Abstract
The liquified mash of milled grains from the Canadian wheat cultivar, AC Andrew, was fermented to determine whether α-glycerylphosphorylcholine (α-GPC) accumulated and whether the accumulation was dependent on fermentation-related factors. Fermentation was conducted at a temperature of 37 °C for 7 days (168 h) with samples collected every 24 h. The samples were analyzed using a proton nuclear magnetic resonance water suppression pulse sequence to allow the quantitation of ethanol, acetic acid, lactic acid, succinic acid, glycerol, phenethyl alcohol, betaine, and α-GPC. A Gompertz model was used to interpret fermentation kinetics for each analyte, and during fermentation, ethanol accumulated to a concentration of 72.1 g/L while α-GPC accumulated to a concentration of 1.68 g/L over 72 h. There were significant and positive correlations between the accumulation of α-GPC, ethanol, lactic acid, and glycerol and acetic acid production. Furthermore, there were no significant negative correlations between the productions of these compounds; hence, all the compounds accumulated during fermentation were produced simultaneously with no observed decrease measured in any compound. This indicates that α-GPC can be successfully produced industrially without any negative impact on ethanol or other useful compounds.
1. Introduction
Wheat (Triticum aestivum L.) is the most cultivated crop in Canada with an average production of 30 million tonnes per year.1 Wheat production and sales contribute approximately 11 billion dollars annually to Canada’s economy.2 Canada is one of the world’s top five wheat-exporting nations and the largest producer of high-protein milling wheat.1
The Prairie provinces grow the majority of Canadian wheat, with relatively smaller quantities grown in eastern Canada and British Columbia.3 In order to ensure sustainability and profitability from wheat production, there is a need for technology that adds value and supports growth of the industry.1 Use of wheat for ethanol production favors sustainable economic development because it ensures that all grades of wheat are utilized. Ethanol production from cereal grain has proliferated and has had a positive environmental impact through the production of renewable fuel and reduction of greenhouse gas emissions.4
α-Glycerylphosphorylcholine (α-GPC) is a potent nootropic, employed to combat the onset of Alzheimer’s disease and dementia and stimulate cognitive recovery, improved learning, and neurological function.5,6 This compound comprises glycerol, choline, and phosphate moieties linked by ester bonds. It is potentially one of the most valuable compounds that can be recovered from wheat fermentation.7,8 This compound is of interest for its application in medicine as a precursor to acetylcholine.9 It is possible to treat psychiatric and neurological conditions that are associated with lower concentration of acetylcholine (i.e., Alzheimer’s disease, bipolar affective disorder, and schizophrenia) through the consumption of α-GPC.10−12 Other nootropics, such as 6-paradol,13,14 also demonstrate neuroprotective effects, although its production from ginger fermentation, with Schizosaccharomyces pombe,13−15 can demonstrate slow kinetic fermentation16 and high acetic acid production,17,18 thereby making purification processes more expensive.
Phosphatidylcholine (PC) is one of the most abundant phospholipids in eukaryotic cells,19,20 which can be further converted to α-GPC. PC can be synthesized through two different pathways: the Kennedy pathway and through a methylation pathway.20 In the Kennedy pathway, free choline is phosphorylated by choline kinase to form cytidine diphosphate choline, which is then condensed with diacylglycerol to produce PC.20,21 Through the methylation pathway, PC is synthesized through sequential methylation of phosphatidylethanolamine.20,21 PC can then be converted to 1-acyl-GPC and eventually α-GPC through enzymatic hydrolysis by phospholipase A1 and A2.19 α-GPC is then converted to free choline and glycerol-3-phosphate by the enzyme glycerophosphocholine phosphodiesterase.19
α-GPC can also be produced chemically or using enzymatic methods. The chemical methods typically involve hydrolysis of PC or condensation of glycerol derivatives with phosphocholine donors using basic catalysts.22−24 However, the toxicity of the substrates and catalysts makes the α-GPC-produced food safe.25 Alternatively, α-GPC has been enzymatically produced by the hydrolysis of PC in aqueous media,26−28 employing phospholipases.25 Enzymatic preparation of α-GPC is advantageous because the amounts of chemical reagents are reduced, thereby making it comparably expensive and of food quality.25 Further purification of α-GPC can be accomplished via chromatographic method.29 Therefore, alcoholic fermentation of wheat cultivars is highly advantageous because of the inexpensive processes involved, and that α-GPC can be concurrently produced with ethanol during fermentation.
The number of people, who have Alzheimer’s disease worldwide, is predicted to increase from the current 46.8 to 131.5 million by 2050.30 The value and demand for α-GPC could increase correspondingly.31 Also, α-GPC is a biosynthetic precursor of the neurotransmitter acetylcholine and membrane phospholipids.32 Dietary α-GPC can improve cognitive abilities, whereas other cholinergic precursors such as lecithin and choline did not show similar positive effects.33 It can also be used as a cosmetic ingredient for moisturizers, nutritive creams, elasticizers, and emollients. This beneficial compound rarely occurs naturally; hence, there is value in developing alternative and sustainable means for its production.
The objective of this study was two-fold; first to determine how much α-GPC can accumulate during fermentation, and second, to determine whether the quantity of α-GPC that accumulates in stillage can be increased by altering fermentation conditions.
2. Results
2.1. Gompertz Model Fitting to Fermentation Products
The model was used to determine the predicted values for the fermentation products, and these values are shown in Tables 1 and 2. The model was also used to obtain values for the rate of fermentation, the lag period, and the maximum product accumulated. Experimental and predicted values for ethanol accumulation are shown in Table 1. Ethanol accumulation peaks at 96 h of fermentation; although the exponential increase in ethanol production starts as early as 24 h and from that time, production declined. Similarly, the accumulation of α-GPC was fitted to the Gompertz equation and the experimental and predicted values shown in Table 2.
Table 1. Experimental and Predicted Values for Ethanol Accumulated from Fermentation with AC Andrewa.
| WWF (g/L) |
ELF (g/L) |
PLF (g/L) |
PCF (g/L) |
|||||
|---|---|---|---|---|---|---|---|---|
| time (h) | experimental | predicted | experimental | predicted | experimental | predicted | experimental | predicted |
| 0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 2.29 ± 0.89 | 0.00 ± 0.0 | 0.96 ± 0.74 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 24 | 62.7 ± 7.6 | 61.4 ± 7.2 | 58.4 ± 2.7 | 55.2 ± 3.6 | 55.7 ± 3.9 | 54.9 ± 4.2 | 31.7 ± 3.0 | 30.7 ± 2.5 |
| 48 | 65.4 ± 1.7 | 68.9 ± 1.6 | 58.0 ± 4.6 | 70.2 ± 2.1 | 58.0 ± 1.7 | 64.7 ± 2.9 | 58.9 ± 4.9 | 57.3 ± 4.1 |
| 72 | 70.0 ± 2.9 | 69.6 ± 1.9 | 69.6 ± 3.1 | 71.8 ± 2.0 | 66.6 ± 5.7 | 65.9 ± 3.7 | 60.1 ± 1.9 | 59.9 ± 1.8 |
| 96 | 71.6 ± 3.5 | 69.6 ± 1.9 | 74.8 ± 2.4 | 72.0 ± 2.0 | 68.4 ± 4.0 | 66.0 ± 3.8 | 59.3 ± 1.7 | 60.2 ± 1.7 |
| 120 | 69.5 ± 2.8 | 69.6 ± 1.9 | 77.2 ± 1.8 | 72.1 ± 2.0 | 66.7 ± 3.3 | 66.1 ± 3.8 | 60.0 ± 1.8 | 60.3 ± 1.6 |
| 144 | 70.5 ± 3.7 | 69.6 ± 1.9 | 73.4 ± 2.7 | 72.1 ± 2.0 | 67.7 ± 3.5 | 66.1 ± 3.8 | 59.1 ± 1.0 | 60.3 ± 1.6 |
| 168 | 68.6 ± 3.3 | 69.6 ± 1.9 | 74.8 ± 2.5 | 72.1 ± 2.0 | 66.7 ± 3.8 | 66.1 ± 3.8 | 59.6 ± 1.3 | 60.3 ± 1.6 |
Values were determined in four replicates, and the values are presented as the mean ± SD (n = 4).
Table 2. Experimental and Predicted Values for α-GPC Accumulated from Fermentation with AC Andrewa.
| WWF (g/L) |
ELF (g/L) |
PLF (g/L) |
PCF (g/L) |
|||||
|---|---|---|---|---|---|---|---|---|
| time (h) | experimental | predicted | experimental | predicted | experimental | predicted | experimental | predicted |
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 1.09 ± 0.46 | 0.95 ± 0.35 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.35 ± 0.35 | 0.00 ± 0.00 |
| 48 | 1.38 ± 0.24 | 1.33 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.73 ± 0.42 | 0.71 ± 0.41 | 0.98 ± 0.33 | 0.91 ± 0.31 |
| 72 | 1.25 ± 0.08 | 1.33 ± 0.15 | 1.03 ± 0.37 | 0.85 ± 0.29 | 1.66 ± 0.18 | 1.66 ± 0.14 | 1.26 ± 0.05 | 1.34 ± 0.02 |
| 96 | 1.27 ± 0.12 | 1.33 ± 0.15 | 0.86 ± 0.29 | 0.88 ± 0.29 | 1.74 ± 0.15 | 1.68 ± 0.15 | 1.29 ± 0.05 | 1.37 ± 0.05 |
| 120 | 1.27 ± 0.12 | 1.33 ± 0.15 | 1.35 ± 0.23 | 1.35 ± 0.17 | 1.64 ± 0.14 | 1.68 ± 0.15 | 1.39 ± 0.05 | 1.37 ± 0.05 |
| 144 | 1.33 ± 0.13 | 1.33 ± 0.15 | 1.30 ± 0.17 | 1.35 ± 0.17 | 1.72 ± 0.14 | 1.68 ± 0.15 | 1.39 ± 0.04 | 1.37 ± 0.05 |
| 168 | 1.35 ± 0.13 | 1.33 ± 0.15 | 1.24 ± 0.18 | 1.35 ± 0.17 | 1.60 ± 0.13 | 1.68 ± 0.15 | 1.42 ± 0.07 | 1.37 ± 0.05 |
Values were determined in four replicates, and the values are presented as the mean ± SD (n = 4).
2.2. Effects of Treatment Conditions on the Amax Values of Fermentation Compounds
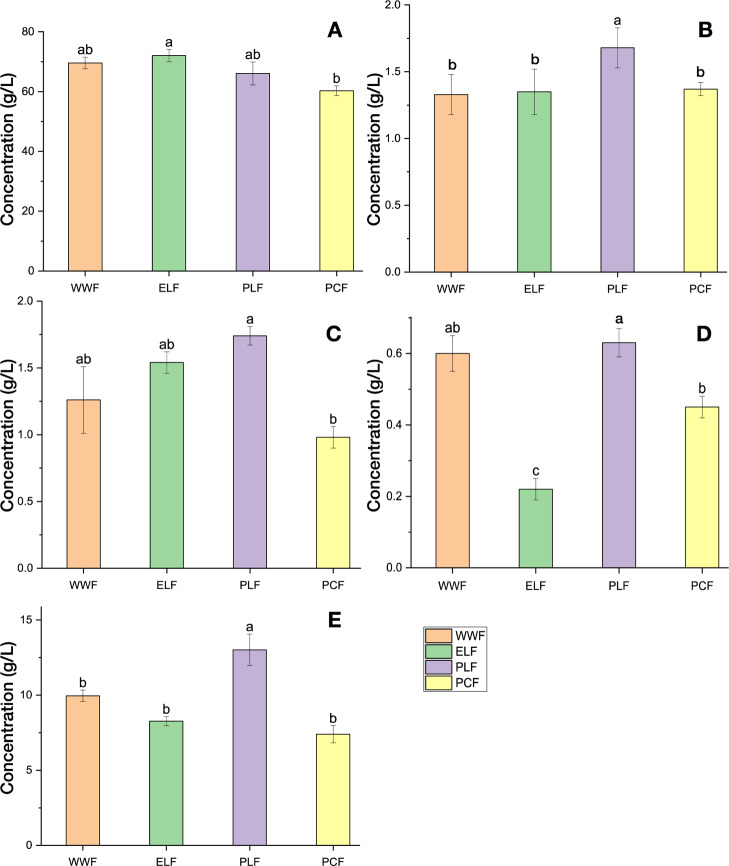
The maximum ethanol accumulated was 72.1 g/L in endosperm layer fermentation (ELF) (Table 3). There was a statistically significant difference between the different treatment conditions (p < 0.05). There was no difference between fermentation with whole wheat fermentation (WWF) and phospholipase A1 fermentation (PLF), and they accumulated 69.6 and 66.1 g/L of ethanol, respectively. The lowest ethanol accumulated was observed with phosphatidylcholine fermentation (PCF) which is at 60.3 g/L concentration (Figure 1A). The Amax value for the accumulation of α-GPC from the different treatments shows a statistically significant difference p < 0.05 (Figure 1B, Table 3). There was no statistically significant difference between the maximum accumulation of lactic acid and succinic acid among the different treatments (p > 0.05). Acetic acid accumulation was statistically significant between treatments (p < 0.01), with PLF producing the highest acetic acid concentration (1.74 g/L), WWF and ELF accumulated similar quantities while the lowest acetic acid accumulation was 0.98 g/L in PCF (Figure 1C, Table 3). Also, the highest glycerol accumulation (Figure 1E) was found with PLF treatment at 13.02 g/L.
Table 3. Parameters Obtained from Gompertz Model for Compounds Measured from Fermentation.
| kinetic parameters | treatment condition | ethanol | α-GPC | lactic acid | acetic acid | succinic acid | betaine | glycerol | PEA |
|---|---|---|---|---|---|---|---|---|---|
| Amax (g/L) | WWF | 69.6a,b± 1.9 | 1.33b± 0.15 | 0.44a± 0.10 | 1.26a,b± 0.25 | 0.65a± 0.10 | 0.60a,b± 0.05 | 9.96b± 0.38 | 0.54b± 0.03 |
| ELF | 72.1a± 2.0 | 1.35b± 0.17 | 0.72a± 0.02 | 1.54a,b± 0.08 | 0.49a± 0.01 | 0.22c± 0.03 | 8.27b± 0.30 | 0.93a± 0.03 | |
| PLF | 66.1a,b± 3.8 | 1.68a± 0.15 | 0.70a± 0.08 | 1.74a± 0.07 | 0.58a± 0.07 | 0.63a± 0.04 | 13.0a± 1.1 | 0.84a± 0.05 | |
| PCF | 60.3b± 1.6 | 1.37b± 0.05 | 0.57a± 0.07 | 0.98b± 0.08 | 0.45a± 0.04 | 0.45b± 0.03 | 7.40b± 0.57 | 0.60b± 0.02 | |
| Rmax (g/L/h) | WWF | 17.6a± 4.9 | 0.34a± 0.08 | 0.07a± 0.02 | 0.06a± 0.03 | 0.25a± 0.05 | 0.32a± 0.02 | 4.39a± 0.28 | 0.03a± 0.01 |
| ELF | 2.93b± 0.36 | 0.48a± 0.15 | 0.08a± 0.05 | 0.28a± 0.11 | 0.25a± 0.02 | 0.05b± 0.02 | 2.33b± 0.17 | 0.03a± 0.00 | |
| PLF | 3.69b± 0.66 | 0.39a± 0.06 | 0.06a± 0.03 | 0.13a± 0.06 | 0.20a± 0.06 | 0.19a,b± 0.07 | 0.78c± 0.33 | 0.02a± 0.00 | |
| PCF | 9.1a,b± 2.4 | 0.41a± 0.05 | 0.09a± 0.04 | 0.02a± 0.00 | 0.07a± 0.03 | 0.16b± 0.01 | 1.83b,c± 0.33 | 0.01a± 0.00 | |
| L (h) | WWF | 4.8b± 2.0 | ND | 8.1a± 5.0 | 1.94a± 0.92 | 15.9a±2.7 | ND | 11.7a± 4.5 | 9.2a± 9.0 |
| ELF | 2.28b± 0.82 | ND | 2.22a± 0.72 | 2.43a± 0.36 | 9.7a,b± 1.2 | ND | 1.73a± 0.04 | 2.08a± 0.72 | |
| PLF | 3.61b± 0.62 | ND | 6.8a± 2.1 | 2.87a± 0.63 | 8.9a,b± 1.8 | ND | 6.7a± 4.3 | 0.53a± 0.53 | |
| PCF | 20.0a± 1.3 | ND | 13.7a± 4.0 | 4.2a± 1.5 | 5.3b± 3.2 | ND | 24a± 11 | 4.6a± 1.8 |
Values were determined in four replicates, and the values are presented as the mean ± SD (n = 4).
Values followed by different letters are significantly different (p < 0.05).
Amax: potential maximum fermentation accumulation; Rmax: maximum fermentation productivity rate or productivity; L: lag phase; ND: not detected.
Figure 1.
Maximum (A) ethanol, (B) α-GPC, (C) acetic acid, (D) betaine, and (E) glycerol accumulated from fermentation of AC Andrew under the different treatment conditions. Values followed by different letters are significantly different (p < 0.05).
2.3. Rate of Substrate Conversion
There were no significant differences among the rates of accumulation of α-GPC, lactic acid, acetic acid, succinic acid, and phenethyl alcohol (PEA) accumulation, but the rate of ethanol accumulation differed significantly (p < 0.05, Table 3), the maximum rate of ethanol accumulation was observed in WWF at 17.6 g/L/h, and the rate of accumulation in ELF and PLF did not differ significantly, while PCF accumulated ethanol at 9.1 g/L/h.
2.4. Lag Phase
The Gompertz model was used to describe fermentation and identify the time between when the fermentation begins and when fermentation products are accumulated. Ethanol accumulation with the different treatment conditions showed a significant difference in the lag phase (L, p < 0.001). The lag phase with treatments WWF, ELF, and PLF are similar with values of 4.8, 2.28, and 3.61 h, respectively; however, the lag phase was increased significantly with the addition of PC to 20.0 h (Table 3). There was no lag period observed from the accumulation of α-GPC from fermentation, but at the early stages of fermentation, α-GPC concentrations were below detection, and the peak position overlapped partially with glucose peaks. Also, there was no significant difference between the lag phases in the accumulation of lactic acid, acetic acid, glycerol, and PEA, but succinic acid accumulation did show a significant difference in its lag phase with a p-value < 0.05. WWF has a lag period of 15.9 h maximum, ELF and PLF phases were similar at 9.7 and 8.9 h, respectively, and PCF with a minimum of 5.3 h.
2.5. Maximum Time to Reach α-GPC and Betaine
The accumulation of α-GPC reached the maximum at different times for the treatments, after which, there was no further conversion of substrates to yield additional α-GPC. There is no statistically significant difference in the maximum α-GPC accumulation (p > 0.05), but there was a statistically significant difference in the time taken to reach its maximum accumulation (p < 0.05). WWF peaked at the shortest time of 42 h, and ELF reached its maximum accumulation at 90 h. Also, there was no significant difference between the Tmax values from PLF and PCF which were 72 and 66 h, respectively (Table 4).
Table 4. Time Taken to Attain Maximum Accumulation of α-GPC and Betainec.
| Tmax (h) | α-GPC | betaine |
|---|---|---|
| WWF | 42b± 6 | 30b± 6 |
| ELF | 90a± 11 | 96a± 10 |
| PLF | 72a,b± 10 | 96a± 10 |
| PCF | 66a,b± 11 | 54b± 6 |
Values were determined in four replicates, and the values are presented as the mean ± SD (n = 4).
Values followed by different letters are significantly different (p < 0.05).
Tmax, time at which the maximum compound was obtained or time taken to reach Amax accumulation in the fermentation broth.
2.6. Correlation Coefficients of Compounds Accumulating during Fermentation
There were statistically significant and positive correlations on the compounds accumulated from fermentation (Table 5). α-GPC is equally correlated with acetic acid (p < 0.0001, r = 0.652) and glycerol accumulation (p < 0.0001, r = 0.671). Lactic acid accumulation is also significantly correlated with α-GPC (p = 0.092, r = 0.229), betaine (p = 0.023, r = 0.307), and PEA (p < 0.0001, r = 0.484). Similarly, betaine and α-GPC both correlated significantly with glycerol accumulation during fermentation (Table 5).
Table 5. Pearson Ranked Correlation Coefficients on All the Compounds Accumulated from Fermentation.
| ethanol | α-GPC | lactic acid | acetic acid | succinic acid | betaine | glycerol | PEA | |
|---|---|---|---|---|---|---|---|---|
| ethanol | 1.000 | 0.711 | 0.130 | 0.463 | 0.226 | 0.298 | 0.592 | –0.166 |
| <0.0001 | 0.346 | 0.0004 | 0.097 | 0.027 | <0.0001 | 0.225 | ||
| α-GPC | 0.711 | 1.000 | 0.229 | 0.652 | 0.355 | 0.324 | 0.671 | –0.036 |
| <0.0001 | 0.092 | <0.0001 | 0.0078 | 0.016 | <0.0001 | 0.795 | ||
| lactic acid | 0.130 | 0.229 | 1.000 | 0.257 | 0.108 | 0.307 | 0.328 | 0.484 |
| 0.346 | 0.092 | 0.058 | 0.435 | 0.023 | 0.015 | <0.0001 | ||
| acetic acid | 0.463 | 0.652 | 0.257 | 1.000 | 0.0749 | 0.557 | 0.714 | 0.295 |
| 0.0004 | <0.0001 | 0.058 | 0.59 | <0.0001 | <0.0001 | 0.029 | ||
| succinic acid | 0.226 | 0.355 | 0.108 | 0.0749 | 1.000 | 0.363 | 0.520 | 0.111 |
| 0.097 | 0.008 | 0.435 | 0.587 | 0.007 | <0.0001 | 0.420 | ||
| betaine | 0.298 | 0.324 | 0.307 | 0.557 | 0.363 | 1.000 | 0.603 | 0.155 |
| 0.027 | 0.016 | 0.023 | <0.0001 | 0.007 | <0.0001 | 0.258 | ||
| glycerol | 0.592 | 0.671 | 0.328 | 0.714 | 0.520 | 0.603 | 1.000 | 0.186 |
| <0.0001 | <0.0001 | 0.015 | <0.0001 | <0.0001 | <0.0001 | 0.175 | ||
| PEA | –0.166 | –0.036 | 0.484 | 0.295 | 0.111 | 0.155 | 0.186 | 1.000 |
| 0.225 | 0.795 | <0.0001 | 0.029 | 0.420 | 0.258 | 0.175 |
2.7. Correlation Coefficients of Kinetic Parameters for α-GPC Accumulation
There was a significant correlation between the maximum α-GPC accumulation, Amaxag, and the rate of accumulation, Rmaxag (p = 0.03, r = 0.55) (Table 6). When the rate of accumulation increases, there is a corresponding increase in the maximum α-GPC accumulated from fermentation. There was no significant correlation between the time taken to reach the maximum accumulation and the Amaxag and Tmaxag values.
Table 6. Pearson Ranked Correlation Coefficients on α-GPC Kinetic Parametersa.
| Amaxag | Rmaxag | Tmaxag | |
|---|---|---|---|
| Amaxag | 1 | 0.55 | 0.32 |
| 0.03 | 0.23 | ||
| Rmaxag | 0.55 | 1 | 0.20 |
| 0.03 | 0.47 | ||
| Tmaxag | 0.32 | 0.20 | 1 |
| 0.23 | 0.47 |
Amaxag, the maximum α-GPC accumulation value; Rmaxag, the maximum α-GPC productivity rate or productivity; Tmaxag, time at which the maximum compound was obtained or time taken to reach Amaxag accumulation in the α-GPC broth.
3. Discussion
Fermentation is characterized by substrate transformation to products. There are three stages in fermentation: (1) a lag phase before fermentation has commenced; (2) an exponential phase where the accumulation rate increases; and (3) a final stationary phase where the yeast cannot metabolize more substrates. The most common wheat cultivar used for fermentation is AC Andrew because of its high starch content.34 Therefore, AC Andrew was used for this fermentation study and resulted in the highest accumulation of α-GPC (1.68 g/L).
3.1. Correlations of Compound Accumulation during Fermentation
The correlation coefficient shows that there was a significant positive correlation between acetic acid accumulation and ethanol accumulation during fermentation (Table 5). Acetic acid and ethanol accumulation decreased fermentation rates; acetic acid lowers the fermenter pH.35 Ethanol accumulates until it reaches a constant maximum concentration (usually 72 h; Table 1), and then, there is no further increase in fermentation products. This stationary phase could be the result of the reduction in the simple sugars available for conversion or decrease in the fermenter pH which in turn reduces the yeast activity. Studies have shown that there is a positive correlation between the accumulation of acetic acid and ethanol, and both compounds are described as toxic to yeast. Ethanol does not accumulate in yeast cells. However, acetic acid may accumulate during fermentation and is toxic to glucose-grown yeast cells.35,36
3.2. Compound Accumulation in Bran Layer-Based Media
Wheat mashes were prepared from whole grains except for the ELF treatment that utilized debranned flour for fermentation mash. Table 3 and Figure 1D show the accumulation of betaine, and there was no significant difference between the treatment conditions except ELF which accumulated 0.22 g/L of betaine. Wheat is a predominant source of betaine, and it has been found predominantly in whole wheat flour but not in the refined endosperm wheat flour.37 Betaine was detected in commercial thin stillage samples,38,39 and these findings correspond with this research.
Ethanol accumulation was highest when fermentation was conducted in a medium with only the endosperm layer included (ELF; 72.1 g/L, Table 3). This accumulation is possible because the endosperm layer is starch-rich flour that lacks the bran layer from the whole grain. Bran constitutes 14–19% of the whole grain and comprises mostly arabinoxylans, cellulose, protein, and lignin.40 The enzymes used for preparing the medium for fermentation are not able to hydrolyze the cellulose and hemicellulose fractions of the bran layer into simple sugars.40 Ethanol accumulation with the other treatments utilized whole wheat; hence, there is a significant difference in the maximum ethanol concentration.
3.3. α-GPC Production Efficiency
α-GPC accumulation correlated positively and significantly with acetic acid and glycerol accumulation. Acetic acid accumulation is toxic to yeast cell cultures and reduces fermentation rates.35 The peak of acetic acid accumulation corresponds to the peak of α-GPC accumulation. PLF accumulated 1.68 g/L of α-GPC and 1.74 g/L of acetic acid, while WWF accumulated 1.33 g/L of α-GPC and 1.26 g/L of acetic acid (Table 3).
Phospholipase A1 treatment accumulated the most α-GPC from the fermentation broth, but there is no significant statistical difference between the quantities of α-GPC accumulated by the other treatment conditions, WWF, ELF, and PLF. It was hypothesized that the yeast might convert PC into additional α-GPC, but the addition of the precursor compound did not increase its production.
Glycerol is a significant component of α-GPC, in addition to choline and phosphate. Glycerol accumulates naturally as a byproduct of yeast in ethanol fermentation.41 In yeast fermentation, glycerol is accumulated by the redox-neutral process or fermentation when pH is above 7.42 PLF accumulated the highest glycerol concentration (13.0 g/L) and the highest accumulation of α-GPC (1.68 g/L; Table 3). There is an increase in α-GPC accumulated from fermentation after 72 h for PLF, 90 h for ELF, 66 h for PCF, and 42 h for WWF (Table 4). In ethanol production industries, fermentation typically lasts for about 72 h, and commercial production of α-GPC can also be obtained simultaneously without any change in the regular process or fermentation times.
4. Conclusions
The main contributions of this research are the validation of the potential for obtaining α-GPC from wheat fermentation in a cost-effective manner and provision for a less energy intensive means of its production from conventional fermentation. A Gompertz model was used to successfully describe the products obtained via fermentation of the wheat cultivar AC Andrew. The observed maximum accumulation of 1.68 g/L of α-GPC was measured in the PLE, but there were no significant differences among the other treatment conditions WWF, ELF, PCF aimed at increasing the quantity of α-GPC produced. Fermentation aimed to produce α-GPC will not have any effect on the ethanol production as 72.1 g/L of ethanol was produced simultaneously. Further research should include an investigation of the effect of different wheat cultivars, other grain sources, and increased sampling frequency as this might influence the kinetic parameters.
5. Experimental Section
5.1. Experimental Conditions
5.1.1. WWF
Fermentation for this treatment was conducted using media prepared from the whole wheat grain. The grain was milled with Glen mill type (C/11/1) dry grind disc mill. This procedure was designed to determine the quantity of α-GPC that accumulated during fermentation in AC Andrew-based media.
5.1.2. ELF
The grain (AC Andrew) used for fermentation for this study was milled with a modified Quadrumat Senior mill (C.W. Brabender Instruments, South Hackensack, NJ, USA). In the mill, there were two sieves used on the “Break” side: #35 (500 μm) and #100 (150 μm), while on the “Reduction” side, a #80 screen (180 μm) was used to recover flour. This process involves separation of bran from germ of the whole wheat kernel. Milling is then followed by sieving which converts flour to an off-white product free of bran and germ. This process was selected to determine the effects the bran layer might have as a source of α-GPC.
5.1.3. PLF
Phospholipase A1 has been used in the hydrolysis of soy PC and lecithin to release α-GPC.43,44 To determine whether treatment of mash with phospholipase A1 affected α-GPC accumulation, this enzyme was used to pretreat wheat mash. Experimental conditions were similar to the control using the whole wheat grain for fermentation; however, after liquefaction, 2000 μL of phospholipase A1 enzyme (EC 3.1.1.32) purchased from Sigma-Aldrich (Oakville, ON, Canada) was added. The enzyme was allowed to act at 30 °C for 4 h before simultaneous saccharification, and fermentation was initiated. This experiment will determine the effect of phospholipase A1 on α-GPC accumulation.
5.1.4. PCF
It was considered that yeast endogenous enzyme (phospholipase B) might convert lecithin, added prior to fermentation, to α-GPC. Therefore, additional α-GPC precursor compound l-α-PC purchased from Sigma-Aldrich (Oakville, ON, Canada) was added to the whole wheat mash. An increase in α-GPC concentration accumulation after fermentation might indicate conversion of the substrate by wheat enzymes.
5.2. Experimental Design
A completely randomized experimental design was selected with four replicated fermentation treatments. Sufficient mash was prepared from the wheat cultivar AC Andrew for four treatments. The first treatment involved fermenting the whole grain flour (WWF) with the bran layer present. The second treatment separated the bran layer prior to fermentation with only the endosperm layer (ELF) used as a fermentation substrate. The third treatment included whole wheat as in the first treatment, but the enzyme phospholipase A1 was added, as described above (phospholipase fermentation; PLF). The fourth treatment involved fermentation of the whole wheat grain with the addition of a precursor compound, l-α-phosphatidylcholine (PCF). Fermentation was conducted to mimic industrial standard conditions and determine the quantity of α-GPC accumulated/released over time. ELF treatment was to determine whether the bran layer contributed significantly to α-GPC accumulation, while the PLF and PCF were fermented to determine the effects of enzyme and precursor compound on α-GPC accumulation or release. The accumulation of compounds was measured over periods of 0, 24, 48, 72, 96, 120, and 168 h.
5.3. Fermentation
Milled whole wheat was gelatinized with boiled distilled water (36%, w/v) at 70 °C for 10 min. The gelatinized starch was then sterilized at 130 °C for 15 min in an autoclave. The temperature was then decreased to 80 °C prior to saccharification with α-amylase (0.2%, v/v) for 60 min. Afterward, the samples were cooled to 55 °C, a 25:75 mixture of glucanase/xylanase was added (0.01%, v/v), and saccharification proceeded for an additional 30 min. The samples were then cooled to 37 °C, and final additions of glucoamylase (0.1%, v/v), 5 g of yeast (Saccharomyces cerevisiae), 500 mg of urea, and 50 mg of antibiotics were added prior to fermentation. All enzymes, yeast, urea, and antibiotics were obtained from Terra Grain Fuels (Belle Plaine, SK, Canada). After inoculation, fermented samples were incubated at 37 °C for 168 h, and the sample aliquots were collected every 24 h using disposable transfer pipets (VWR International, Mississauga, ON, Canada).
5.4. Chemical Analysis
Proton resonances from organic compounds in stillage were measured during fermentation by a proton nuclear magnetic resonance (1H NMR) spectroscopy with a water suppression pulse sequence. The compounds α-GPC, ethanol, lactic acid, acetic acid, succinic acid, betaine, glycerol, and PEA were measured quantitatively by resonance signals at 3.05, 1.07, 1.25, 1.95, 2.5, 3.09, 3.45, 7.2 ppm, respectively. The strong water resonance present in the spectra was suppressed using double pulse field gradient spin echo, as provided by the Bruker XWIN-NMR software (Bruker, Mississauga, ON, Canada). The samples were collected every 24 h for seven days of incubation, and before analysis, samples were centrifuged (Beckman Coulter Canada Inc., Mississauga, ON, Canada) at 10,000 rpm for 10 min, and supernatant samples were filtered with 0.45 μm PTFE filters (Pall Corp, Ann Arbor, MI). Finally, filtered samples (0.5 mL) were added to clean NMR tubes. Deuterium oxide (50 μL, D2O, 99.8%) was mixed with each sample to provide a locking signal, 40 μL of pyrazine (C4H4N2) was added as an internal standard, and 500 μL of filtered samples were pipetted into each NMR tube. 1H NMR was used to record spectra (16 scans), and the concentration was determined by comparison with the internal standard resonance at 8.5 ppm.
5.5. Kinetic Model
The Gompertz model was used to describe ethanol production in the whole wheat mash.45 α-GPC accumulation from WWF was also modeled successfully via a model that considers a diminishing value in the variable contributing to accumulation over time. Fermentation took place as simultaneous saccharification and fermentation; no additional nutrients were supplied during fermentation; hence, no increase in the substrate concentration was observed. A sigmoidal curve model describes biological growth phenomenon that explains how a variable increases over different time intervals until it reaches saturation.46 Data were fitted to the model by nonlinear least squares to the equation representing the Gompertz model.46
Growth models generally are represented as a process monitored by dynamic characteristics of a variable as a function of increasing time. The Gompertz model provided in eq 2 was used to describe α-GPC accumulation.47 This model has been modified to fit the fermentation data as the variables of the original equation cannot be used to describe fermentation.
| 1 |
where Amaxe is the potential maximum ethanol accumulation (g/L), Rmaxe is the maximum ethanol productivity rate or productivity (g/L/h), and Le is the lag phase or the time to exponential ethanol accumulation (h).
| 2 |
where Amaxag is the potential maximum α-GPC accumulation (g/L), Rmaxag is the maximum α-GPC productivity rate or productivity (g/L/h), and Lag is the lag phase or the time to exponential α-GPC accumulation (h).
| 3 |
where Amaxl is the potential maximum lactic acid accumulation (g/L), Rmaxl is the maximum lactic acid productivity rate or productivity (g/L/h), and Ll is the lag phase or the time to exponential lactic acid accumulation (h).
| 4 |
where Amaxa is the potential maximum acetic acid accumulation (g/L), Rmaxa is the maximum acetic acid productivity rate or productivity (g/L/h), and La is the lag phase or the time to exponential acetic acid accumulation (h).
| 5 |
where Amaxs is the potential maximum succinic acid accumulation (g/L), Rmaxs is the maximum succinic acid productivity rate or productivity (g/L/h), and Ls is the lag phase or the time to exponential succinic acid accumulation (h).
| 6 |
where Amaxb is the potential maximum betaine accumulation (g/L), Rmaxb is the maximum betaine productivity rate or productivity (g/L/h), and Lb is the lag phase or the time to exponential betaine accumulation (h).
| 7 |
where Amaxg is the potential maximum glycerol accumulation (g/L), Rmaxg is the maximum glycerol productivity rate or productivity (g/L/h), and Lg is the lag phase or the time to exponential glycerol accumulation (h).
| 8 |
where Amaxp is the potential maximum PEA accumulation (g/L), Rmaxp is the maximum PEA productivity rate or productivity (g/L/h), and Lp is the lag phase or the time to exponential PEA accumulation (h).
Also, from the kinetic model, Tmax was derived for α-GPC and betaine because their respective lag periods were not defined from the analytical instrumentation measurements, where Tmax is time at which the maximum compound was obtained or time taken to reach Amax accumulation in the fermentation broth.
5.6. Statistical Analysis
Regression analyses of the Gompertz model (eqs 1–8 to determine the parameters Amax, Rmax, and L) were performed for all products accumulated from four treatments (WWF, ELF, PLF, and PCF) using Microsoft Excel with the Solver add-in. The root means square (rms) error was calculated, and this was further used to obtain the rms of the model in comparison to the experimental data. The Excel Solver add-in was then used to obtain the minimum rms error of the model by adjusting the initial values with a constraint of only obtaining positive values (≥0). The final values of each parameter were then used to obtain a new value for the predicted model. The parameters obtained were also analyzed using the SAS 9.4 package with a completely randomized design (eq 9).
| 9 |
where y is the dependent variable under examination, i is four treatments (1–4), ti is the fixed effect of the treatment, and eij is the error term specific to the wheat assigned to the treatment. The experiment was conducted with four replicates, and differences between the treatments were analyzed using the Tukey test.
Acknowledgments
The authors acknowledge the kind contribution of thin stillage samples from North West Terminal Ltd. (Unity, SK, Canada) and Terra Grain Fuels (Belle Plaine, SK, Canada) for enzymes and yeast.
Glossary
Abbreviations
- α-GPC
glycerylphosphorylcholine
- WWF
whole wheat fermentation
- ELF
endosperm layer fermentation
- PC
phosphatidylcholine
- PCF
l-α phosphatidylcholine fermentation
- PEA
phenethyl alcohol
- PLF
phospholipase A1 fermentation
- 1H NMR
proton nuclear magnetic resonance
This research was supported by the Strategic Research Program, Agricultural Development Funds of the Saskatchewan Ministry of Agriculture (20140277 and 20160233).
The authors declare no competing financial interest.
References
- Cereals Canada . Cereals Canada-Agriculture and Agri-food Canada and Cereals Canada Launch a National Report That Aims to Improve the Profitability of Canada’s Wheat Industry. https://cerealscanada.ca/news-policies/51-agriculture-and-agri-food-canada-and-cereals-canada-launch-a-national-report-that-aims-to-improve-the-profitability-of-canada-s-wheat-industry (accessed on April 12, 2020).
- National Research Council Canada . Factsheet-The Canadian Wheat Alliance. https://www.nrc-cnrc.gc.ca/eng/news/releases/2013/wheat_nrc_factsheet.html (accessed on April 12, 2020).
- McCallum B. D.; DePauw R. M. A review of wheat cultivars grown in the Canadian prairies. Can. J. Plant Sci. 2008, 88, 649–677. 10.4141/cjps07159. [DOI] [Google Scholar]
- Natural Resources Canada . Ethanol. http://www.nrcan.gc.ca/energy/alternative-fuels/fuel-facts/ethanol/3493 (accessed on April 12, 2020).
- Sangiorgi G. B.; Barbagallo M.; Giordano M.; Meli M.; Panzarasa R. α-Glycerophosphocholine in the mental recovery of cerebral ischemic attacks. Ann. N. Y. Acad. Sci. 1994, 717, 253–269. 10.1111/j.1749-6632.1994.tb12095.x. [DOI] [PubMed] [Google Scholar]
- Schettini G.; Ventra C.; Florio T.; Grimaldi M.; Meucci O.; Scorziello A.; et al. Molecular mechanisms mediating the effects of L-alpha-glycerylphosphorylcholine, a new cognition-enhancing drug, on behavioral and biochemical parameters in young and aged rats. Pharmacol. Biochem. Behav. 1992, 43, 139–151. 10.1016/0091-3057(92)90650-5. [DOI] [PubMed] [Google Scholar]
- Ratanapariyanuch K.; Shim Y. Y.; Emami S.; Reaney M. J. T. Industrial clarification of wheat-based distillers’ solubles and thin stillage. Ind. Crops Prod. 2017, 109, 828–835. 10.1016/j.indcrop.2017.09.029. [DOI] [Google Scholar]
- Ratanapariyanuch K.; Shim Y. Y.; Wiens D. J.; Reaney M. J. T. Grain thin stillage protein utilization: A review. J. Am. Oil Chem. Soc. 2018, 95, 933–942. 10.1002/aocs.12056. [DOI] [Google Scholar]
- Amenta F.; Parnetti L.; Gallai V.; Wallin A. Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches?. Mech. Ageing Dev. 2001, 122, 2025–2040. 10.1016/s0047-6374(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Grimm M. O. W.; Grösgen S.; Riemenschneider M.; Tanila H.; Grimm H. S.; Hartmann T. From brain to food: Analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in Alzheimer’s disease human post mortem brains and mice model via mass spectrometry. J. Chromatogr. A 2011, 1218, 7713–7722. 10.1016/j.chroma.2011.07.073. [DOI] [PubMed] [Google Scholar]
- De Jesus Moreno M. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial. Clin. Ther. 2003, 25, 178–193. 10.1016/s0149-2918(03)90023-3. [DOI] [PubMed] [Google Scholar]
- Parnetti L.; Amenta F.; Gallai V. Choline alphoscerate in cognitive decline and in cerebrovascular disease: an analysis of published clinical data. Mech. Ageing Dev. 2001, 122, 2041–2055. 10.1016/s0047-6374(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Choi J. W.; Park H.-Y.; Oh M. S.; Yoo H. H.; Lee S.-H.; Ha S. K. Neuroprotective effect of 6-paradol enriched ginger extract by fermentation using Schizosaccharomyces pombe. J. Funct. Foods 2017, 31, 304–310. 10.1016/j.jff.2017.02.010. [DOI] [Google Scholar]
- Gaire B. P.; Kwon O. W.; Hyuk P. S.; Kwang-Hoon C.; Yeou K. S.; Yun S. D.; Woong C. J. Neuroprotective effect of 6-paradol in focal cerebral ischemia involves the attenuation of neuroinflammatory responses in activated microglia. PLoS One 2015, 10, e0120203 10.1371/journal.pone.0120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019, 103, 4291–4312. 10.1007/s00253-019-09827-7. [DOI] [PubMed] [Google Scholar]
- Du Plessis H.; Du Toit M.; Nieuwoudt H.; Van Der Rijst M.; Kidd M.; Jolly N. Effect of Saccharomyces, non-Saccharomyces yeasts and malolactic fermentation strategies on fermentation kinetics and flavor of Shiraz wines. Fermentation 2017, 3, 64. 10.3390/fermentation3040064. [DOI] [Google Scholar]
- Minnaar P. P.; Jolly N. P.; Paulsen V.; Du Plessis H. W.; Van Der Rijst M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237. 10.1016/j.ijfoodmicro.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Miljić U.; Puškaš V.; Vučurović V.; Muzalevski A. Fermentation characteristics and aromatic profile of plum wines produced with indigeous microbiota and pure cultures of selected yeasts. J. Food Sci. 2017, 82, 1443–1450. 10.1111/1750-3841.13736. [DOI] [PubMed] [Google Scholar]
- Sonkar K.; Ayyappan V.; Tressler C. M.; Adelaja O.; Cai R.; Cheng M.; Glunde K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112 10.1002/nbm.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Murray J. P.; McMaster C. R. Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy Pathway. J. Biol. Chem. 2005, 280, 38290–38296. 10.1074/jbc.m507700200. [DOI] [PubMed] [Google Scholar]
- Li Z.; Vance D. E. Thematic Review Series: Glycerolipids.Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. 10.1194/jlr.r700019-jlr200. [DOI] [PubMed] [Google Scholar]
- Baer E.; Kates M. L-α-Glycerylphosphorylcholine. J. Am. Chem. Soc. 1948, 70, 1394–1399. 10.1021/ja01184a031. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H.; Yurkowski M. Simplified preparation of L-α-glycerylphosphoryl choline. Can. J. Biochem. 1965, 43, 1777. 10.1139/o65-197. [DOI] [PubMed] [Google Scholar]
- Kim H. J.; Song Y. S.; Song E. S.; Kang D. S.; Song I. W.; Kang P. G.; Oh S. S.; Moon S. C.; Lee B. G.. A process for preparation of L-alpha-glycerophosphoeyl choline. WO 2007145476 A1, 2007.
- Kim J.; Song Y.; Lee S. J.; Lee J. E.; Chung M.-Y.; Kim I.-H.; Kim B. H. Enzymatic preparation of food-grade L-α-glycerylphosphorylcholine from soy phosphatidylcholine or fractionated soy lecithin. Biotechnol. Prog. 2020, 36, e2910 10.1002/btpr.2910. [DOI] [PubMed] [Google Scholar]
- Blasi F.; Cossignani L.; Simonetti M. S.; Brutti M.; Ventura F.; Damiani P. Enzymatic deacylation of 1,2-diacyl-sn-glycero-3-phosphocholines to sn-glycerol-3-phosphocholine. Enzyme Microb. Technol. 2006, 39, 1405–1408. 10.1016/j.enzmictec.2006.03.026. [DOI] [Google Scholar]
- Zhang K.; Liu Y.; Wang X. Enzymatic preparation of L-α-glycerylphosphorylcholine in an aqueous medium. Eur. J. Lipid Sci. Technol. 2012, 114, 1254–1260. 10.1002/ejlt.201100219. [DOI] [Google Scholar]
- Zhang K.; Wang X.; Liu Y. Aqueous medium enzymatic preparation of L-alpha glycerylphosphorylcholine optimized by response surface methodology. Eur. Food Res. Technol. 2012, 234, 485–491. 10.1007/s00217-011-1655-x. [DOI] [Google Scholar]
- Zhang K.; Wang X.; Huang J.; Liu Y. Purification of L-alpha-glycerylphosphorylcholine by column chromatography. J. Chromatogr. A 2012, 1220, 108–114. 10.1016/j.chroma.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Prince M.; Wimo A.; Guerchet M.; Ali G.; Wu Y.; Prina M.. World Alzheimer report 2015-The global impact of dementia; Alzheimer Disease International, 2015; pp 1–82.
- AC Immune . Alzheimer’s disease. http://www.acimmune.com/en/alzheimer-s-disease/ (accessed on April 12, 2020).
- Zhang K.; Wang X.; Liu Y. Aqueous medium enzymatic preparation of L–alpha glycerylphosphorylcholine optimized by response surface methodology. Eur. Food Res. Technol. 2012, 234, 435–441. 10.1007/s00217-011-1655-x. [DOI] [Google Scholar]
- Parnetti L.; Amenta F.; Gallai V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: an analysis of published clinical data. Mech. Ageing Dev. 2001, 122, 2041–2055. 10.1016/s0047-6374(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Saunders J.Physicochemical properties of wheat starches and their relationship to liquefaction and fermentative bioethanol performance. Master Thesis, University of Manitoba: Winnipeg, MB, Canada, 2010. [Google Scholar]
- Pampulha M. E.; Loureiro-Dias M. C. Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biotechnol. 1989, 31, 547–550. 10.1007/BF00270792. [DOI] [Google Scholar]
- Pinto I.; Cardoso H.; Leão C.; van Uden N. High enthalpy and low enthalpy death in Saccharomyces cerevisiae induced by acetic acid. Biotechnol. Bioeng. 1989, 33, 1350–1352. 10.1002/bit.260331019. [DOI] [PubMed] [Google Scholar]
- Likes R.; Madl R. L.; Zeisel S. H.; Craig S. A. S. The betaine and choline content of a whole wheat flour compared to other mill streams. J. Cereal Sci. 2007, 46, 93–95. 10.1016/j.jcs.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanapariyanuch K.; Shen J.; Jia Y.; Tyler R. T.; Shim Y. Y.; Reaney M. J. T. Rapid NMR method for the quantification of organic compounds in thin stillage. J. Agric. Food Chem. 2011, 59, 10454–10460. 10.1021/jf2026007. [DOI] [PubMed] [Google Scholar]
- Ratanapariyanuch K.; Shim Y. Y.; Emami S.; Reaney M. J. T. Production of protein concentrate and 1,3-propanediol by wheat-based thin stillage fermentation. J. Agric. Food Chem. 2017, 65, 3858–3867. 10.1021/acs.jafc.7b00772. [DOI] [PubMed] [Google Scholar]
- Palmarola-Adrados B.; Chotěborská P.; Galbe M.; Zacchi G. Ethanol production from non-starch carbohydrates of wheat bran. Bioresour. Technol. 2005, 96, 843–850. 10.1016/j.biortech.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Tse J. T.; Shen J.; Shim Y. Y.; Reaney M. J. Changes in bacterial populations and their metabolism over ninety sequential cultures on wheat-based thin stillage. J. Agric. Food Chem. 2020, 68, 4717–4729. 10.1021/acs.jafc.9b07414. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhuge J.; Fang H.; Prior B. A. Glycerol production by microbial fermentation. Biotechnol. Adv. 2001, 19, 201–223. 10.1016/s0734-9750(01)00060-x. [DOI] [PubMed] [Google Scholar]
- Bang H.-J.; Kim I.-H.; Kim B. H. Phospholipase A-catalyzed hydrolysis of soy phosphatidylcholine to prepare L-α-glycerylphosphorylcholine in organic-aqueous media. Food Chem. 2016, 190, 201–206. 10.1016/j.foodchem.2015.05.093. [DOI] [PubMed] [Google Scholar]
- Chu H. P. The lecithinase of Bacillus cereus and its comparison with Clostridium welchii alpha-toxin. J. Gen. Microbiol. 1949, 3, 255–273. 10.1099/00221287-3-2-255. [DOI] [PubMed] [Google Scholar]
- O’Neill B.; van Heeswijck T.; Muhlack R.. Models for predicting wine fermentation kinetics. Proceedings of CHEMECA, 2011; pp 18–21.
- Carrillo M.; González J. M. A new approach to modelling sigmoidal curves. Technol. Forecast. Soc. Change 2002, 69, 233–241. 10.1016/s0040-1625(01)00150-0. [DOI] [Google Scholar]
- Groot J. C. J.; Cone J. W.; Williams B. A.; Debersaques F. M. A.; Lantinga E. A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. 10.1016/s0377-8401(96)01012-7. [DOI] [Google Scholar]