Abstract

The effect of cosolvent on trace free water in the determination of the contamination degree of jet fuel was investigated. The interference of trace free water on the automatic particle counter can be eliminated by adding isopropanol as a cosolvent to the measured oil sample. Isopropanol can dissolve trace free water in oil. Addition of isopropanol could stabilize the pollution grade of particles with size ≥30 μm (c) at the same level, which is most obviously affected by free water without isopropanol. The standard uncertainty u(X1) is slightly reduced with the addition of isopropanol, and the repeatability and accuracy of the automatic particle counting method are obviously improved. The results show that isopropanol should be added as a cosolvent to eliminate the interference of free water when the contamination degree of jet fuel oil samples with obvious free water is determined by the automatic particle counting method.
Introduction
The automatic particle counter is an instrument that uses optical sensors to measure solid particles in oil, which can directly detect oil in the laboratory or on-line. It can measure the distribution of particle number and size.1−3 According to the equivalent projection particle size, the light flux, which is blocked by particles, is received through the shading sensor and converted into electrical signals, which are transmitted to the counter through the preamplifier for counting. However, the trace free water suspended in jet fuel can also shield the light, which interferes with the results of the automatic particle counting method.4−7
Domestic research lacks the application of automatic particle counter in the field of jet fuel.8−10 In contrast, foreign research on the application of particle counter in the field of jet fuel is more, mainly exploring the feasibility of using this method, but few standards have been formed. In recent years, British Energy Institute has proposed a test standard for detecting fuel particulate pollutants by sensors.11−13 There are three standards: IP 564 (Cleanliness Measurement of Aviation Turbine Fuel—Laboratory Automatic Particle Counter Method), IP 565 (Cleanliness Measurement of Aviation Turbine Fuel—Portable Automatic Particle Counter Method), and IP 577 (Cleanliness Measurement of Aviation Turbine Fuel—Shaded Automatic Particle Counter Method).
Because the application time of the particle counter method in the jet fuel field is still short, there is no mature and effective standard method for monitoring jet fuel quality using sensors in the United States. Only TARDEC of the U.S. Army put forward a monitoring method proposal,12 which uses the 19/17/14/13 grade of 4 μm (c)/6 μm (c)/14 μm (c)/30 μm (c) particle size in ISO 4406 pollution level standard for the quality control requirement of jet fuel contamination. The USA TARDEC has systematically studied the feasibility of applying a particle counter in the jet fuel field and evaluated the application prospects of particle counter from many aspects, especially eliminating the influence of free water on the results of particle counter.12 Therefore, isopropanol was selected as the cosolvent to evaluate the effect of cosolvent on the determination results.
Results and Discussion
Effect of Container Materials on the Determination of the Contamination Degree of Jet Fuel
As Table 1 shows, the contamination degrees of oil samples have not changed significantly, and the repeatability of the test is good. The influence of free water on the automatic particle counter is basically eliminated by using a glass bottle. The chemical composition of the glass bottle is mainly silica, which may absorb part of water from oil samples by a hydrogen bond. When free water is adsorbed on the wall of the glass bottle, the result of particle counting of oil samples would naturally decrease. Although the glass bottle eliminated the influence of free water, it had a negative impact on the determination of contamination degrees, and the automatic particle counter could not truly reflect the situation of free water in oil samples, which is not conducive to the comprehensive evaluation of the quality of fuel.
Table 1. Determination of Particle Quantity and Grade of Samples in a Glass Bottle.
| ACM
20 |
||||||
|---|---|---|---|---|---|---|
| sample | particle (mg/L) | free water (ppm) | ≥4 μm (c) | ≥6 μm (c) | ≥14 μm (c) | ≥30 μm (c) |
| 1 | 1.0 | 0 | 2410.4/18 | 913.6/17 | 49.8/13 | 1.0/7 |
| 2 | 1.0 | 5 | 2150.4/18 | 953.3/17 | 58.9/13 | 2.0/8 |
| 3 | 1.0 | 10 | 2616.6/19 | 1116.1/17 | 66.7/13 | 2.7/9 |
| 4 | 1.0 | 20 | 2363.9/18 | 1026.5/17 | 65.4/13 | 3.6/9 |
| 5 | 1.0 | 30 | 2848.6/19 | 1200.9/17 | 59.4/13 | 2.6/9 |
| 6 | 2.0 | 0 | 4341.4/19 | 1446.6/18 | 62.1/13 | 3.6/9 |
| 7 | 2.0 | 5 | 4982.1/19 | 1795.7/18 | 76.5/13 | 4.1/9 |
| 8 | 2.0 | 10 | 5108.1/20 | 1685.6/18 | 60.4/13 | 2.0/8 |
| 9 | 2.0 | 20 | 5333.9/20 | 1906.4/18 | 85.8/14 | 3.9/9 |
| 10 | 2.0 | 30 | 4395.2/19 | 1431.9/18 | 57.3/13 | 2.5/8 |
| 11 | 3.0 | 0 | 8109.1/20 | 3206.1/19 | 141.6/14 | 8.7/10 |
| 12 | 3.0 | 5 | 8536.6/20 | 3554.9/19 | 440.4/16 | 13.1/11 |
| 13 | 3.0 | 10 | 7805.3/20 | 3115.7/19 | 183.9/15 | 4.7/9 |
| 14 | 3.0 | 20 | 6903.4/20 | 2618.9/19 | 154.0/14 | 8.0/10 |
| 15 | 3.0 | 30 | 5716.6/20 | 2018.0/18 | 114.4/14 | 8.6/10 |
As shown in Table 2, although the contamination degrees of oil samples have a certain linear relationship, there are also some fluctuations. For example, compared with the no. 2 oil sample, the number of particles in the no. 4 oil sample decreases. There is a linear relationship between the number of particles with different particle sizes [≥4 μm (c) and ≥6 μm (c)]. However, the results of contamination degrees fluctuate when there are particles with large sizes [≥14 μm (c) and ≥30 μm (c)] in oil samples, especially, oil samples with particle size ≥30 μm (c). The main reason may be that the larger the particle size, the more likely it is to be unevenly distributed in the oils. The results of contamination degrees of oil samples are basically kept at the same pollution level, except oil samples with particle size ≥30 μm (c).
Table 2. Determination of Particle Quantity and Grade of Samples in a Metal Bottle.
| ACM
20 |
||||||
|---|---|---|---|---|---|---|
| sample | particle (mg/L) | free water (ppm) | ≥4 μm (c) | ≥6 μm (c) | ≥14 μm (c) | ≥30 μm (c) |
| 1 | 1.0 | 0 | 3940.3/19 | 1751.4/18 | 126.6/14 | 4.1/9 |
| 2 | 1.0 | 5 | 3761.7/19 | 1571.3/18 | 105.0/14 | 4.9/9 |
| 3 | 1.0 | 10 | 4187.1/19 | 1756.4/18 | 108.7/14 | 3.7/9 |
| 4 | 1.0 | 20 | 5455.6/19 | 1855.8/18 | 71.4/13 | 4.7/9 |
| 5 | 1.0 | 30 | 5768.9/19 | 1915.1/18 | 109.0/14 | 4.4/9 |
| 6 | 2.0 | 0 | 3895.9/19 | 1586.9/18 | 141.6/14 | 21.6/12 |
| 7 | 2.0 | 5 | 4768.7/19 | 1986.9/18 | 126.1/14 | 6.9/10 |
| 8 | 2.0 | 10 | 6788.6/20 | 2581.4/19 | 141.4/14 | 3.4/9 |
| 9 | 2.0 | 20 | 7573.9/20 | 2989.0/19 | 168.4/15 | 8.0/10 |
| 10 | 2.0 | 30 | 8492.8/19 | 2758.0/18 | 171.8/14 | 6.0/10 |
| 11 | 3.0 | 0 | 6180.0/20 | 2460.2/18 | 138.9/14 | 15.1/11 |
| 12 | 3.0 | 5 | 7499.0/20 | 3197.1/19 | 183.6/15 | 6.4/10 |
| 13 | 3.0 | 10 | 7852.6/20 | 3264.4/19 | 166.7/15 | 12.6/11 |
| 14 | 3.0 | 20 | 8389.5/20 | 4000.0/19 | 180.4/15 | 7.4/10 |
| 15 | 3.0 | 30 | 10,152.9/21 | 4420.4/19 | 329.2/16 | 18.9/11 |
As Table 3 exhibits, the number of particles shows a certain linear relationship, especially, the number of particles with particle sizes ≥4 μm (c) and ≥6 μm (c). Theoretically, when the number of small size particles is more, their distribution is more uniform. Compared with the results of plastic bottles and metal bottles, it seems that the results of plastic bottles were better. Similar to the results of metal bottles, the contamination degrees of oil samples are at the same level within the repeatability range.
Table 3. Determination of Particle Quantity and Grade of Samples in a Plastic Bottle without Isopropanol.
| ACM
20 |
||||||
|---|---|---|---|---|---|---|
| sample | particle (mg/L) | free water (ppm) | ≥4 μm (c) | ≥6 μm (c) | ≥14 μm (c) | ≥30 μm (c) |
| 1 | 1.0 | 0 | 2513.1/19 | 1141.7/17 | 74.2/13 | 1.1/7 |
| 2 | 1.0 | 5 | 2006.8/18 | 947.3/17 | 65.7/13 | 1.4/8 |
| 3 | 1.0 | 10 | 3020.6/19 | 1366.2/18 | 107.0/14 | 1.6/8 |
| 4 | 1.0 | 20 | 2712.3/19 | 1205.1/17 | 75.9/13 | 0.9/7 |
| 5 | 1.0 | 30 | 3371.8/19 | 1548.1/18 | 107.4/14 | 2.6/9 |
| 6 | 2.0 | 0 | 3884.4/19 | 1355.0/18 | 77.7/13 | 3.4/9 |
| 7 | 2.0 | 5 | 3639.3/19 | 1261.4/17 | 73.1/13 | 9.6/10 |
| 8 | 2.0 | 10 | 4434.9/19 | 1499.1/18 | 60.1/13 | 3.6/9 |
| 9 | 2.0 | 20 | 4442.2/19 | 1474.6/18 | 58.9/13 | 2.2/8 |
| 10 | 2.0 | 30 | 5189.0/20 | 1789.4/18 | 110.8/14 | 12.4/11 |
| 11 | 3.0 | 0 | 3120.2/19 | 1146.7/17 | 90.7/14 | 7.4/10 |
| 12 | 3.0 | 5 | 5322.4/20 | 1909.1/18 | 73.4/13 | 3.4/9 |
| 13 | 3.0 | 10 | 5979.3/20 | 2244.1/18 | 110.0/14 | 7.9/10 |
| 14 | 3.0 | 20 | 6216.4/20 | 2308.1/18 | 117.3/14 | 7.3/10 |
| 15 | 3.0 | 30 | 7301.9/20 | 2844.4/19 | 155.2/14 | 5.4/10 |
Above all, plastic material is more suitable for the material of oil container and would be used to investigate the effect of cosolvent in eliminating the interference of free water on the determination of the contamination degree of jet fuel.
Effect of Additives on the Elimination of Free Water for Particle Counting
The contamination degrees of oil samples without or with cosolvents are determined sequentially and listed in Tables 3 and 4 respectively. According to the U.S. Army’s recommendations for the detection of the contamination degree of jet fuel, the contamination degrees of particles with different sizes [≥4 μm (c), ≥6 μm (c), ≥14 μm (c), and ≥30 μm (c)] are detected, which were determined according to ISO 4406 standard for its corresponding number and grade.
Table 4. Determination of Particle Quantity and Grade of Samples in a Plastic Bottle with Isopropanol.
| ACM
20 |
||||||
|---|---|---|---|---|---|---|
| sample | particle (mg/L) | free water (ppm) | ≥4 μm (c) | ≥6 μm (c) | ≥14 μm (c) | ≥30 μm (c) |
| 1 | 1.0 | 0 | 3288.9/19 | 1460.3/18 | 149.1/14 | 8.5/10 |
| 2 | 1.0 | 5 | 3423.6/19 | 1375.9/18 | 92.7/14 | 3.0/9 |
| 3 | 1.0 | 10 | 3353.6/19 | 1387.1/18 | 82.1/14 | 2.1/8 |
| 4 | 1.0 | 20 | 3446.4/19 | 1408.6/18 | 92.7/14 | 2.6/9 |
| 5 | 1.0 | 30 | 3534.9/19 | 1462.2/18 | 92.1/14 | 2.9/9 |
| 6 | 2.0 | 0 | 3486.7/19 | 1523.0/18 | 91.1/14 | 2.8/9 |
| 7 | 2.0 | 5 | 4021.4/19 | 1816.6/18 | 146.1/14 | 4.6/9 |
| 8 | 2.0 | 10 | 3792.2/19 | 1648.9/18 | 137.1/14 | 4.9/9 |
| 9 | 2.0 | 20 | 3766.7/19 | 1576.4/18 | 107.2/14 | 2.9/9 |
| 10 | 2.0 | 30 | 3697.1/19 | 1630.4/18 | 159.3/14 | 4.7/9 |
| 11 | 3.0 | 0 | 7753.1/20 | 3355.9/19 | 190.7/15 | 5.0/9 |
| 12 | 3.0 | 5 | 7721.1/20 | 3214.9/19 | 152.1/14 | 2.0/8 |
| 13 | 3.0 | 10 | 7543.9/20 | 3235.1/19 | 164.4/15 | 3.6/9 |
| 14 | 3.0 | 20 | 7590.4/20 | 3225.6/19 | 155.7/14 | 3.1/9 |
| 15 | 3.0 | 30 | 7691.3/20 | 3258.9/19 | 180.4/15 | 3.6/9 |
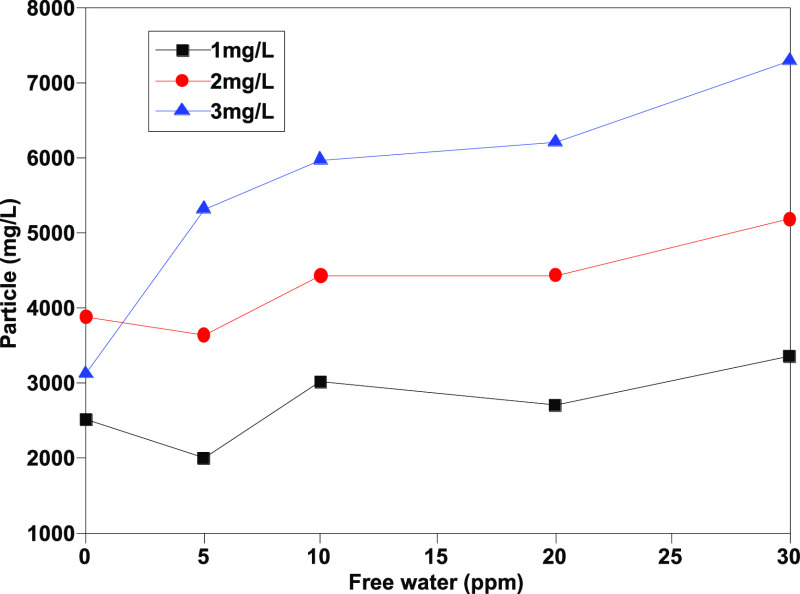
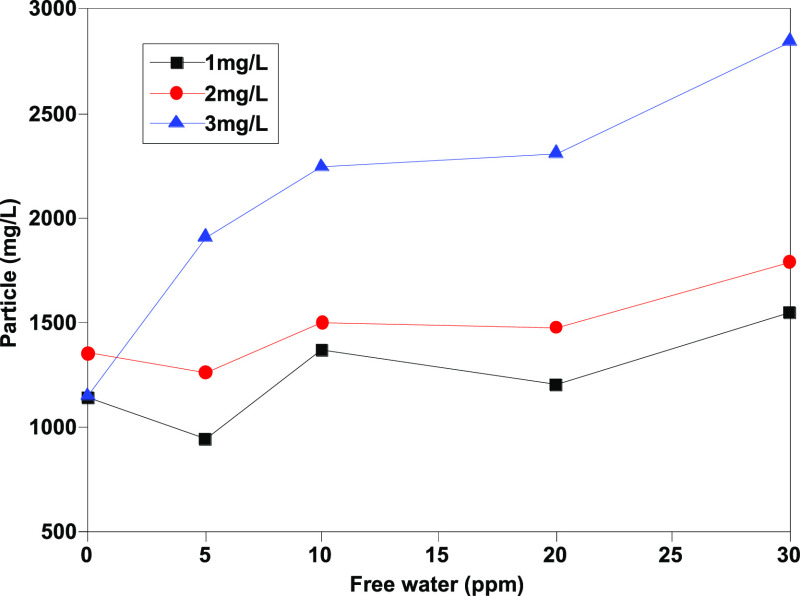
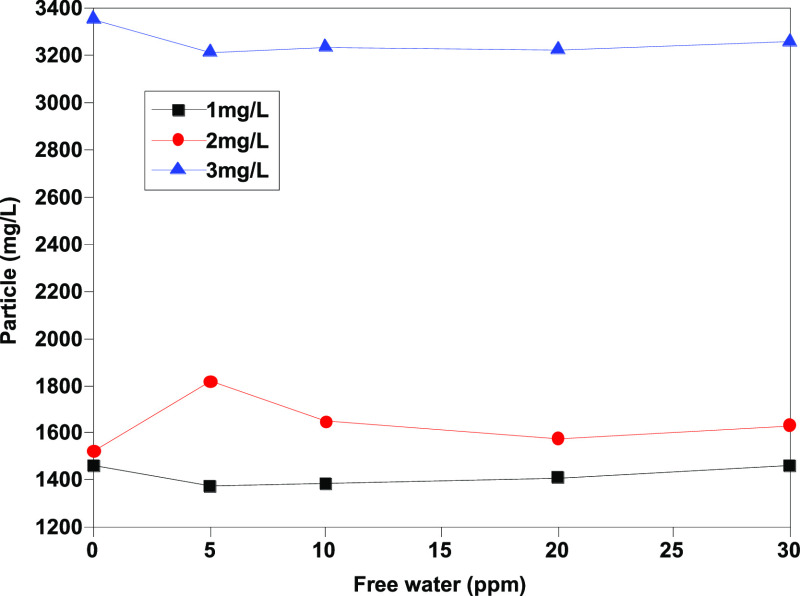
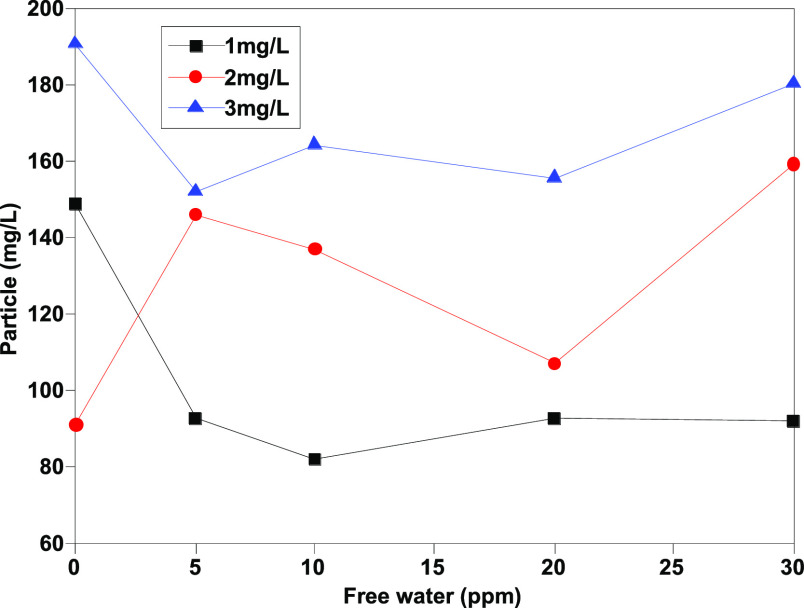
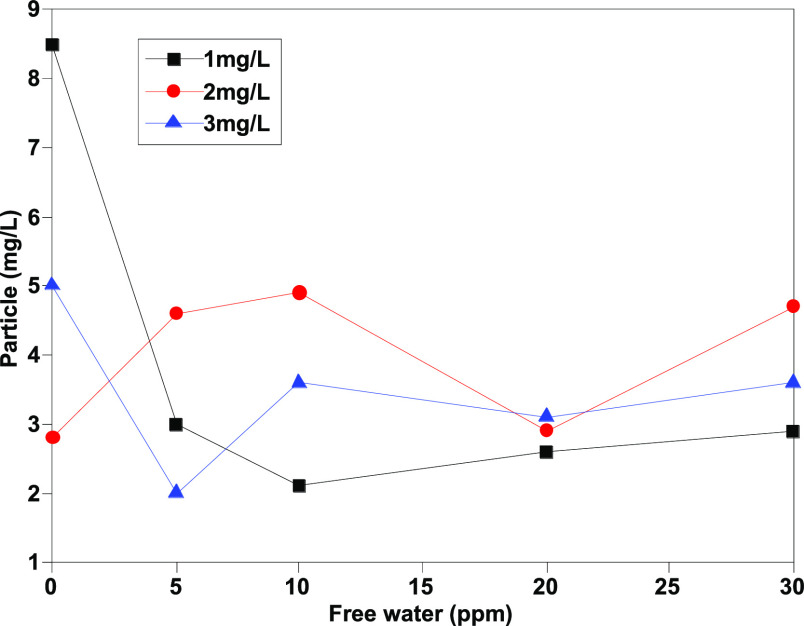
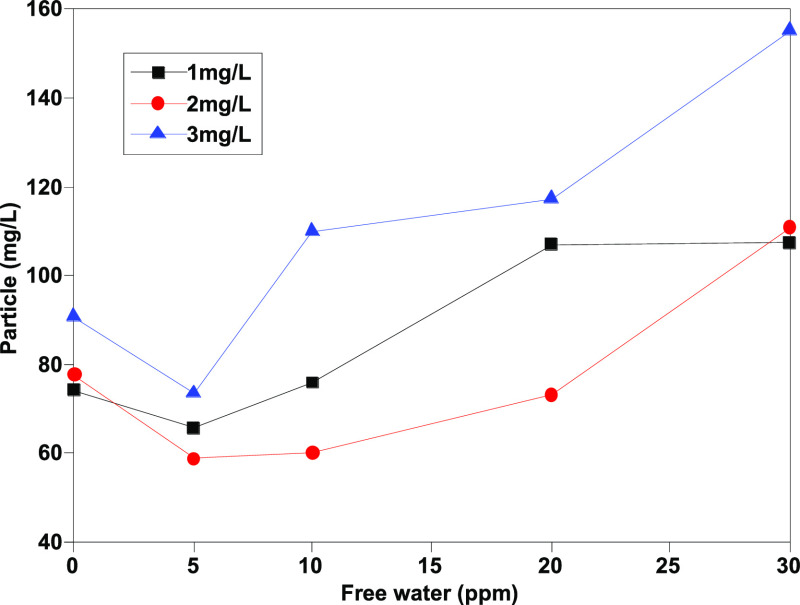
As Figures 1–4 show, the number of particles [≥4 μm (c), ≥6 μm (c), ≥14 μm (c), and ≥30 μm (c)] shows a certain linear relationship with free water, and especially, the number of particles ≥4 μm (c) and ≥6 μm (c) has a better linear relationship. Theoretically, more small size particles could result in their more uniform distribution. The linear relationship shows that the existence of free water can improve the counting results of the automatic particle counter, which has an interference effect on the determination of jet fuel contamination. Moreover, the analysis results show that except for particles with particle size ≥30 μm (c), the pollution levels of other particles are at the same level, and the results are within the repeatability range, indicating that the error effect of free water can be partially eliminated when the pollution level is divided.
Figure 1.
Determination results of ≥4 μm (c) granules before addition of isopropanol.
Figure 4.
Determination results of ≥30 μm (c) granules before addition of isopropanol.
Figure 2.
Determination results of ≥6 μm (c) granules before addition of isopropanol.
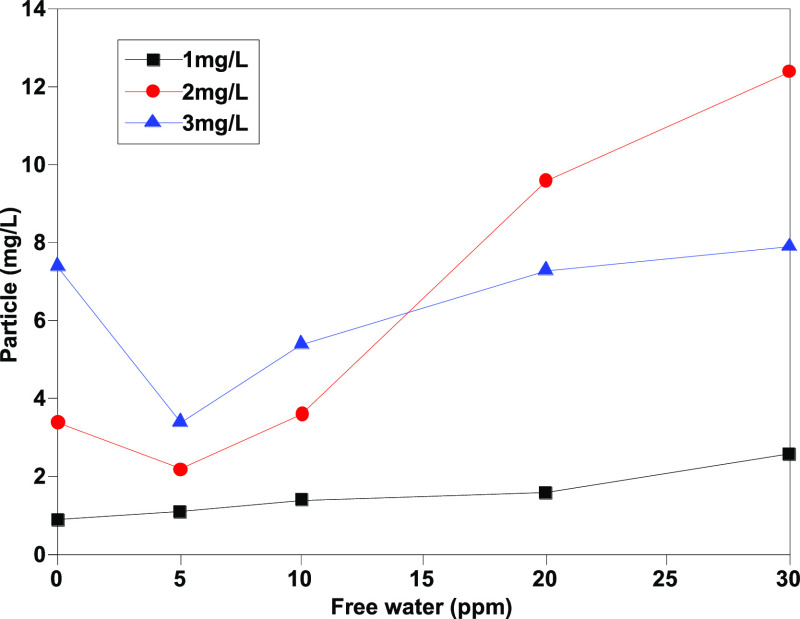
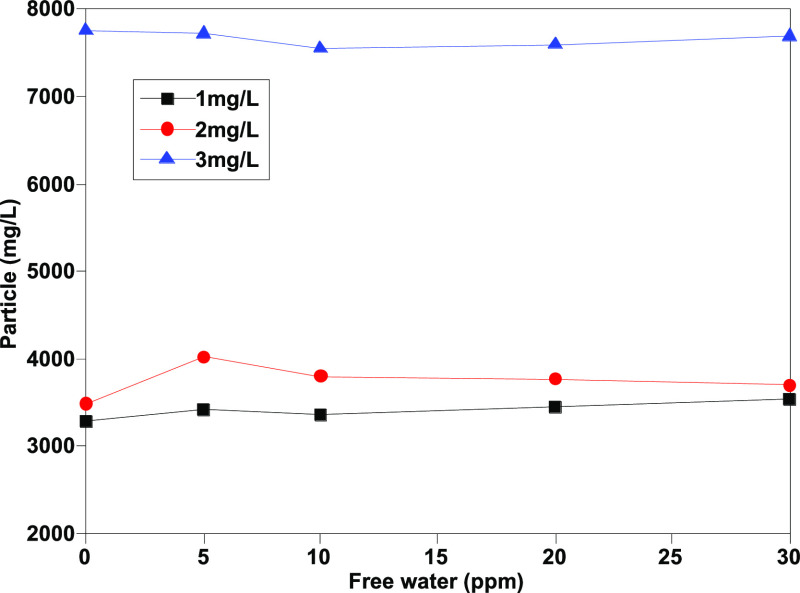
As exhibited in Figures 5 and 6, the counting results of particles with sizes 4 μm (c) and 6 μm (c) tend to be stable. The stability of the measured results of particles ≥4 μm (c) and (≥6 μm (c) is obviously better than those particles ≥14 μm (c) and ≥30 μm (c). In theory, according to the statistical analysis, less number of large size particles could cause uneven distribution. Even so, comparing Figures 7 and 8 with Figures 3 and 4, the results of determination tend to be more stable after adding isopropanol, and the impact of impurities in free water is basically eliminated.
Figure 5.
Determination results of ≥4 μm (c) granules after addition of isopropanol.
Figure 6.
Determination results of ≥6 μm (c) granules after addition of isopropanol.
Figure 7.
Determination results of ≥14 μm (c) granules after addition of isopropanol.
Figure 8.
Determination results of ≥30 μm (c) granules after addition of isopropanol.
Figure 3.
Determination results of ≥14 μm (c) granules before addition of isopropanol.
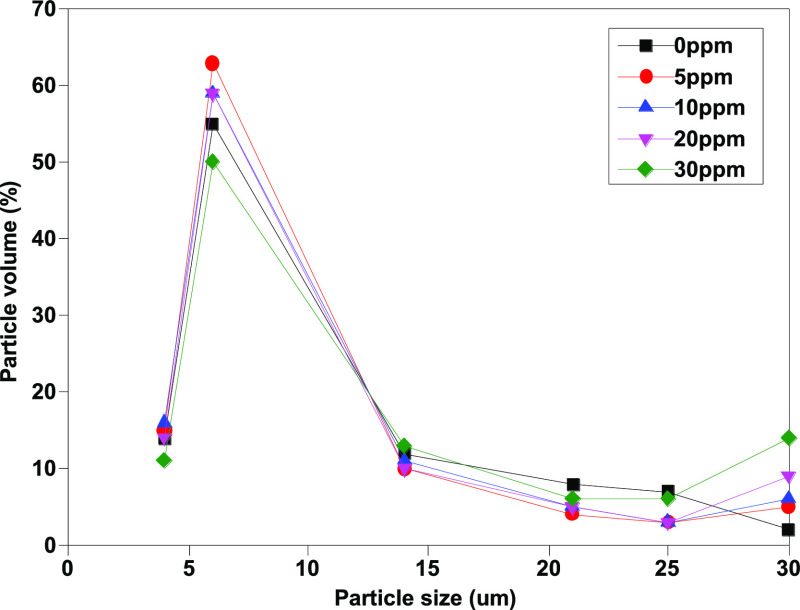
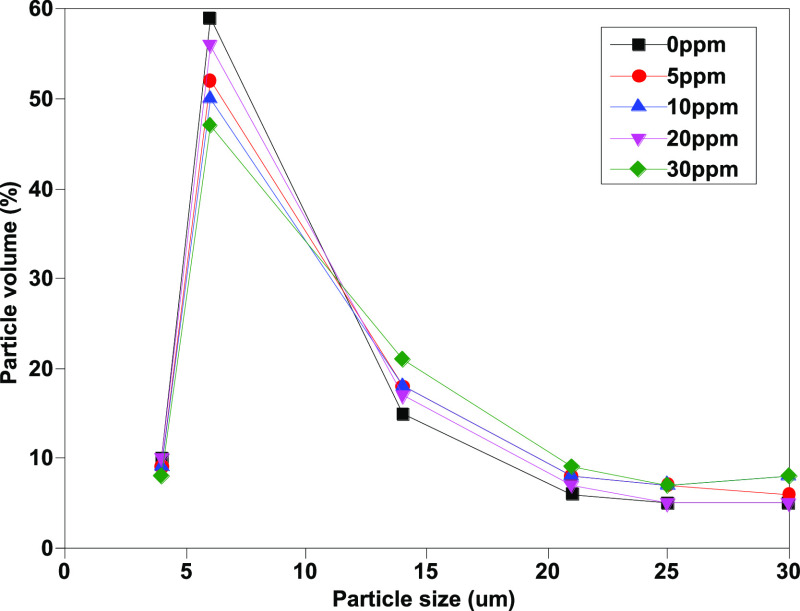
As Figures 9 and 10 show, the number of large-sized particles [mainly >30 μm (c)] in samples without isopropanol increases along with the increase of water content, indicating that the existence of free water will lead to changes in the counting results. In contrast, after adding isopropanol, the volume fraction of particles [>30 μm (c)] decreases significantly and drops to less than 10%. Moreover, the difference between the determination results of different moisture contents is significantly reduced, which shows that isopropanol as a cosolvent can effectively eliminate the interference effect of free water and significantly improve the repeatability of the determination method.
Figure 9.
Particle volume percent of different particle sizes before addition of isopropanol.
Figure 10.
Particle volume percent of different particle sizes after addition of isopropanol.
In summary, the results in Table 4 and Figures 5–8 show that the counting results are more stable. Particles with sizes greater than 4, 6, and 16 μm (c) are almost maintained at the same pollution level, which is more reproducible than those results without cosolvents. Moreover, the counting level of particles with sizes greater than 30 μm (c) is stabilized at the same level by adding cosolvents with less than 1 level of deviation. The results show that the addition of cosolvent basically eliminates the influence of free water on the automatic particle counter with a remarkable effect. The effect of isopropanol is attributed to the addition of isopropanol in jet fuel. Isopropanol contains not only the polar group hydroxyl (−OH) but also the nonpolar group methyl (−CH3). The polar group in isopropanol can be dissolved in water, whereas the nonpolar group isobutyl can be dissolved in jet fuel. As a result, the solubility of water in jet fuel increases due to the presence of isopropanol, which leads to the increase of water jet fuel solubility, and the phase separation zone decreases and the maximum temperature of mutual solubility decreases. Therefore, isopropanol combined with water can be dissolved in jet fuel so that the free water in jet fuel is eliminated and the influence of free water is eliminated, and isopropanol, as a cosolvent, is better than isobutanol, butanol, and other alcohol cosolvents.
Uncertainty Analysis
In this paper, uncertainty analysis is introduced to evaluate the test data in order to verify the accuracy and repeatability of the object of inquiry in determining the degree of jet fuel pollution. Uncertainty can be evaluated according to the error of test data, which is an index to measure the quality of test data. Generally, the less uncertain the data, the better its accuracy and repeatability. Therefore, the test data are analyzed with uncertainty analysis.
Because the repeatability of the experimental results is mainly evaluated, only the standard uncertainty u(X1) is introduced to evaluate the different repeatability of the results in the uncertainty analysis process. The uncertainty of the determination results is calculated, and the schematic diagram is made as follows.
As Figure 11 shows, the uncertainty of particle counting results of each particle size decreases slightly with the addition of isopropanol as a cosolvent, and there is a decrease of about 1%, which shows that the addition of isopropanol can not only dissolve free water in oil but also improve the repeatability of the test results. In fact, because the shape of free water is not fixed, the shape of free water may change when it is dispersed in oil, resulting in poor repeatability.
Figure 11.
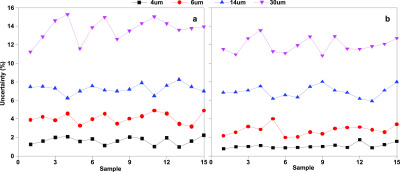
Uncertainty analysis without (a) and with (b) isopropanol.
Conclusions
The interference of trace free water on the automatic particle counter can be eliminated by adding isopropanol as a cosolvent to the measured oil sample. Isopropanol can dissolve trace free water in oil. Addition of isopropanol could stabilize the pollution grade of particles with size ≥30 μm (c) at the same level, which is most obviously affected by free water without isopropanol. The standard uncertainty u(X1) is slightly reduced with the addition of isopropanol, and the repeatability and accuracy of the automatic particle counting method are obviously improved.
The results show that isopropanol should be added as a cosolvent to eliminate the interference of free water when the contamination degree of jet fuel oil samples with obvious free water is determined by the automatic particle counting method.
Materials and Methods
Reagents and Materials
No. 3 jet fuel was purchased from Shanghai Gaoqiao Refinery. N-heptane was purified for market analysis. Isopropanol and 2,2-dimethoxypropane (DMP) were purchased from Shanghai China National Medicines Corporation Ltd and purified for market analysis. ISO MTD dust with ISO 12103-1 standard was purchased from Shanghai Rebey Trading Co., Ltd. Distilled water was laboratory self-made. Three kinds of common container materials are selected: glass, metal, and plastic. Each material container has 1 L capacity.
Test Instrument
ACM 20 automatic particle counter, manufactured by American Parker Company, was specially used to detect the contamination of jet fuel in accordance with the equivalent projection particle size, which is a portable instrument for rapid detection of aviation fuel pollutants and can be connected to pipeline on-line measurement and measure solid particles in jet fuel after sampling.
Preparation of Jet Fuel Containing Saturated Dissolved Water
Jet fuel containing saturated dissolved water was prepared as follows.14 A 1000 mL glass jug was carefully filled with 100 mL of distilled water so that the sides of the jug would not been clung by water droplets above the water level. Whatman filter papers were inserted in the glass jug, keeping the bottom of the paper in the water. Then, 800 mL of fuel was slowly poured over the water, avoiding intermingling of the water and fuel. The top of the filter paper was protruded into the fuel, bringing water up into the fuel. A closed vent system is provided to allow only water-saturated air to enter the system to maintain the fuel in a water-saturated condition. The combination of fuel and water was left in the jug for more than 24 h, and then the water-saturated fuel was removed by a siphon. Then, the content of saturated dissolved water was determined in accordance with the method described in our previous study.15,16 The content of saturated dissolved water was detected with DMP as the titrant. DMP underwent an endothermic reaction with water under the catalytic action of acids, and its enthalpy is +27.6 kJ/mol. The reaction equation is exhibited as eq 1.
| 1 |
Determination of the Contamination Degree of Jet Fuel
The initial oil sample was filtered to ensure that the original particles were removed, followed by preparation of the oil sample with saturated dissolved water. Then, three batches of oil samples were prepared by adding 1, 2, and 3 mg/L of ISO MTD dust. Distilled water of 0, 5, 10, 20, and 30 ppm as free water was added into each batch of oil sample.
Before starting the test, the containers were precleaned to store the oil samples. The instrument was rinsed three times with clean n-heptane solution. Then, the cleaned instrument was carries out with a test operation according to the normal test operation steps to ensure that the cleanliness of the instrument catheter is lower enough with the oil sample. Before each test, the contamination degree of oil samples from 1 to 15 was measured by the automatic particle counting method with shaking manually for 60 s. The contamination of no. 1 to no. 15 oil samples was determined repeatedly by adding isopropanol at a volume fraction of 5.9%. All oil samples were measured three times with or without isopropanol to obtain the average value.
Acknowledgments
This work was subsidized by the Fund from National Natural Science Foundation of China (grant 51575525), and the Natural Science Foundation of Jiangsu Province (grant BK20191155). The project was supported by the Tribology Science Fund of State Key Laboratory of Tribology (grant SKLTKF17B16).
The authors declare no competing financial interest.
References
- Chen L.; Gao K.; Zhang C.; Lang W.. Alternative fuels for IC engines and jet engines and comparison of their gaseous and particulate matter emissions. Advanced Biofuels; Elsevier, 2019; pp 17–64. [Google Scholar]
- Wei H.; Liu W.; Chen X.; Yang Q.; Li J.; Chen H. Renewable bio-jet fuel production for aviation: A review. Fuel 2019, 254, 115599. 10.1016/j.fuel.2019.06.007. [DOI] [Google Scholar]
- Zhang R.; Xu Q.; Fan W. Effect of swirl field on the fuel concentration distribution and combustion characteristics in gas turbine combustor with cavity. Energy 2018, 162, 83–98. 10.1016/j.energy.2018.07.170. [DOI] [Google Scholar]
- Adekitan A. I.; Shomefun T.; John T. M.; Adetokun B.; Aligbe A. Dataset on statistical analysis of jet A-1 fuel laboratory properties for on-spec into-plane operations. Data in Brief 2018, 19, 826–834. 10.1016/j.dib.2018.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistoni P.; Popovichev S.; Crowe R.; Cufar A.; Ghani Z.; Keogh K.; Peacock A.; Price R.; Baranov A.; Korotkov S.; Lykin P.; Samoshin A. Technical preparations for the in-vessel 14 MeV neutron calibration at JET. Fusion Eng. Des. 2017, 117, 107–114. 10.1016/j.fusengdes.2017.01.023. [DOI] [Google Scholar]
- Chang R. C. Examination of excessive fuel consumption for transport jet aircraft based on fuzzy-logic models of flight data. Fuzzy Set Syst. 2015, 269, 115–134. 10.1016/j.fss.2014.04.021. [DOI] [Google Scholar]
- Hileman J. I.; Stratton R. W. Alternative jet fuel feasibility. Trans. Pol. 2014, 34, 52–62. 10.1016/j.tranpol.2014.02.018. [DOI] [Google Scholar]
- Baena-Zambrana S.; Repetto S. L.; Lawson C. P.; Lam J. K.-W. Behaviour of water in jet fuel-a literature review. Prog. Aero. Sci. 2013, 60, 35–44. 10.1016/j.paerosci.2012.12.001. [DOI] [Google Scholar]
- Bhan O. K.; Tang S. Y.; Brinkman D. W.; Carley B. Causes of poor filterability in jet fuels. Fuel 1988, 67, 227–237. 10.1016/0016-2361(88)90268-2. [DOI] [Google Scholar]
- Petersen K. M.Inline Monitoring of Free Water and Particulate Contamination of Jet-a fuel; U.S. Army TARDEC Fuels and Lubricants Research Facility (SwRI): San Antonio, 2015.
- Schmitigal J.Field Evaluation of Particle Counter Technology for Aviation Fuel Contamination Detection—Fort Rucker; U.S. Army Tank Automotive Research Development and Engineering Center: Warren, 2013.
- Schmitigal J.Evaluation of Additives to Eliminate Free Water from Aviation Fuel Light Obscuration Particle Counts; Army TARDEC RDTA SIE-ES-FPT-PSD: Warren, 2015.
- Naya S.; Cao R.; Francisco-Fernandez M. Estimating water andsolid impurities in jet fuel from ISO codes. Energy Fuels 2013, 27, 7858–7867. 10.1021/ef401378z. [DOI] [Google Scholar]
- Williams W. R.; Keehan C.. Evaluation of Commercial Water-in-Fuel Test Kits; U.S. Army Tank Automotive Research Development and Engineering Center: Warren, 1994.
- Hu J.-Q.; Zhang J.-J.; Yang S.-Z.; Xin Y.-L.; Guo L.; Yao T. Thermometric titration for rapid determination of trace water in jet fuel. J. Spectrosc. 2017, 2017, 1–5. 10.1155/2017/8429525. [DOI] [Google Scholar]
- Hu J.-Q.; Yang S.-Z.; Zhang J.-J.; Guo L.; Xin Y.-L. The determination of lower acidity in several coloured oils by catalyzed thermometric titration. Pet. Chem. 2017, 57, 1099–1104. 10.1134/s0965544117120052. [DOI] [Google Scholar]