This randomized clinical trial investigates the efficacy and safety of abrocitinib in adolescents and adults with moderate-to-severe atopic dermatitis.
Key Points
Question
What is the efficacy and safety of abrocitinib monotherapy in patients with moderate-to-severe atopic dermatitis?
Findings
In this randomized clinical trial of 391 patients 12 years or older with moderate-to-severe atopic dermatitis, significantly greater proportions of patients treated with abrocitinib (200 mg or 100 mg) compared with placebo achieved an Investigator Global Assessment response of clear or almost clear with improvement of at least 2 grades and/or at least 75% improvement in Eczema Area and Severity Index scores. Serious adverse events were reported for 2 patients (1.3%) in the 200-mg group, 5 (3.2%) in the 100-mg group, and 1 (1.3%) in the placebo group.
Meaning
Abrocitinib was effective and well tolerated in adolescents and adults with moderate-to-severe atopic dermatitis.
Abstract
Importance
Abrocitinib, an oral, once-daily Janus kinase 1 selective inhibitor, was effective and well tolerated in a phase 3 monotherapy trial of patients with moderate-to-severe atopic dermatitis (AD).
Objective
To investigate the efficacy and safety of abrocitinib in adolescents and adults with moderate-to-severe AD in an identically designed trial.
Design, Setting, and Participants
This phase 3, double-blinded, placebo-controlled, parallel-group randomized clinical trial included patients 12 years or older with a clinical diagnosis of moderate-to-severe AD for at least 1 year and inadequate response to topical medications given for at least 4 weeks within 6 months. Patients were enrolled from 115 centers in Australia, Bulgaria, Canada, China, Czechia, Germany, Hungary, Japan, South Korea, Latvia, Poland, United Kingdom, and the United States from June 29, 2018, to August 13, 2019. Data were analyzed from September 13 to October 25, 2019.
Interventions
Patients were randomly assigned (2:2:1) to receive once-daily oral abrocitinib in 200- or 100-mg doses or placebo for 12 weeks.
Main Outcomes and Measures
The coprimary end points were the proportion of patients achieving Investigator Global Assessment (IGA) response (ie, clear [0] or almost clear [1], with improvement of ≥2 grades) and the proportion of patients achieving at least 75% improvement in Eczema Area and Severity Index score (EASI-75) at week 12. Key secondary end points included the proportion of patients achieving a Peak Pruritus Numerical Rating Scale (PP-NRS) response (ie, improvement of ≥4 points) at week 12. Other secondary end points included the proportion of patients achieving at least 90% improvement in EASI score (EASI-90). Safety was assessed via adverse events and laboratory monitoring.
Results
A total of 391 patients (229 male [58.6%]; mean [SD] age, 35.1 [15.1] years) were included in the analysis; of these, 155 received abrocitinib, 200 mg/d; 158, abrocitinib, 100 mg/d; and 78, placebo. Among patients with available data at week 12, greater proportions of patients in the 200- and 100-mg abrocitinib groups vs the placebo group achieved IGA (59 of 155 [38.1%] and 44 of 155 [28.4%] vs 7 of 77 [9.1%]; P < .001) and EASI-75 (94 of 154 [61.0%] and 69 of 155 [44.5%] vs 8 of 77 [10.4%]; P < .001), greater estimated proportions achieved PP-NRS (55.3% [95% CI, 47.2%-63.5%] and 45.2% [95% CI, 37.1%-53.3%] vs 11.5% [95% CI, 4.1%-19.0%]; P < .001), and/or greater proportions achieved EASI-90 (58 of 154 [37.7%] and 37 of 155 [23.9%] vs 3 of 77 [3.9%]) responses. Adverse events were reported for 102 patients (65.8%) in the 200-mg group, 99 (62.7%) in the 100-mg group, and 42 (53.8%) in the placebo group; serious adverse events were reported for 2 patients (1.3%) in the 200-mg group, 5 (3.2%) in the 100-mg group, and 1 (1.3%) in the placebo group. Decreases in platelet count (2 [1.3%]) and laboratory values indicating thrombocytopenia (5 [3.2%]) were reported in the 200-mg group.
Conclusions and Relevance
Monotherapy with once-daily oral abrocitinib was effective and well tolerated in adolescents and adults with moderate-to-severe AD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03575871
Introduction
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin condition with immune dysfunction that affects lesional and nonlesional skin.1 It is characterized by pruritus, skin pain, eczematous lesions, and dry skin.2,3,4,5 Atopic dermatitis affects as many as 20% of children and adolescents and approximately 5% to 10% of adults6,7,8,9 and is associated with substantial comorbidity and impairment in patient and caregiver quality of life.10,11
Systemic therapy is used to treat patients with moderate-to-severe AD on failure or excessive use of topical treatments. Immunosuppressive agents can have modest efficacy in patients with moderate-to-severe AD but are associated with adverse events that often limit long-term use.12 Dupilumab, a subcutaneous interleukin 4 (IL-4) receptor-α subunit antagonist that blocks IL-4 and IL-13 signaling, is approved to treat patients with moderate-to-severe AD. Not all patients respond to dupilumab, and some lose response to treatment over time.13 Dupilumab use is limited in patients unwilling to receive injections, as is the potential for flexible or episodic treatment. Furthermore, dupilumab treatment is associated with injection site reactions, conjunctivitis,14 and/or face and neck erythema.15 Hence, additional treatments for patients with moderate-to-severe AD are needed.
Abrocitinib is an oral, once-daily Janus kinase 1 (JAK1) selective inhibitor under investigation for the treatment of AD. Selective inhibition of JAK1 with abrocitinib modulates signaling by IL-4, IL-13, and other cytokines (eg, IL-31, IL-22, and thymic stromal lymphopoietin) involved in the pathogenesis of AD and pruritus while sparing JAK2 inhibition and minimizing the risk for neutropenia and anemia.16 Inhibition of neuronal JAK1 pathways has also been shown to ameliorate pruritus.17 Monotherapy with once-daily abrocitinib in 200-mg or 100-mg oral doses was effective and well tolerated in a dose-ranging phase 2b study in adults with moderate-to-severe AD.18 Similar results were observed in JADE MONO-1, a phase 3 trial of abrocitinib (200 mg or 100 mg) vs placebo in adolescents and adults with moderate-to-severe AD.19,20 Herein, we report the results of JADE MONO-2, a second replicate phase 3 trial of monotherapy with once-daily oral abrocitinib (200 mg or 100 mg) vs placebo in patients 12 years or older with moderate-to-severe AD.
Methods
The study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki21 and in compliance with all International Council for Harmonisation Good Clinical Practice Guidelines.22 All local regulatory requirements were followed. This research was approved by institutional review boards or ethics committees at each study site. An external data monitoring review committee monitored the safety of patients throughout the study. All patients or parents and guardians provided written informed consent. There were no substantial changes to methods or trial outcomes after the study was initiated. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Design and Participants
This multicenter, international, phase 3, placebo-controlled, parallel-group randomized clinical trial was conducted from June 29, 2018, to August 13, 2019, in Australia, Bulgaria, Canada, China, Czechia, Germany, Hungary, Japan, South Korea, Latvia, Poland, United Kingdom, and the United States. Eligible patients were 12 years or older, with body weight of at least 40 kg. Adolescent patients younger than 18 years (or country-specific age of majority) were eligible on a country-by-country basis as approved by the country or regulatory/health authority. Eligible patients had a confirmed diagnosis of chronic AD23 for at least 1 year before the first dose of study drug and moderate-to-severe AD (Investigator’s Global Assessment [IGA] score ≥3, Eczema Area and Severity Index [EASI] score24≥16, affected body surface area ≥10%, and Peak Pruritus Numerical Rating Scale [PP-NRS, used with permission of Regeneron Pharmaceuticals, Inc, and Sanofi SA] score25≥4) at the baseline visit. Eligible patients also had a documented recent history (within 6 months before screening) of inadequate response to treatment with topical corticosteroids or topical calcineurin inhibitors given for at least 4 weeks, a history of topical AD treatments being considered medically inadvisable, or a history of receiving systemic therapies for AD. The study protocol is found in Supplement 1.
Patients with a medical history of conditions associated with thrombocytopenia, coagulopathy, or platelet dysfunction were excluded from the study. Patients who had used any systemic JAK inhibitor, had used a systemic corticosteroid within 4 weeks of the first dose of study medication, had used topical treatments for AD within 72 hours of the first dose of study medication, or had received treatment with dupilumab within 6 weeks of the first dose of study medication were excluded. Concomitant use of topical (corticosteroids, calcineurin inhibitors, tars, antibiotic creams, or topical antihistamines) or systemic therapies for AD or rescue medication was prohibited. Patients were permitted to use oral antihistamines and topical nonmedicated emollients. Additional exclusion criteria are presented in eMethods in Supplement 2.
Randomization and Masking
Patients were randomly assigned (2:2:1) to receive once-daily oral abrocitinib in 200-mg or 100-mg doses or matching placebo for 12 weeks with a computer-generated randomization schedule using interactive response technology (Almac Group). Randomization was stratified by baseline disease severity (IGA score of 3 or 4) and age group (<18 or ≥18 years) and was administered via an interactive response technology system. Patients, investigators, and sponsors were blinded to study treatment.
Outcomes Measures
Multiplicity-controlled coprimary end points were the proportion of patients achieving IGA response (clear [0] or almost clear [1] with an improvement of ≥2 grades from baseline) and proportion of patients achieving EASI-75 response (≥75% improvement from baseline), both at week 12. Multiplicity-controlled key secondary end points were the proportion of patients achieving PP-NRS response (improvement of ≥4 points from baseline) at weeks 2, 4, and 12 and change from baseline in Pruritus and Symptoms Assessment for AD (an 11-item questionnaire developed to measure daily symptoms of AD) total score26 at week 12.
Other secondary end points included the proportion of patients achieving IGA response at all other scheduled points, the proportion of patients achieving EASI-75 at all other scheduled points, the proportion of patients achieving EASI-50 response (improvement of ≥50% from baseline) and/or EASI-90 response (improvement of ≥90% from baseline) at all scheduled points, the proportion of patients with PP-NRS response at all other scheduled points, time to PP-NRS response, and change from baseline in Scoring of AD index27 and EASI scores at all scheduled points.
The incidence of adverse events and clinical abnormalities as well as changes from baseline in clinical laboratory values, electrocardiographic measurements, and vital signs were recorded from the first dose of study drug through 28 days after the last dose of study drug. Patient-reported outcomes included change from baseline at week 12 and all other points in Dermatology Life Quality Index (DLQI) total score28 (in patients aged ≥18 years); Children’s DLQI (CDLQI) total score29 (in patients aged <18 years), Patient-Oriented Eczema Measure total score,30 Patient Global Assessment score, and Hospital Anxiety and Depression Scale anxiety and depression scores.31
Statistical Analysis
Data were analyzed from September 13 to October 25, 2019. Sample size calculation was based on Fisher exact test for comparing 2 proportions (eMethods in Supplement 2). The family-wise type 1 error rate for testing the coprimary and key secondary end points was strictly controlled at 5% using a sequential, Bonferroni-based procedure (eFigure 1 in Supplement 2). Testing of all other secondary end points was performed at the nominal 5% significance level and not controlled for multiplicity. Additional details of the statistical analysis are presented in eMethods in Supplement 2. Two-sided P < .05 indicated significance. Binary end points were tested with the Cochran-Mantel-Haenszel test. Continuous measures were tested based on a t distribution.
Results
Patients
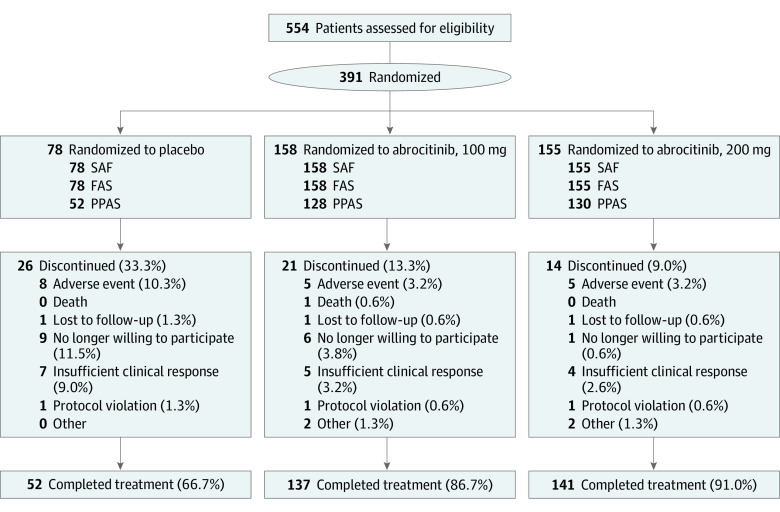
Overall, 554 patients were screened and 391 were randomized to treatment groups (229 male [58.6%] and 162 female [41.4%]; mean [SD] age, 35.1 [15.1] years). The treatment discontinuation rate was higher in the placebo group (26 of 78 [33.3%]) than in the abrocitinib groups (200-mg group, 14 of 155 [9.0%]; 100-mg group, 21 of 158 [13.3%]) (Figure 1).
Figure 1. CONSORT Diagram.
FAS indicates full analysis set; PPAS, per protocol analysis set; and SAF, safety analysis set.
Demographics and baseline characteristics were balanced across treatment arms (Table 1). White (232 [59.3%]), Asian (129 [33.0%]), black (21 [5.4%]), and Hispanic (9 [2.3%]) patients were included. Overall, 265 patients (67.8%) had moderate disease and 126 (32.2%) had severe disease, assessed by IGA score; 162 (41.4%) had received systemic treatment with or without topical treatment; and 226 (57.8%) had received topical agents alone within 1 year before the current study.
Table 1. Demographic and Baseline Characteristics.
| Characteristic | Treatment groupa | |||
|---|---|---|---|---|
| Placebo (n = 78) | Abrocitinib | All (n = 391) | ||
| 100 mg (n = 158) | 200 mg (n = 155) | |||
| Age, mean (SD), y | 33.4 (13.8) | 37.4 (15.8) | 33.5 (14.7) | 35.1 (15.1) |
| Age group, y | ||||
| <18 | 8 (10.3) | 17 (10.8) | 15 (9.7) | 40 (10.2) |
| 18-64 | 69 (88.5) | 130 (82.3) | 133 (85.8) | 332 (84.9) |
| ≥65 | 1 (1.3) | 11 (7.0) | 7 (4.5) | 19 (4.9) |
| Male | 47 (60.3) | 94 (59.5) | 88 (56.8) | 229 (58.6) |
| Race | ||||
| White | 40 (51.3) | 101 (63.9) | 91 (58.7) | 232 (59.3) |
| Asian | 29 (37.2) | 46 (29.1) | 54 (34.8) | 129 (33.0) |
| Black or African American | 6 (7.7) | 9 (5.7) | 6 (3.9) | 21 (5.4) |
| Multiracial | 1 (1.3) | 1 (0.6) | 2 (1.3) | 4 (1.0) |
| Not reported | 2 (2.6) | 1 (0.6) | 2 (1.3) | 5 (1.3) |
| Ethnicity | ||||
| Not Hispanic or Latino | 73 (93.6) | 154 (97.5) | 150 (96.8) | 377 (96.4) |
| Hispanic or Latino | 2 (2.6) | 3 (1.9) | 4 (2.6) | 9 (2.3) |
| Not reported | 3 (3.8) | 1 (0.6) | 1 (0.6) | 5 (1.3) |
| Disease duration, mean (SD), y | 21.7 (14.3) | 21.1 (14.8) | 20.5 (14.8) | 21.0 (14.7) |
| Prior prescribed or OTC medication for ADb | ||||
| Any | 78 (100) | 157 (99.4) | 153 (98.7) | 388 (99.2) |
| Anti-inflammatory topical agents alone (corticosteroids, crisaborole, calcineurin inhibitors) | 46 (59.0) | 87 (55.1) | 93 (60.0) | 226 (57.8) |
| Systemic agents (corticosteroids, cyclosporin, nonbiologics), biologics, and/or topical agents | 32 (41.0) | 70 (44.3) | 60 (38.7) | 162 (41.4) |
| Dupilumab | 2 (2.6) | 7 (4.4) | 5 (3.2) | 14 (3.6) |
| IGA scorec | ||||
| 3 | 52 (66.7) | 107 (67.7) | 106 (68.4) | 265 (67.8) |
| 4 | 26 (33.3) | 51 (32.3) | 49 (31.6) | 126 (32.2) |
| EASI score, mean (SD)d | 28.0 (10.2) | 28.4 (11.2) | 29.0 (12.4) | 28.5 (11.5) |
| BSA affected, mean (SD), % | 48.2 (20.8) | 48.7 (21.4) | 47.7 (22.3) | 48.2 (21.6) |
| SCORAD | ||||
| No. of patients | 78 | 158 | 155 | 391 |
| Mean (SD) scoree | 64.3 (12.4) | 63.8 (11.4) | 64.1 (13.1) | 64.0 (12.3) |
| PP-NRS | ||||
| No. of patients | 78 | 158 | 155 | 391 |
| Mean (SD) scoref | 6.7 (1.9) | 7.1 (1.6) | 7.0 (1.6) | 7.0 (1.7) |
| PSAAD | ||||
| No. of patients | 77 | 156 | 155 | 388 |
| Mean (SD) total scoreg | 5.1 (2.1) | 5.4 (2.1) | 5.2 (2.0) | 5.2 (2.1) |
| DLQIh | ||||
| No. of patients | 70 | 140 | 139 | 349 |
| Mean (SD) total scorei | 15.0 (7.1) | 15.4 (7.3) | 14.8 (6.0) | 15.0 (6.8) |
| CDLQIj | ||||
| No. of patients | 8 | 16 | 15 | 39 |
| Mean (SD) total scorek | 10.1 (3.8) | 13.8 (5.8) | 12.9 (5.7) | 12.7 (5.4) |
| POEM | ||||
| No. of patients | 78 | 156 | 154 | 388 |
| Mean (SD) total scorel | 19.2 (5.5) | 20.9 (5.7) | 19.7 (5.7) | 20.1 (5.7) |
| HADS | ||||
| Anxiety | ||||
| No. of patients | 78 | 156 | 153 | 387 |
| Mean (SD) scorem | 6.0 (3.7) | 5.5 (4.2) | 5.9 (3.9) | 5.7 (4.0) |
| Depression | ||||
| No. of patients | 78 | 156 | 153 | 387 |
| Mean (SD) scoren | 4.4 (3.3) | 4.1 (4.0) | 4.0 (3.7) | 4.1 (3.8) |
Abbreviations: AD, atopic dermatitis; BSA, body surface area; CDLQI, Children’s Dermatology Life Quality Index (DLQI); EASI, Eczema Area and Severity Index; HADS, Hospital Anxiety and Depression Scale; IGA, Investigator’s Global Assessment; OTC, over-the-counter; POEM, Patient-Oriented Eczema Measure; PP-NRS, Peak Pruritus Numerical Rating Scale; PSAAD, Pruritus and Symptoms Assessment for Atopic Dermatitis; SCORAD, Scoring of Atopic Dermatitis index.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
Patients were counted once for each main category in an exclusive manner, with systemic agents, biologics, and/or topical agents taking precedence over anti-inflammatory topical agents alone.
Scores range from 0 (clear) to 4 (severe), with 3 indicating moderate.
Scores range from 0 to 72, with higher scores indicating greater extent and severity of atopic dermatitis.
Scores range from 0 to 103, with higher scores indicating greater extent and severity of atopic dermatitis.
Scores range from 0 to 10, with higher scores indicating worse itch.
Scores range from 0 to 10, with higher scores indicating worse daily symptoms of atopic dermatitis.
For patients 18 years or older.
Scores range from 0 to 30, with higher scores indicating worse quality of life.
For patients aged younger than 18 years.
Scores range from 0 to 30, with higher scores indicating worst quality of life.
Scores range from 0 to 28, with higher scores indicating higher severity of atopic dermatitis.
Scores range from 0 to 21, with higher scores indicating increased anxiety.
Scores range from 0 to 21, with higher scores indicating increased depression.
Efficacy
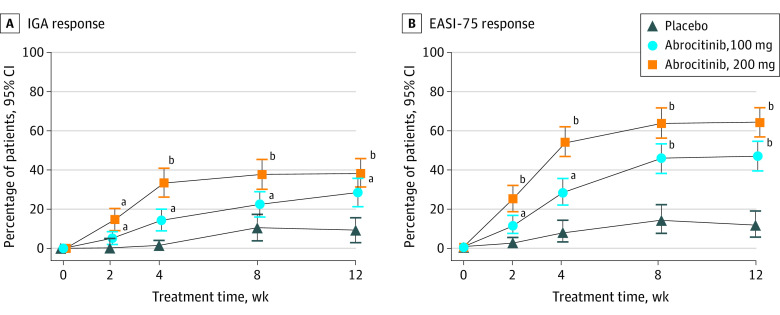
Among patients with data available at week 12 for the efficacy analysis, 59 of 155 patients in the 200-mg group (38.1%), 44 of 155 in the 100-mg group (28.4%), and 7 of 77 in the placebo group (9.1%) had an IGA response (Figure 2A); differences from placebo were 28.7% (95% CI, 18.6%-38.8%; P < .001) for the 200-mg group and 19.3% (95% CI, 9.6%-29.0%; P < .001) for the 100-mg group (eTable 1 in Supplement 2). At week 12, 94 of 154 patients in the 200-mg group (61.0%), 69 of 155 in the 100-mg group (44.5%), and 8 of 77 in the placebo group (10.4%) had an EASI-75 response (Figure 2B); differences from placebo were 50.5% (95% CI, 40.0%-60.9%; P < .001) for the 200-mg group and 33.9% (95% CI, 23.3%-44.4%; P < .001) for the 100-mg group (eTable 1 in Supplement 2). Sensitivity analyses performed for the coprimary end points at week 12 yielded similar conclusions (IGA response in the per protocol analysis set, 50 of 130 in the 200-mg group [38.5%], 39 of 128 in the 100-mg group [30.5%], and 6 of 52 in the placebo group [11.5%]; EASI-75 response in the per protocol analysis set, 81 of 130 in the 200-mg group [62.3%], 63 of 128 in the 100-mg group [49.2%], and 7 of 52 in the placebo group [13.5%]) (eTables 2 and 3 in Supplement 2). The proportions of patients achieving IGA (<18 years, 6 of 15 in the 200-mg group [40.0%], 2 of 16 patients in the 100-mg group [12.5%], and 0 of 7 patients in the placebo group [0%]; ≥18 years, 53 of 140 in the 200-mg group [37.9%], 42 of 139 patients in the 100-mg group [30.2%], and 7 of 70 patients in the placebo group [10.0%]) and/or EASI-75 (<18 years, 9 of 15 in the 200-mg group [60.0%], 7 of 16 patients in the 100-mg group [43.8%], and 0 of 7 patients in the placebo group [0%]; ≥18 years, 85 of 139 in the 200-mg group [61.2%], 62 of 139 patients in the 100-mg group [44.6%], and 8 of 70 patients in the placebo group [11.4%]) responses were higher with abrocitinib treatment vs placebo for all age groups (eTable 4 in Supplement 2). From weeks 2 to 12, the proportions of patients achieving IGA (week 2, 22 of 152 in the 200-mg group [14.5%], 8 of 157 in the 100-mg group [5.1%], and 0 of 76 in the placebo group; week 12, 59 of 155 in the 200-mg group [38.1%], 44 of 155 in the 100-mg group [28.4%], and 7 of 77 in the placebo group [9.1%]) and/or EASI-75 (week 2, 37 of 152 in the 200-mg group [24.3%], 16 of 157 in the 100-mg group [10.2%], and 1 of 76 in the placebo group [1.3%]; week 12, 94 of 154 in the 200-mg group [61.0%], 69 of 155 in the 100-mg group [44.5%], and 8 of 77 in the placebo group [10.4%]) responses increased and were higher for abrocitinib at each time point than with placebo (Figure 2).
Figure 2. Coprimary End Points at Week 12.
Data are represented as the percentage of patients achieving Investigator Global Assessment (IGA) response (ie, clear [0] or almost clear [1], with improvement of ≥2 grades) and at least 75% improvement in Eczema Area and Severity Index (EASI-75) score from baseline. IGA response (A) was achieved in 38.1% of patients in the 200-mg group (59 of 155), 28.4% in the 100-mg group (44 of 155), and 9.1% in the placebo group (7 of 77). EASI-75 response (B) was achieved by 61.0% in the 200-mg group (94 of 154), 44.5% in the 100-mg group (69 of 155), and 10.4% in the placebo group (8 of 77). Error bars represent 95% CIs. Conclusion of statistical significance was controlled for multiplicity only at week 12.
aP < .05 vs placebo.
bP < .001 vs placebo.
Percentage decreases from baseline in EASI scores were greater for both abrocitinib doses than for placebo at all time points (week 2, −51.3 [95% CI, −55.9 to −46.7] in the 200-mg group, −39.2 [95% CI, −43.8 to −34.7] in the 100-mg group, and −9.0 [95% CI, −15.4 to −2.5] in the placebo group; week 12, −73.3 [95% CI, −79.7 to −66.9] in the 200-mg group, −60.0 [95% CI, −66.5 to −53.6] in the 100-mg group, and −28.6 [95% CI, −38.4 to −18.8] in the placebo group) (eFigure 2A in Supplement 2). From weeks 2 to 12, the proportions of patients achieving EASI-50 (week 2, 84 of 152 in the 200-mg group [55.3%], 56 of 157 in the 100-mg group [35.7%], and 8 of 76 in the placebo group [10.5%]; week 12, 123 of 154 in the 200-mg group [79.9%], 106 of 155 in the 100-mg group [68.4%], and 15 of 77 in the placebo group [19.5%]) and/or EASI-90 (week 2, 14 of 152 in the 200-mg group [9.2%], 4 of 157 in the 100-mg group [2.5%], and 0 of 76 in the placebo group; week 12, 58 of 154 in the 200-mg group [37.7%], 37 of 155 in the 100-mg group [23.9%], and 3 of 77 in the placebo group [3.9%]) responses increased and were higher for abrocitinib treatment groups at each point than for placebo (eTable 1 and eFigure 3 in Supplement 2).
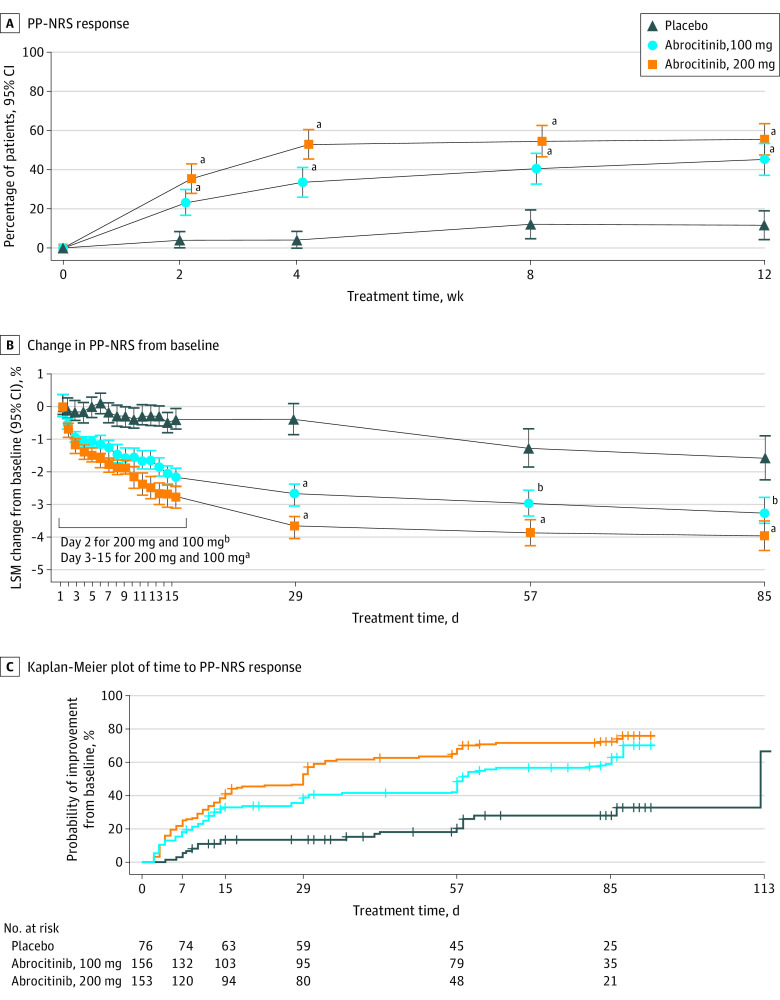
Proportions of patients achieving a PP-NRS response increased from weeks 2 to 12 for both abrocitinib doses (estimated response rate at week 2, 35.3% [95% CI, 27.7%-42.9%] in the 200-mg group, 23.1% [95% CI, 16.5%-29.7%] in the 100-mg group, and 3.9% [95% CI, 0.0%-8.3%] in the placebo group; estimated response rate at week 12, 55.3% [95% CI, 47.2%-63.5%] in the 200-mg group, 45.2% [95% CI, 37.1%-53.3%] in the 100-mg group, and 11.5% [95% CI, 4.1%-19.0%] in the placebo group) (Figure 3A and eTable 1 in Supplement 2). Decreases from baseline in PP-NRS scores were greater for both abrocitinib groups than the placebo group at all time points (week 12, −4.0 [95% CI, −4.4 to −3.6] in the 200-mg group, −3.3 [95% CI, −3.7 to −2.8] in the 100-mg group, and −1.6 [95% CI, −2.3 to −0.9] in the placebo group). Significant differences in PP-NRS scores between both doses of abrocitinib and placebo were observed within 24 hours of the first dose of treatment (ie, by day 2; −0.7 [95% CI, −0.9 to −0.5] in the 200-mg group, −0.6 [95% CI, −0.8 to −0.4] in the 100-mg group, and −0.1 [95% CI, −0.4 to 0.2] in the placebo group; P < .05 for 200-mg and 100-mg compared with placebo groups) (Figure 3B). Median time to PP-NRS response was 29.0 (95% CI, 16.0-31.0) days in the 200-mg group, 58.0 (95% CI, 56.0-83.0) days in the 100-mg group, and 112.0 (95% CI, 112.0-not evaluable [NE]) in the placebo group (Figure 3C). Decreases from baseline in Pruritus and Symptoms Assessment for AD (week 12, −3.0 [95% CI, −3.3 to −2.7] in the 200-mg group, −2.4 [95% CI, −2.8 to −2.1] in the 100-mg group, and −0.8 [95% CI, −1.3 to −0.3] in the placebo group) and Scoring of AD (week 12, −56.2 [95% CI, −61.2 to −51.1] in the 200-mg group, −45.8 [95% CI, −50.9 to −40.7] in the 100-mg group, and −22.7 [95% CI, −30.4 to −15.1] in the placebo group) index scores were greater for both abrocitinib groups vs placebo group at all points (eFigure 2B-C in Supplement 2).
Figure 3. Peak Pruritus Numerical Rating Scale (PP-NRS) Outcomes.
A, PP-NRS response is defined as improvement of at least 4 points from baseline. Response was achieved in the 200-mg group by 35.3% of patients at week 2, 52.8% at week 4, and 55.3% at week 12 and in the 100-mg group by 23.1% at week 2, 33.4% at week 4, and 45.2% at week 12 compared with 11.5% of the placebo group at week 12. Conclusion of statistical significance was not controlled for multiplicity at week 8. Error bars indicate 95% CIs. B, Change from baseline in PP-NRS was measured as least-squares mean (LSM). Conclusion of statistical significance was not controlled for multiplicity. Error bars indicate 95% CIs. C, In Kaplan-Meier plot of time to PP-NRS response, plot is based on observed data only (no imputation), and times to event were censored at treatment discontinuation or last observation if response had not been achieved. Twenty events were observed in the placebo group, 90 in the 100-mg group, and 110 in the 200-mg group.
aP ≤ .001 vs placebo.
bP < .05 vs placebo.
Safety
Overall, 102 patients in the 200-mg group (65.8%), 99 in the 100-mg group (62.7%), and 42 in the placebo group (53.8%) reported treatment-emergent adverse events (TEAEs) of any causality. The most frequently reported TEAEs were nausea in the 200-mg group (22 of 155 [14.2%]), nasopharyngitis in the 100-mg group (20 of 158 [12.7%]), and dermatitis atopic in the placebo group (12 of 78 [15.4%]) (Table 2). Median time to cessation of nausea was 7.0 (95% CI, 1.0-14.0) days in the 200-mg group, 2.5 (95% CI, 1.0-7.0) days in the 100-mg group, and 1.0 (95% CI, NE-NE) days in the placebo group.
Table 2. Summary of Adverse Events .
| Event | Treatment group, No. (%) | ||
|---|---|---|---|
| Placebo (n = 78) | Abrocitinib | ||
| 100 mg (n = 158) | 200 mg (n = 155) | ||
| Deaths | 0 | 1 (0.6) | 0 |
| Serious adverse events of any cause | 1 (1.3) | 5 (3.2) | 2 (1.3) |
| Most frequently reported TEAEs of any cause (≥3% in any treatment group) | |||
| Nausea | 2 (2.6) | 12 (7.6) | 22 (14.2) |
| Nasopharyngitis | 5 (6.4) | 20 (12.7) | 12 (7.7) |
| Headache | 2 (2.6) | 9 (5.7) | 12 (7.7) |
| Upper respiratory tract infection | 3 (3.8) | 14 (8.9) | 5 (3.2) |
| Dermatitis atopic | 12 (15.4) | 9 (5.7) | 6 (3.9) |
| Acne | 0 | 2 (1.3) | 9 (5.8) |
| Vomiting | 1 (1.3) | 2 (1.3) | 8 (5.2) |
| Upper abdominal pain | 0 | 2 (1.3) | 6 (3.9) |
| Blood creatine phosphokinase increased | 2 (2.6) | 3 (1.9) | 5 (3.2) |
| Folliculitis | 2 (2.6) | 0 | 5 (3.2) |
| Thrombocytopenia | 0 | 0 | 5 (3.2) |
Abbreviation: TEAE, treatment-emergent adverse event.
Serious adverse events were reported for 2 patients in the 200-mg group (1.3%), 5 in the 100-mg group (3.2%), and 1 in the placebo group (1.3%) (Table 2). Most serious adverse events were considered not related to treatment. There were no treatment-related serious adverse events in the 200-mg group. Serious adverse events that were considered related to treatment were reported for 2 patients in the 100-mg group: 1 patient developed herpangina, and 1 developed pneumonia (in both instances, abrocitinib treatment was discontinued). Two cases of serious adverse events considered related to treatment were reported for 1 patient in the placebo group: 1 case of eczema herpeticum and 1 case of staphylococcal infection (treatment was discontinued, and the patient recovered) (eTable 5 in Supplement 2).
Overall, 5 patients in the 200-mg group (3.2%), 6 in the 100-mg group (3.8%), and 10 in the placebo group (12.8%) discontinued treatment because of adverse events. The most frequently reported adverse events leading to treatment discontinuation were headache (2 [1.3%]) in the 200-mg group, dermatitis atopic (2 [1.3%]) in the 100-mg group, and dermatitis atopic (6 [7.7%]) in the placebo group.
There were no cases of malignant neoplasms or venous thromboembolism in any treatment group. Sudden cardiac death occurred in a woman in her 70s 3 weeks after discontinuation of the 100-mg dose of abrocitinib. This patient had a history of aortic valve sclerosis and untreated hypertension; the event was considered not related to treatment by the investigator (Table 2).
Serious infections were reported for no patients in the 200-mg group, 3 in the 100-mg group (1.9%), and 1 in the placebo group (1.3%). Herpes zoster TEAEs were reported for 2 patients in the 200-mg group (1.3%) (1 case of 2 adjacent dermatomes and 1 multidermatomal case; no treatment was given, and the cases resolved); no cases were reported in the 100-mg or placebo groups. Eczema herpeticum/Kaposi varicelliform eruption TEAEs were reported for 2 patients in the 100-mg group (1.3%), patients in the 100-mg group (1 patient discontinued treatment), and 1 in the placebo group (1.3%); no cases were reported in the 200-mg group. Other TEAEs of interest reported were acne (9 in the 200-mg group [5.8%], 2 in the 100-mg group [1.3%], and 0 in the placebo group), folliculitis (5 in the 200-mg group [3.2%], 0 in the 100-mg group, and 2 in the placebo group [2.6%]), vomiting (8 in the 200-mg group [5.2%], 2 in the 100-mg group [1.3%], and 1 in the placebo group [1.3%]), and upper abdominal pain (6 in the 200-mg group [3.9%], 2 in the 100-mg group [1.3%], and 0 in the placebo group) (Table 2).
Dose-related numeric decreases in median platelet counts were observed in patients treated with abrocitinib. A nadir in median platelet count was observed at week 4, with decreases of 26% in the 200-mg group, 19% in the 100-mg group, and less than 1% in the placebo group. Platelet counts returned toward baseline values thereafter despite continuation of treatment (eFigure 4 in Supplement 2). Most patients maintained platelet counts within the reference range; however, 2 patients required monitoring because of platelet counts of less than 75 × 103/μL (to convert to ×109/L, multiply by 0.001). Thrombocytopenia (assessed with laboratory values; 5 [3.2%]) and decreased platelet count (assessed objectively; 2 [1.3%]) TEAEs were reported in the 200-mg group; no events were reported in the 100-mg or placebo groups. The 2 patients with mild reductions in platelet counts recovered with continued treatment. One patient in the 200-mg group discontinued treatment for 8 days because of mild thrombocytopenia (approximately 83 × 103/μL); the patient recovered, and abrocitinib treatment was reinitiated without additional reports of thrombocytopenia. There were no reports of bleeding disorders in any treatment group.
There were no clinically significant changes in hemoglobin levels or neutrophil or lymphocyte counts. There were dose-related increases of approximately 10% in high- and low-density lipoprotein levels for both abrocitinib doses compared with placebo. Increases in creatinine kinase levels also occurred in the 200-mg and 100-mg groups. All other observed changes in clinical laboratory values, electrocardiographic measurements, and vital signs were not clinically meaningful.
Patient-Reported Outcomes
From weeks 2 to 12, adult (≥18 years) and adolescent (<18 years) patients reported improvement in their quality of life, with greater improvements in DLQI (week 2, −7.9 [95% CI, −8.8 to −7.1] in the 200-mg group, −5.6 [95% CI, −6.5 to −4.8] in the 100-mg group, and −1.6 [95% CI, −2.8 to −0.4] in the placebo group; week 12, −9.8 [95% CI, −10.7 to −8.8] in the 200-mg group, −8.3 [95% CI, −9.3 to −7.3] in the 100-mg group, and −3.9 [95% CI, −5.3 to −2.4] in the placebo group) and CDLQI (week 2, −7.1 [95% CI, −9.6 to −4.6] in the 200-mg group, −3.3 [95% CI, −5.8 to −0.9] in the 100-mg group, and −2.4 [95% CI, −5.8 to 1.1] in the placebo group; week 12, −9.7 [95% CI, −12.1 to −7.4] in the 200-mg group, −4.8 [95% CI, −7.2 to −2.5] in the 100-mg group, and −2.7 [95% CI, −6.1 to 0.8] in the placebo group) scores for both abrocitinib doses compared with placebo (eTable 1 and eFigure 5A-B in Supplement 2). From weeks 2 to 12, abrocitinib-treated patients reported greater improvements in AD severity than placebo-treated patients, measured by Patient-Oriented Eczema Measure (week 2, −7.5 [95% CI, −8.4 to −6.6] in the 200-mg group, −5.2 [95% CI, −6.1 to −4.3] in the 100-mg group, and −0.6 [95% CI, −2.0 to 0.7] in the placebo group; week 12, −11.0 [95% CI, −12.1 to −9.8] in the 200-mg group, −8.7 [95% CI, −9.9 to −7.5] in the 100-mg group, and −3.6 [95% CI, −5.3 to −1.9] in the placebo group) and Patient Global Assessment (week 2, −1.0 [95% CI, −1.1 to −0.9] in the 200-mg group, −0.6 [95% CI, −0.7 to −0.5] in the 100-mg group, and 0.0 [95% CI, −0.2 to 0.1] in the placebo group; week 12, −1.4 [95% CI, −1.6 to −1.2] in the 200-mg group, −1.0 [95% CI, −1.2 to −0.8] in the 100-mg group, and −0.4 [95% CI, −0.7 to −0.1] in the placebo group) (eTable 1 and eFigure 5C-D in Supplement 2), and improvement in anxiety (week 2, −1.3 [95% CI, −1.7 to −0.9] in the 200-mg group, −1.1 [95% CI, −1.5 to −0.7] in the 100-mg group, and −0.3 [95% CI, −0.8 to 0.3] in the placebo group; week 12, −1.7 [95% CI, −2.2 to −1.2] in the 200-mg group, −1.6 [95% CI, −2.1 to −1.1] in the 100-mg group, and −0.6 [95% CI, −1.3 to 0.2] in the placebo group) and depression (week 2, −0.9 [95% CI, −1.3 to −0.6] in the 200-mg group, −0.8 [95% CI, −1.2 to −0.4] in the 100-mg group, and 0.9 [95% CI, 0.3 to 1.4] in the placebo group; week 12, −1.4 [95% CI, −1.8 to −1.0] in the 200-mg group, −1.0 [95% CI, −1.5 to −0.6] in the 100-mg group, and 0.3 [95% CI, −0.3 to 0.9] in the placebo group) measured with the Hospital Anxiety and Depression Scale (eTable 1 and eFigure 5E-F in Supplement 2).
Discussion
Oral abrocitinib in a 200-mg or a 100-mg dose significantly improved AD signs and symptoms compared with placebo in adolescents and adults with moderate-to-severe AD. Similar to the JADE MONO-1 study, IGA and EASI-75 responses were observed as early as week 2 of treatment and were sustained until week 12. Furthermore, similar IGA and EASI-75 responses were observed in analyses for patients younger than 18 years or 18 years or older.
Abrocitinib was well tolerated in this study; the incidences of serious adverse events, adverse events leading to discontinuation, and serious infections were low in the abrocitinib groups compared with the placebo group. Nausea was transient and resulted in treatment discontinuation in 1 patient in the 200-mg group. The incidences of herpes zoster and eczema herpeticum in patients treated with abrocitinib were also low and comparable with those of the placebo group. There were no significant clinical changes in hemoglobin level or neutrophil or lymphocyte counts, consistent with the selective inhibition of JAK1 vs JAK2 for abrocitinib. However, dose-related decreases in median platelet counts were observed at week 4. Platelet count stabilized by week 12 of treatment without clinical sequalae or treatment cessation. Most patients had platelet counts within the reference range throughout the study, but patients with thrombocytopenia, coagulopathy, or platelet dysfunction were excluded from the study. The mechanism that leads to changes in platelet count with abrocitinib treatment is not known. However, it could be a pharmacologic effect of abrocitinib, potentially mediated by the inhibition of JAK1 and downstream inhibition of thrombopoietin production or by the inhibition of Ashwell-Morrell receptors and downstream effects on platelet production.32,33
Pruritus is one of the most bothersome symptoms of AD.9,34 In this study, abrocitinib reduced the severity of pruritus compared with placebo within 24 hours after treatment initiation and through week 12. This reduction could explain the substantial quality-of-life benefits observed in this study. Abrocitinib may mediate this effect through various mechanisms, including relief of inflammation and/or direct inhibition of neuronal JAK1 signaling of pruritogenic cytokines.17,35
The results of the JADE MONO-1 and JADE MONO-2 studies followed similar trends. In both studies, higher proportions of abrocitinib-treated than placebo-treated patients achieved IGA and/or EASI-75 responses at week 12. Abrocitinib was well tolerated, with a low incidence of serious adverse events, similar to placebo. However, compared with the JADE MONO-1 study, the JADE MONO-2 study included a greater proportion of Asian patients (15.0% vs 33.0%, respectively), who have been reported to have different subsets of helper T cell–driven inflammatory activity compared with patients of European descent.36 Together, the results of both trials support the efficacy and safety profile of abrocitinib in a broad range of patients, possibly related to the inhibition of a range of AD-associated cytokines. Comparisons of efficacy and safety results between abrocitinib and dupilumab are complicated by differences in administration route, treatment period, efficacy end point definitions, analysis of efficacy end points, and patient inclusion criteria. However, the efficacy of abrocitinib appears at least comparable to that of dupilumab in a broadly similar patient population.
Limitations
This study was limited by the short duration of the treatment and follow-up periods, which did not address the long-term efficacy and safety of abrocitinib. More patients might have achieved IGA and/or EASI-75 responses with longer treatment. The adolescent and nonwhite patient populations of the study were small, limiting the applicability of abrocitinib in these patient populations. In addition, this study excluded patients with platelet dysfunction, limiting the applicability of these results in this patient population.
Conclusions
Once-daily oral abrocitinib, 200 mg or 100 mg, was well tolerated and significantly improved signs and symptoms of AD compared with placebo in adolescent and adult patients with moderate-to-severe disease. Future studies and analyses should focus on the long-term efficacy and safety of abrocitinib in patients with moderate-to-severe AD and in adolescents and nonwhite patients and explore the use of abrocitinib in combination with topical therapies.
Trial Protocol
eMethods. Additional Exclusion Criteria, Sample Size Calculation, Bonferroni-Based Procedure for Testing the Coprimary and Key Secondary End Points, and Additional Statistical Analyses
eFigure 1. Bonferroni-Based Procedure for Testing the Coprimary and Key Secondary End Points
eFigure 2. Change From Baseline in (A) EASI, (B) PSAAD, and (C) SCORAD
eFigure 3. Proportions of Patients Achieving (A) EASI-50 and (B) EASI-90 Responses
eFigure 4. Median Absolute Platelet Count
eFigure 5. Patient-Reported Outcomes
eTable 1. Summary of Efficacy End Points and Patient-Reported Outcomes
eTable 2. Sensitivity Analysis Using the Per-Protocol Analysis Set for Coprimary End Points
eTable 3. Sensitivity Analysis Using Tipping Point Analysis for Coprimary End Points
eTable 4. Proportion of Patients Achieving Coprimary End Points by Age Group
eTable 5. Serious Adverse Events of Any Causality
Data Sharing Statement
References
- 1.Suárez-Fariñas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127(4):954-64.e1, 4. doi: 10.1016/j.jaci.2010.12.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10-22.e2. doi: 10.1016/j.anai.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 3.Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699-2706.e7. doi: 10.1016/j.jaip.2019.05.055 [DOI] [PubMed] [Google Scholar]
- 4.Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42. doi: 10.1186/s41687-019-0128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548-552.e3. doi: 10.1016/j.anai.2017.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132-1138. doi: 10.1016/j.jaci.2013.08.031 [DOI] [PubMed] [Google Scholar]
- 7.Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1 pt 1):125-138. doi: 10.1016/S0091-6749(99)70536-1 [DOI] [PubMed] [Google Scholar]
- 8.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583-590. doi: 10.1016/j.jid.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 9.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340-347. doi: 10.1016/j.anai.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192-199. doi: 10.1111/j.1525-1470.2005.22303.x [DOI] [PubMed] [Google Scholar]
- 11.Rønnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448-456.e30. doi: 10.1016/j.jaad.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 12.Wollenberg A, Barbarot S, Bieber T, et al. ; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS) . Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850-878. doi: 10.1111/jdv.14888 [DOI] [PubMed] [Google Scholar]
- 13.Hendricks AJ, Lio PA, Shi VY. Management recommendations for dupilumab partial and non-durable responders in atopic dermatitis. Am J Clin Dermatol. 2019;20(4):565-569. doi: 10.1007/s40257-019-00436-8 [DOI] [PubMed] [Google Scholar]
- 14.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459-473. doi: 10.1111/bjd.17869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albader SS, Alharbi AA, Alenezi RF, Alsaif FM. Dupilumab side effect in a patient with atopic dermatitis: a case report study. Biologics. 2019;13:79-82. doi: 10.2147/BTT.S195512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462(1):1-13. doi: 10.1042/BJ20140712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217-228.e13. doi: 10.1016/j.cell.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. Published online October 2, 2019. doi: 10.1001/jamadermatol.2019.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson E, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis (AD): results from the phase 3, JADE MONO-1 study. Poster presented at: European Academy of Dermatology and Venereology 28th Congress; October 9-13, 2019; Madrid, Spain. [Google Scholar]
- 20.ClinicalTrials.gov . Study to Evaluate Efficacy and Safety of PF-04965842 in Subjects Aged 12 Years and Older With Moderate to Severe Atopic Dermatitis (JADE Mono-1). NCT03349060. Accessed X. https://clinicaltrials.gov/ct2/show/NCT03349060
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Dixon JR Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 23.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(92):44-47. [Google Scholar]
- 24.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M; EASI Evaluator Group . The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10(1):11-18. doi: 10.1034/j.1600-0625.2001.100102.x [DOI] [PubMed] [Google Scholar]
- 25.Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761-769. doi: 10.1111/bjd.17744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebwohl MG, Simpson EL, Bushmakin AG, et al. Validation of the pruritus and symptoms assessment for atopic dermatitis in adults with moderate to severe atopic dermatitis [ISAD abstract P071]. Br J Dermatol. 2018;179:e44. https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.16718 [Google Scholar]
- 27.European Task Force on Atopic Dermatitis . Severity scoring of atopic dermatitis: the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23-31. doi: 10.1159/000247298 [DOI] [PubMed] [Google Scholar]
- 28.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216. doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 29.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132(6):942-949. doi: 10.1111/j.1365-2133.1995.tb16953.x [DOI] [PubMed] [Google Scholar]
- 30.Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513-1519. doi: 10.1001/archderm.140.12.1513 [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 32.Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47-54. doi: 10.1038/nm.3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grozovsky R, Giannini S, Falet H, Hoffmeister KM. Novel mechanisms of platelet clearance and thrombopoietin regulation. Curr Opin Hematol. 2015;22(5):445-451. doi: 10.1097/MOH.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51(3):263-292. doi: 10.1007/s12016-015-8488-5 [DOI] [PubMed] [Google Scholar]
- 35.Rabenhorst A, Hartmann K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep. 2014;14(4):423. doi: 10.1007/s11882-014-0423-y [DOI] [PubMed] [Google Scholar]
- 36.Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254-1264. doi: 10.1016/j.jaci.2015.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Additional Exclusion Criteria, Sample Size Calculation, Bonferroni-Based Procedure for Testing the Coprimary and Key Secondary End Points, and Additional Statistical Analyses
eFigure 1. Bonferroni-Based Procedure for Testing the Coprimary and Key Secondary End Points
eFigure 2. Change From Baseline in (A) EASI, (B) PSAAD, and (C) SCORAD
eFigure 3. Proportions of Patients Achieving (A) EASI-50 and (B) EASI-90 Responses
eFigure 4. Median Absolute Platelet Count
eFigure 5. Patient-Reported Outcomes
eTable 1. Summary of Efficacy End Points and Patient-Reported Outcomes
eTable 2. Sensitivity Analysis Using the Per-Protocol Analysis Set for Coprimary End Points
eTable 3. Sensitivity Analysis Using Tipping Point Analysis for Coprimary End Points
eTable 4. Proportion of Patients Achieving Coprimary End Points by Age Group
eTable 5. Serious Adverse Events of Any Causality
Data Sharing Statement