaltenes and solid can be realized effectively by ozone- catalyzed oxidation. To explore the mechanism of the ozone-catalyzed oxidation process, the XPS core-level spectra of O1s in oily sludge before the catalyzed oxidation process and after the catalyzed oxidation process was determined, and the result is shown in Figure 11. As can be seen from Figure 11a, the O1s spectra of was decomposed into three peaks after deconvolution, located at 530.3 eV (O-1), 531.7 eV (O-2), and 533.0 eV (O-3), which correspond to highly conjugated forms of carbonyl oxygen (from asphaltene), —C=O groups (from asphaltene), and C–OH groups or O–H···O groups (between asphaltenes and soil), respectively,34,46,49 while, from Figure 11b, it could be noted that peaks of O-1, O-2, and O-3 all became weakened significantly after catalyzed oxidation, which proved that the hydrogen bond between asphaltenes and solid was broken and realized the asphaltene–solid separation. With further observation, two new peaks appeared after the catalyzed oxidation process, located at 531.1 eV (O-4) and 532.5 eV (O-5),49 which were ascribed to Al–O (from soil) and Si–O (from soil). One possible reason for this was that XPS could not detect the composition of the soil surface before the catalyzed oxidation process due to asphaltene coverage, which proved the asphaltene removal effectiveness by catalyzed oxidation from a different perspective.
Figure 11.
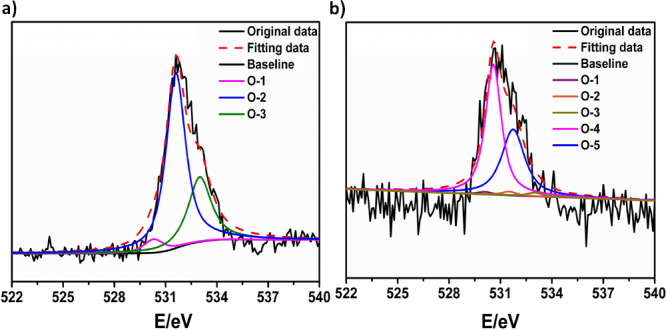
XPS core-level spectra of O1s (a) before the catalyzed oxidation process and (b) after the catalyzed oxidation process.
Therefore, one possible pathway of oil removal during the treatment process by ball milling and ozone-catalyzed oxidation was proposed, as shown in Figure 12. Through the combined forces of surfactant and ball milling, the van der Waals force between saturates, aromatics, resins, and soil was destroyed, resulting in the three-component desorption from the surface of soil particles. However, ball milling or surfactant has no significant effect on the asphaltenes, so the asphaltenes remained firmly attached to the soil surface. Then, during ozonation, O3 and •OH attacked the asphaltenes, making them transform into smaller molecules (saturates or aromatics). Thus, the hydrogen or chemical bond between asphaltenes and soil was broken, resulting in the asphaltene desorption from the surface of soil particles.
3. Conclusions
Overall, the combined process with “ball milling + ozone-catalyzed oxidation” as the core was investigated to solve the two problems in the treatment of oily sludge from the tank bottom by the thermochemical cleaning process, and this novel combined process could solve the two problems brilliantly. Under the optimal conditions, the oil content of treated oily sludge from the tank bottom could be reduced to 0.28%, which met the treatment and disposal requirements stipulated in GB4284-2018. Accordingly, the recovered oil during the process had a high utilization value. Also, the reaction kinetics and mechanism also were described. The reaction met the first-order reaction kinetics model. Compared with SOP, the reaction activation energy of CAO was significantly reduced from 69.062 to 10.884 kJ/mol and decreased by ∼82.26%, and the reaction rate constant k could be increased by ∼3 times. The combined process with “ball milling + ozone-catalyzed oxidation” as the core provides a reference for the harmless and resourceful treatment of oily sludge from the tank bottom.
4. Materials and Methods
4.1. Materials
The main raw materials included sodium hydroxide (NaOH), hydrochloric acid (HCl), anhydrous sodium sulfate (Na2SO4), soluble starch, potassium iodide (KI), sodium silicate (Na2SiO3·9H2O), and sodium dodecylbenzene sulfonate (SDS, C18H29NaO3S), all of which were analytically pure and purchased from Sinopharm Group Chemical Reagent Beijing Co. Ltd.; carbon tetrachloride (CCl4) was of chromatographic grade and purchased from Tianjin Guangfu Fine Chemical Research Institute; and Tween 80, washing powder, and compound surfactant were of industrial grade.
4.2. Methods
4.2.1. Property Analysis of Oily Sludge
Oily sludge was supplied by Huabei Oilfield in China and was obtained during the tank clearing process, and the oil content was tested by an infrared oil meter (GB/T16488-1996) through the oily sludge treated by Soxhlet extraction using carbon tetrachloride as the solvent (the recovered oil by this method was called crude oil),50 as shown in Figure 13. The water content was determined according to the ASTM D95-13 standard,51 whereas the soil content was calculated by difference. The SARA (saturates, aromatics, resins, and asphaltenes) fractions were separated according to the Chinese Standard procedure (SY/T 5119-2008).52 The elemental content (C, H, O, N, and S) was determined using a VARIO EL III elemental analyzer.53 Thermogravimetric analysis of the recovered oil was performed in a Linseis STA PT1600 thermogravimetric analyzer (TGA); before analysis, a thermogravimetric baseline was needed to be created to reduce measurement errors. The particle size was analyzed by wet sieving, the heavy metal content was analyzed by ICP-MS (Prodigy7, Leeman), and the morphology analysis was done by scanning electron microscopy (SEM; field-emission scanning electron microscope, Merlin Compact). All the measurements were done in duplicate.
Figure 13.
Soxhlet extraction of oily sludge.
The solid content (X1), oil content of dry oil sludge (X2), and deoiling efficiency (X3) were calculated respectively according to the following formula:
| 3 |
| 4 |
| 5 |
where M is the total mass of the sample (g), M1 is the water content in the original sample, and M2 and M3 represent the oil content of the sample before and after treatment, respectively.
4.2.2. Preparation and Characterization of Catalysts
The natural aluminum ore (NAO; density = 3.92 g/cm3, hardness = 2.5) used in this study was obtained from a local mine. NAO was washed with distilled water to remove the debris after being thoroughly dried at room temperature, and it was calcined, referred to as calcined aluminum ore (CAO) hereafter, at 1075.15 K for 6 h. Both the NAO and CAO were used as catalysts. Scanning electron microscopy (SEM) was applied for the morphology analysis. The pH of zero point charge (pHpzc) was measured by the zeta electric potential method, where the net charge of the catalyst surface was zero. The bulk chemical compositions were measured by X-ray fluorescence (XRF) (ZSX 100e, Rigaku, Japan). N2 adsorption–desorption was used to measure the surface area and pore size distribution (ASAP2000, Micromeritics, USA). The surface functional groups were performed with Fourier transform infrared (FTIR) spectroscopy in the range of 4000–400 cm–1 (MagnaIR 560 E.S.P., Nicolet, USA).
4.2.3. Experimental Setup and Procedure
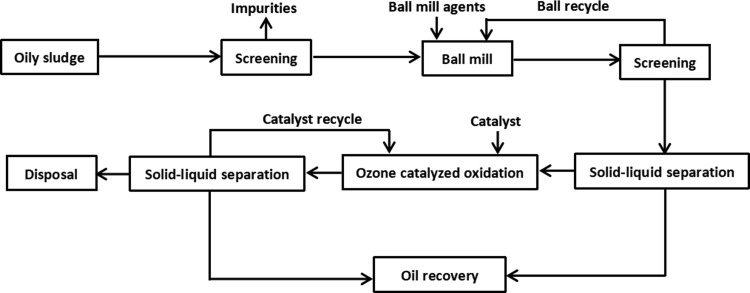
The impurities in oily sludge were removed through screening, 300 g of oily sludge sample was loaded into a 2000 mL beaker, and then fresh water, surfactant, and glass balls were added in turn. After thorough stirring, the beaker was put into a constant temperature water bath for the ball milling test. After the test, the slurry in the beaker was separated from the glass ball with a vibrating screen, and the separated glass ball was reused. The slurry was centrifuged at 3000 rpm to complete the solid–liquid separation operation. The liquid phase was separated from oil and water under the static condition. Five grams of the solid phase was taken out and put into a vacuum oven (−0.1 MPa, 60 °C) after drying, and the oil content of the solid phase was tested.
The rest of the solid phase was put into a column reactor to test ozone-catalyzed oxidation. The liquid–solid ratio of the system was maintained at 3:1, and the pH of the system was adjusted with HCl and NaOH. After the reaction, the catalyst was recovered and reused by screening, the slurry was poured into the centrifugal tube smoothly, and the solid–liquid separation was completed under the centrifugal force of 3000 rpm. The liquid phase was separated from oil and water under static conditions, the solid phase was taken out and put into a vacuum oven (−0.1 MPa, 60 °C) after drying, and the oil content of solid phase was tested. The overall test flow is shown in Figure 14.
Figure 14.
Overall process of oily sludge treatment.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant 21077002).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00958.
Additional information as noted in the text, catalyst preparation process (Figure S1), micromorphology of the oily sludge (Figure S2), thermal gravimetric analysis of CO, BO, and BOO from oily sludge (Figure S3), heavy metal content in the oily sludge particle (Table S1), size distribution of the solid from oily sludge (Table S2), properties of NAO and CAO catalysts Table S3), reaction parameters of ozone oxidation and ozone catalytic oxidation processes (Table S4), and analysis and comparison of CO, BO, and BOO from oily soil (Table S5) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Taiwo E. A.; Otolorin J. A. Oil Recovery from Petroleum Sludge by Solvent Extraction. Pet. Sci. Technol. 2009, 27, 836–844. 10.1080/10916460802455582. [DOI] [Google Scholar]
- Ramaswamy D.; Kar D. D.; De S. A Study on Recovery of Oil from Sludge Containing Oil using Froth Flotation. J. Environ. Manage. 2007, 85, 150–154. 10.1016/j.jenvman.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang X.; Pan Y.; Chen Y. Analysis of Oil Content in Drying Petroleum Sludge of Tank Bottom. Int. J. Hydrogen Energy 2017, 42, 18681–18684. 10.1016/j.ijhydene.2017.04.153. [DOI] [Google Scholar]
- Qin H.; Ma J.; Wang Q.; Li J.; Chi M.; Zhang L.; Liu H. Treatment of Oil Shale Sludge using Solvent Extraction and Thermo-Chemistry. Chin. J. Environ. Eng. 2016, 10, 851–857. [Google Scholar]
- Shen Y.; Chen X.; Wang J.; Ge X.; Chen M. Oil Sludge Recycling by Ash-Catalyzed Pyrolysis-Reforming Processes. Fuel 2016, 182, 871–878. 10.1016/j.fuel.2016.05.102. [DOI] [Google Scholar]
- Tiwari P.; Deo M. Detailed Kinetic Analysis of Oil Shale Pyrolysis TGA Data. AIChE J. 2012, 58, 505–515. 10.1002/aic.12589. [DOI] [Google Scholar]
- Guo S.; Li G.; Qu J.; Liu X. Improvement of Acidification on Dewaterability of Oily Sludge from Flotation. Chem. Eng. J. 2011, 168, 746–751. 10.1016/j.cej.2011.01.070. [DOI] [Google Scholar]
- Al-Doury M. M. I. Treatment of Oily Sludge using Solvent Extraction. Pet. Sci. Technol. 2019, 37, 190–196. 10.1080/10916466.2018.1533859. [DOI] [Google Scholar]
- Fan C.; Yan J.; Huang Y.; Han X.; Jiang X. XRD and TG-FTIR Study of the Effect of Mineral Matrix on the Pyrolysis and Combustion of Organic Matter in Shale Char. Fuel 2015, 139, 502–510. 10.1016/j.fuel.2014.09.021. [DOI] [Google Scholar]
- Lai C.-C.; Huang Y.-C.; Wei Y.-H.; Chang J.-S. Biosurfactant-Enhanced Removal of Total Petroleum Hydrocarbons from Contaminated Soil. J. Hazard. Mater. 2009, 167, 609–614. 10.1016/j.jhazmat.2009.01.017. [DOI] [PubMed] [Google Scholar]
- da Rosa J. J.; Rubio J. The FF (flocculation-flotation) Process. Miner. Eng. 2005, 18, 701–707. 10.1016/j.mineng.2004.10.010. [DOI] [Google Scholar]
- Schramm L. L.; Mikula R. J. Froth Flotation of Oil Sand Bitumen. Foam Eng. 2012, 251. 10.1002/9781119954620.ch12. [DOI] [Google Scholar]
- Liang J.; Zhao L.; Du N.; Li H.; Hou W. Solid Effect in Solvent Extraction Treatment of Pre-Treated Oily Sludge. Sep. Purif. Technol. 2014, 130, 28–33. 10.1016/j.seppur.2014.03.027. [DOI] [Google Scholar]
- Al-Zahrani S. M.; Putra M. D. Used Lubricating Oil Regeneration by Various Solvent Extraction Techniques. J. Ind. Eng. Chem. 2013, 19, 536–539. 10.1016/j.jiec.2012.09.007. [DOI] [Google Scholar]
- Song W.; Vidonish J. E.; Kamath R.; Yu P.; Chu C.; Moorthy B.; Gao B.; Zygourakis K.; Alvarez P. J. J. Pilot-scale Pyrolytic Remediation of Crude-Oil-Contaminated Soil in a Continuously-Fed Reactor: Treatment Intensity Trade-Offs. Environ. Sci. Technol. 2019, 53, 2045–2053. 10.1021/acs.est.8b05825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z.; Du A.; Wang Z.; Fang P.; Li X. Experimental Study on Pyrolysis Characteristics of Oil Sludge with a Tube Furnace Reactor. Energy Fuels 2017, 31, 8102–8108. 10.1021/acs.energyfuels.7b01363. [DOI] [Google Scholar]
- Wang Z.; Guo Q.; Liu X.; Cao C. Low Temperature Pyrolysis Characteristics of Oil Sludge under Various Heating Conditions. Energy Fuels 2007, 21, 957–962. 10.1021/ef060628g. [DOI] [Google Scholar]
- Zheng X.; Ying Z.; Cui J.; Wang B.; Chen J.; Zhang Q. Simultaneous Dewatering and Recovering Oil from High-Viscosity Oily Sludge through the Combination Process of Demulsification, Viscosity Reduction, and Centrifugation. Energy Fuels 2017, 31, 14401–14407. 10.1021/acs.energyfuels.7b02481. [DOI] [Google Scholar]
- Liang J.; Zhao L.; Hou W. Solid Effect in Chemical Cleaning Treatment of Oily Sludge. Colloids Surf., A 2017, 522, 38–42. 10.1016/j.colsurfa.2017.02.038. [DOI] [Google Scholar]
- Xu Y.; Zhang Y.; Liu X.; Chen H.; Fang Y. Retrieving Oil and Recycling Surfactant in Surfactant-Enhanced Soil Washing. ACS Sustainable Chem. Eng. 2018, 6, 4981–4986. 10.1021/acssuschemeng.7b04614. [DOI] [Google Scholar]
- Zhang J.; Li J.; Thring R.; Liu L. Application of Ultrasound and Fenton’s Reaction Process for the Treatment of Oily Sludge. Procedia Environ. Sci. 2013, 18, 686–693. 10.1016/j.proenv.2013.04.093. [DOI] [Google Scholar]
- Xu N.; Wang W.; Han P.; Lu X. Effects of Ultrasound on Oily Sludge Deoiling. J. Hazard. Mater. 2009, 171, 914–917. 10.1016/j.jhazmat.2009.06.091. [DOI] [PubMed] [Google Scholar]
- Hu G.; Li J.; Thring R. W.; Arocena J. Ultrasonic Oil Recovery and Salt Removal from Refinery Tank Bottom Sludge. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 1425–1435. 10.1080/10934529.2014.928556. [DOI] [PubMed] [Google Scholar]
- Mullakaev M. S.; Abramov V. O.; Abramova A. V. Development of Ultrasonic Equipment and Technology for Well Stimulation and Enhanced Oil Recovery. J. Pet. Sci. Eng. 2015, 125, 201–208. 10.1016/j.petrol.2014.10.024. [DOI] [Google Scholar]
- Rajaković V.; Skala D. Separation of Water-in-Oil Emulsions by Freeze/Thaw Method and Microwave Radiation. Sep. Purif. Technol. 2006, 49, 192–196. 10.1016/j.seppur.2005.09.012. [DOI] [Google Scholar]
- Elektorowicz M.; Habibi S.; Chifrina R. Effect of Electrical Potential on the Electro-Demulsification of Oily Sludge. J. Colloid Interface Sci. 2006, 295, 535–541. 10.1016/j.jcis.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Mao X.; Jiang R.; Xiao W.; Yu J. Use of Surfactants for the Remediation of Contaminated Soils: a Review. J. Hazard. Mater. 2015, 285, 419–435. 10.1016/j.jhazmat.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Hu G.; Li J.; Zeng G. Recent Development in the Treatment of Oily Sludge from Petroleum Industry: a Review. J. Hazard. Mater. 2013, 261, 470–490. 10.1016/j.jhazmat.2013.07.069. [DOI] [PubMed] [Google Scholar]
- Chen T.; Delgado A. G.; Yavuz B. M.; Proctor A. J.; Maldonado J.; Zuo Y.; Westerhoff P.; Krajmalnik-Brown R.; Rittmann B. E. Ozone Enhances Biodegradability of Heavy Hydrocarbons in Soil. J. Environ. Eng. Sci. 2016, 11, 7–17. 10.1680/jenes.16.00002. [DOI] [Google Scholar]
- Rondón M.; Bouriat P.; Lachaise J.; Salager J.-L. Breaking of Water-in-Crude oil Emulsions: 1. Physicochemical Phenomenology of Demulsifier Action. Energy Fuels 2006, 20, 1600–1604. 10.1021/ef060017o. [DOI] [Google Scholar]
- Trellu C.; Mousset E.; Pechaud Y.; Huguenot D.; van Hullebusch E. D.; Esposito G.; Oturan M. A. Removal of Hydrophobic Organic Pollutants from Soil Washing/Flushing Solutions:a Critical Review. J. Hazard. Mater. 2016, 306, 149–174. 10.1016/j.jhazmat.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Mohammadi S.; Mirghaffari N. A Preliminary Study of the Preparation of Porous Carbon from Oil Sludge for Water Treatment by Simple Pyrolysis or KOH Activation. New Carbon Mater. 2015, 30, 310–318. 10.1016/S1872-5805(15)60192-5. [DOI] [Google Scholar]
- Guritno M. A.; Sihombing R.; Krisnandi Y. K. Synthesis of Porous Activated Carbon from Petroleum Sludge using Mesoporous Silica Template. AIP Conf. Proc. 2016, 1729, 020040 10.1063/1.4946943. [DOI] [Google Scholar]
- Wang J.; Liu T.-L.; Huang Q.-X.; Ma Z.-Y.; Chi Y.; Yan J.-H. Production and Characterization of High Quality Activated Carbon from Oily Sludge. Fuel Process. Technol. 2017, 162, 13–19. 10.1016/j.fuproc.2017.03.017. [DOI] [Google Scholar]
- Dutta Majumdar R.; Montina T.; Mullins O. C.; Gerken M.; Hazendonk P. Insights into Asphaltene Aggregate Structure using Ultrafast MAS Solid-state 1H NMR Spectroscopy. Fuel 2017, 193, 359–368. 10.1016/j.fuel.2016.12.082. [DOI] [Google Scholar]
- Pourmohammadbagher A.; Shaw J. M. Probing the Impact of Asphaltene Contamination on Kaolinite and Illite Clay Behaviors in Water and Organic Solvents: A Calorimetric Study. Energy Fuels 2016, 30, 6561–6569. 10.1021/acs.energyfuels.6b00646. [DOI] [Google Scholar]
- Hu J.; Gan J.; Li J.; Luo Y.; Wang G.; Wu L.; Gong Y. Extraction of Crude Oil from Petrochemical Sludge: Characterization of Products using Thermogravimetric Analysis. Fuel 2017, 188, 166–172. 10.1016/j.fuel.2016.09.068. [DOI] [Google Scholar]
- Liu J.; Xu Z.; Masliyah J. Interaction between Bitumen and Fines in Oil Sands Extraction System: Implication to Bitumen Recovery. Can. J. Chem. Eng. 2004, 82, 655–666. 10.1002/cjce.5450820404. [DOI] [Google Scholar]
- Zhao D.-Z.; Shi C.; Li X.-S.; Zhu A.-M.; Jang B. W.-L. Enhanced Effect of Water Vapor on Complete Oxidation of Formaldehyde in Air with Ozone over MnOx Catalysts at Room Temperature. J. Hazard. Mater. 2012, 239-240, 362–369. 10.1016/j.jhazmat.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Rodríguez J. L.; Poznyak T.; Valenzuela M. A.; Tiznado H.; Chairez I. Surface Interactions and Mechanistic Studies of 2,4-dichlorophenoxyacetic Acid Degradation by Catalytic Ozonation in Presence of Ni/TiO2. Chem. Eng. J. 2013, 222, 426–434. 10.1016/j.cej.2013.02.086. [DOI] [Google Scholar]
- Chen C.; Cai L.-X.; Tan B.; Zhang Y.-J.; Yang X.-D.; Zhang J. Ammonia Detection by using Flexible Lewis Acidic Sites in Luminescent Porous Frameworks Constructed from a Bipyridinium Derivative. Chem. Commun. 2015, 51, 8189–8192. 10.1039/C5CC01239B. [DOI] [PubMed] [Google Scholar]
- Qi F.; Xu B.; Chen Z.; Ma J.; Sun D.; Zhang L. Influence of Aluminum Oxides Surface Properties on Catalyzed Ozonation of 2,4,6-trichloroanisole. Sep. Purif. Technol. 2009, 66, 405–410. 10.1016/j.seppur.2009.01.013. [DOI] [Google Scholar]
- Zhang T.; Ma J. Catalytic Ozonation of Trace Nitrobenzene in Water with Synthetic Goethite. J. Mol. Catal. A: Chem. 2008, 279, 82–89. 10.1016/j.molcata.2007.09.030. [DOI] [Google Scholar]
- Nawrocki J.; Kasprzyk-Hordern B. The Efficiency and Mechanisms of Catalytic Ozonation. Appl. Catal., B 2010, 99, 27–42. 10.1016/j.apcatb.2010.06.033. [DOI] [Google Scholar]
- Liu X.; Yao T.; Lai R.; Xiu J.; Huang L.; Sun S.; Luo Y.; Song Z.; Zhang Z. Recovery of Crude Oil from Oily Sludge in an Oilfield by Sophorolipid. Pet. Sci. Technol. 2019, 37, 1582–1588. 10.1080/10916466.2019.1594286. [DOI] [Google Scholar]
- Li Z.; Wu P.; Hou X.; Liu D.; Wang J.; Lou B.; Kong X. Probing the Essence of Strong Interaction in Oily Sludge with Thermodynamic Analysis. Sep. Purif. Technol. 2017, 187, 84–90. 10.1016/j.seppur.2017.06.044. [DOI] [Google Scholar]
- Pourmohammadbagher A.; Shaw J. M. Probing Contaminant Transport to and from Clay Surfaces in Organic Solvents and Water using Solution Calorimetry. Environ. Sci. Technol. 2015, 49, 10841–10849. 10.1021/acs.est.5b02416. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Z.; Wang Z. U.; Wang H.; Lu J.; Yu S.-H.; Jiang H.-L. Singlet Oxygen-Engaged Selective Photo-Oxidation over Pt Nanocrystals/Porphyrinic MOF: The Roles of Photothermal Effect and Pt Electronic State. J. Am. Chem. Soc. 2017, 139, 2035–2044. 10.1021/jacs.6b12074. [DOI] [PubMed] [Google Scholar]
- Crist B. V.Hand Books of Monochromatic XPS Spectra; XPS International, LLC: 1999. [Google Scholar]
- Chen T.; Delgado A. G.; Yavuz B. M.; Maldonado J.; Zuo Y.; Kamath R.; Westerhoff P.; Krajmalnik-Brown R.; Rittmann B. E. Interpreting Interactions between Ozone and Residual Petroleum Hydrocarbons in Soil. Environ. Sci. Technol. 2017, 51, 506–513. 10.1021/acs.est.6b04534. [DOI] [PubMed] [Google Scholar]
- State Environmental Protection Administration of China (SEPAC), Water Quality Determination of Petroleum Oil, Animal and Vegetable Oils-Infrared Photometric Method (GB16488); 1996.
- Karlsen D. A.; Larter S. R. Analysis of Petroleum Fractions by TlC-FID-Applications to Petroleum Reservoir Description. Org. Geochem. 1991, 17, 603–617. 10.1016/0146-6380(91)90004-4. [DOI] [Google Scholar]
- Jiang C.; Larter S. R.; Noke K. J.; Snowdon L. R. TLC-FID (Iatroscan) Analysis of Heavy Oil and Tar Sand Samples. Org. Geochem. 2008, 39, 1210–1214. 10.1016/j.orggeochem.2008.01.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.