Abstract
Background.
There is mounting evidence that the central nervous system utilizes a modular approach for neuromuscular control of walking by activating groups of muscles in units termed muscle synergies. Examination of muscle synergies in clinical populations may provide insights into alteration of neuromuscular control underlying pathological gait patterns. Previous studies utilizing synergy analysis have reported reduced motor control complexity during walking in those with neurological deficits, revealing the potential clinical utility of this approach.
Methods.
We extracted muscle synergies on a stride-to-stride basis from 20 children with CP (GMFCS I-II) and 8 children without CP, allowing the number of synergies to vary for each stride. Similar muscle synergies across all participants and strides were grouped using a k-means clustering and discriminant analysis.
Results.
In total 10 clusters representing 10 distinct synergies were found across the 28 individuals. Relative to their total number of synergies deployed during walking, synergies from children with CP were present in a higher number of clusters than children with TD, indicating significantly greater stride-to-stride variability. This increased variability was present despite reduced complexity, as measured by the mean number of synergies in each stride. While children with CP demonstrate some novel synergies, they also deploy some of the same muscle synergies as those with TD, although less frequently and with more variability.
Conclusion.
A stride-by-stride approach to muscle synergy analysis expands its clinical utility, and may provide a method to tailor rehabilitation strategies by revealing inconsistent but functional synergies in each child with CP.
Keywords: muscle synergy, gait, cerebral palsy, electromyography, motor function
Introduction
Gait patterns in children with cerebral palsy (CP) can be influenced by muscle weakness, spasticity, poor selective motor control, sensory abnormalities, and secondary musculoskeletal changes.1,2 Abnormal patterns also arise from altered projections from the motor cortex to spinal motor neurons.3 Functional limitations may worsen over time, especially after adolescence and in less functional individuals,4 resulting in diminished mobility with age.5
Walking is a complex motor task that requires coordination of many degrees of freedom. Previous studies suggest that the central nervous system controls gait with a modular approach6,7 whereby groups of muscles are recruited and activated as a single unit8 referred to as a synergy.7,9 Studies in healthy populations report that a small set of synergies can describe muscle activity during walking6,10–13 and that individuals consistently recruit the same muscle synergy during each gait phase. The activation profile and synergy structure, i.e. the relative weighting of the muscles that comprise each synergy, have also been reported to be consistent across studies,9,13 revealing that a small number of synergies (4–6) can account for greater than 90% of the variance observed from individually recorded electromyography (EMG) during walking. Similar synergies may be deployed across related tasks that exhibit distinct kinematics (e.g., running vs. walking) or kinetics (e.g., with or without body weight support), suggesting a neural origin to their organization.9,11,14,15
In individuals with neurological deficits, e.g. stroke and cerebral palsy, the same level of variance in EMG is accounted for by a lower synergy number than in those without deficits16–20 and the variance accounted for (VAF) by a single synergy was significantly higher in CP than in controls with typical development (TD).18 These findings were correlated with clinical measures of decreased function16 and increased spasticity19 and have been attributed to merging of synergies;21,22 however, the evidence for merging of synergies in those with neurological deficits is not conclusive. Cluster analysis of synergy structures extracted from 22 muscles showed the same optimal number of clusters (four) described EMG patterns in children with CP and typical development (TD), although the activation profile of each synergy appeared to be broader in CP than TD, suggesting less precise control of timing.23 Comparable structures were also found in subacute stroke and healthy controls24 and in backward walking in those with CP and TD.25 Thus, the effect of brain injury on the number and structure of synergies deployed during walking remains an open question.
Beyond assessing motor complexity across groups, synergy analysis has also shown promise as a clinical measure. The response to treadmill training in individuals post-stroke was related to synergy number before therapy.26 Similarly, a retrospective synergy analysis in children with CP indicated that dynamic complexity was associated with better treatment outcomes.27 Yet, these findings may be merely a glimpse into this technique’s potential. We recently demonstrated that stride-to-stride muscle synergy analysis can reveal reliable synergies not detected by traditional synergy analyses based solely upon a VAF threshold.28 It is also possible that only examining reliable synergies, regardless of whether they meet the threshold VAF, may omit clinically relevant information. Inconsistent synergies, i.e. those with low repeatability, may not constitute spurious noise but instead could have true neurological underpinnings and clinical relevance, i.e. be indicative of reflex responses or sporadic involuntary movements. Reducing or eliminating this “noise” through intervention may lead to functional benefits. Conversely, inconsistent synergies demonstrating more functional activation profiles and muscle weightings may represent targets for training paradigms which could seek to improve their robustness. Differentiating between synergies that arise from noise and those with physiological underpinnings remains a challenge. One potential solution, which we implemented here, is to eschew aggregation of strides (e.g., averaging or concatenating) which filters out stride-specific features and instead extract synergies from individual strides. The stride-specific synergies could be inspected based on their structure and activation timing, either manually or algorithmically, to verify that they are physiologically meaningful, and then compared across a bout of walking to assess the consistency of the underlying neuromotor control strategies.
Given the high stride-to-stride motor variability exhibited in immature gait,29 combined with the irregular synchrony of motor units30 and loss of selective motor control31 in children with CP, muscle synergies underlying gait in this population may be substantially more variable across strides compared to typically developing peers. A more in depth analysis of these synergies is therefore likely to reveal useful clinical insights. Our aim was to investigate the variability of muscle synergy number and structure during walking in children with CP and those with TD using a different approach than utilized previously. We extracted muscle synergies from individual strides, allowing the number of synergies to vary for each stride. Then clustering and discriminant analyses were used to identify similar synergies across strides. Based on observed motor variability in CP and other brain injuries,30,32 we hypothesized that children with CP would exhibit a larger number of unique muscle synergy structures during walking than children with TD. We also evaluated whether stride-to-stride variability of individual muscle activations differed between children with CP and TD. Finally, we examined whether stride-to-stride variability of synergies was correlated with functional mobility.
Methods
Participants
Participants in this study included 20 children with CP (11 males; mean age, 12.5 ± 3.3 yr; body mass, 43.9 ± 14.5 kg; height, 149.6 ± 16.3 cm) and eight with TD (3 males; mean age, 12.0 ± 2.6 yr; body mass, 45.1 ± 9.8 kg; height, 150.4 ± 11.4 cm). Seventeen children had a diagnosis of hemiplegia, and three had diplegia; 12 were Gross Motor Function Classification System (GMFCS) Level I and 8 were Level II. All participants were able to walk independently. Data were collected during the baseline visit for a planned intervention study, with exclusion criteria for that study including an equinus deformity (less than 0° ankle dorsiflexion with knee extension), botulinum toxin injection within four months, or orthopedic surgery on the legs within a year. The institutional review board approved the study protocol (#90-CC-0168). Informed consent and assent were obtained from a legal guardian and each participant, respectively.
Procedures
Participants completed 5 overground walking trials at self-selected pace while synchronized EMG (Trigno, Delsys, Boston, MA) and joint (hip, knee, and ankle) kinematic data using 10 motion capture cameras (Vicon, Denver, CO) were collected. Bipolar surface electrodes recorded EMG from the tibialis anterior (TA), medial gastrocnemius (MG), rectus femoris (RF), and medial hamstrings (MH) bilaterally. EMG sampling rate was 960 Hz with a fixed low pass filter at 450 Hz within the hardware. Placement of EMG electrodes was per SENIAM guidelines and signals were verified by manual muscle testing to ensure proper location. Kinematic data were used to determine the time point of heel contact in the dominant or less-impaired leg during each trial as the start and end of a single stride or gait cycle. Five gait cycles of EMG data were randomly selected per subject for analysis. Data were analyzed using Visual3D (C-Motion, Germantown, MD) and Matlab (Mathworks, Natick, MA) software.
Muscle synergy analysis
EMG signals were high-pass filtered (3rd order Butterworth) at 35 Hz, full-wave rectified, and low-pass filtered (3rd order Butterworth) with a cutoff of 5 Hz. Then, EMG data were timeinterpolated to 100 points over a gait cycle and normalized by the maximum activation value in each gait cycle resulting in muscle × time matrices (EMGo) ranging from 0 to 1. Non-negative matrix factorization was used to extract muscle synergies from EMGo for single gait cycles.33 This linear decomposition technique computed muscle synergies according to the following formula:
where n is the number of synergies ranging from 1 to 8, W is a synergy structure (muscle × n) indicating weighting values of individual muscles for each synergy, C is a synergy activation (n × time) indicating time-varying synergy activation profiles, and e is residual error. EMGr is a reconstructed EMG matrix (muscle × time) calculated from the multiplication of W and C. To determine the number of muscle synergies, we calculated the variance accounted for (VAF) as follows:
VAF threshold was set at 90% as in previous studies28 for our primary analysis. For each gait cycle we identified the lowest number of synergies that resulted in over 90% total VAF. To assess the sensitivity of our stride-to-stride variability outcome measures we repeated the analysis for VAF thresholds of 75%, 80%, 85%, and 95%. We also computed the variance accounted by 1 synergy and the z-score of the unaccounted variance by a single synergy termed walk-DMC.18
Stride-to-stride variability
The stride-to-stride variability of muscle synergies was examined using synergy structures (W) from the 5 strides for each subject. The number of muscle synergies required to reach the VAF threshold can vary between strides even in healthy individuals,34 and likely also in children with CP, making it impracticable to analyze stride-to-stride variability using conventional methods such as ICC alone (intraclass correlation coefficient). Thus, we combined iterative kmeans clustering with ICC analyses28 to assess the stride-to-stride variability of muscle synergies during walking while allowing the number of synergies to differ between strides. Synergies from individual strides were mapped to the multi-dimensional (n=8) parameter space based on their structure matrices and were then clustered into groups using k-means. ICC was then computed for each cluster to assess the similarity of synergies within it. For an individual, the number of clusters in which their synergies were present provided a measure of synergy variability across strides because disparate synergy structures between strides were grouped into different clusters. For instance, if five gait cycles each required four muscle synergies to satisfy the VAF threshold, and each of those four synergy structures was strongly consistent across the five gait cycles, these synergies would be assigned to four clusters. Conversely, if the four synergy structures were highly variable across the five gait cycles, these synergies would be assigned into as many as 20 distinct clusters.
The size of the data matrix for clustering was 8 muscles × ∑nW, where ∑nW is the total number of synergies across the five strides in all subjects. K-means clustering was performed with ten replicates to avoid local minima.28 The initial value of k (the number of clusters) was set as the maximum number of synergies extracted from a single stride across all individuals. Clustering was repeated with a sequential increase in k and proceeded until the sum of the square of the distances between all points in the cluster and the cluster center were minimized. The value of k was increased if synergy structures extracted from the same gait cycle were not assigned to different clusters. Next, a discriminant analysis was used to revise cluster assignment if necessary. In this supervised learning process, each synergy structure matrix and its cluster assignment were used to optimize the separation between clusters by projecting the data into a subspace that maximized the variance between means of projected classes (clusters) and minimized the variance within each class (cluster).35 The discriminant method was determined by the equality of cluster covariance matrices assessed using the Bartlett test.36 If covariance matrices were equal, linear discriminant analysis (LDA) was used, otherwise quadratic discriminant analysis (QDA) was used.35 Next, the intra-class correlation coefficient (ICC)37 was used to quantify the similarity of the synergy structures assigned to each cluster by discriminant analysis. Those iterative processes were repeated 1,000 times and we selected the case showing the most frequent k and the highest mean ICC value across the clusters. Finally, the number of clusters was determined for each person, and this number was normalized by their total synergy number across all five strides to compute the normalized cluster number for each individual (i.e., normalized cluster number = number of clusters/ total number of synergies).
The stride-to-stride variability of individual muscle EMG and joint angles were also examined using variance and ICC analysis. First, EMG linear envelopes and joint angles were time-interpolated to 100 points over a gait cycle. Next, the variance of each time point was computed for each EMG envelope (8 muscles) and joint angle (6 joints) across the five strides. Then, the mean variance across the 100 time points was computed.
Statistical analysis
The total number of synergies, mean number of synergies per stride, number of clusters, and normalized cluster number were compared between groups using an independent t-test. For muscle activation and joint angles, variance and ICC across the five strides were compared between groups using a two-way mixed analysis of variance (ANOVA) using SPSS version 19.0 (IBM, Armonk, NY). Complexity measures were compared between groups using independent t-tests. To identify whether these data were related to functional mobility, Spearman’s correlation coefficients were computed between these and GMFCS.38 Data were presented with mean ± standard deviation (SD). Statistical significance was set at α = .05.
Results
Mean age was not significantly different between groups (p = 0.72). The mean total number of synergies extracted across the five strides was significantly higher (p = 0.004) in children with TD (19.0 ± 1.0, range: 17–20) compared to CP (17.2 ± 1.5, range: 14–20). On a per stride basis, the mean number of synergies was significantly higher (Fig. 1, p = 0.004) in the TD group (3.80 ± 0.21) compared to CP (3.43 ± 0.30). The mean variance in the number of synergies per stride was not significantly different between the TD (0.15 ± 0.13) and CP (0.23 ± 0.11) groups (p = 0.13). The mean number of synergy clusters per individual was slightly higher in the CP group compared to TD but the difference was not significant (Fig. 1; TD: 6.5 ± 1.3, CP: 7.1 ± 1.3, p = 0.300). However, the mean normalized cluster number, i.e. the ratio of number of clusters to number of synergies for an individual, was significantly higher in CP than TD (Fig. 1; TD: 0.34 ± 0.07, CP: 0.41 ± 0.05, p = 0.012). Significantly lower mean number of synergies per stride and significantly greater normalized cluster number for those with CP compared to TD were also found for VAF threshold values of 80%, 85%, and 95%, consistent with the results at the 90% threshold level (Table 1). However, there were no significant differences in these measures between groups when VAF threshold was 75% (Table 1), with only one or two synergies extracted from all strides in 25 of 28 children at this threshold value.
Fig. 1.
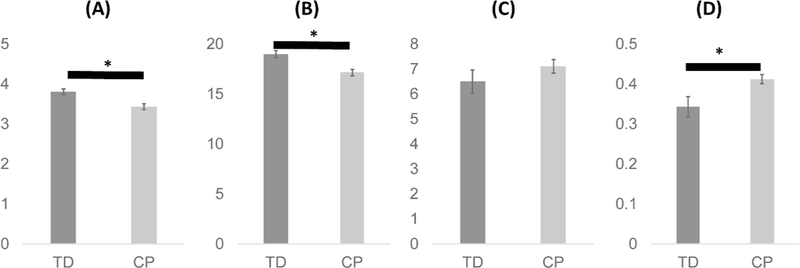
Mean number of synergies per stride (A), total number of synergies (B), number of clusters (C), and normalized cluster number of muscle synergies (D) in five strides. Data are presented as means and standard errors. An asterisk (*) indicates a significant difference (p<0.05) between children with typical development (TD) and cerebral palsy (CP).167×58mm (600 × 600 DPI)
Table 1.
Comparison of Mean number of synergies per stride, number of clusters, and normalized cluster number using different VAF thresholds.
| VAF | Mean number of synergies per stride | Number of synergy clusters | Normalized cluster number | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TD | CP | p | TD | CP | p | TD | CP | p | |
| 75% | 2.1 ± 0.1 | 1.8 ± 0.3 | 0.071 | 3.4 ± 0.7 | 3.5 ± 1.1 | 0.861 | 0.33 ± 0.08 | 0.38 ± 0.11 | 0.265 |
| 80% | 2.6 ± 0.2 | 2.2 ± 0.2 | <0.001 | 4.3 ± 0.5 | 4.2 ± 0.9 | 0.883 | 0.33 ± 0.04 | 0.38 ± 0.06 | 0.048 |
| 85% | 3.1 ± 0.2 | 2.7 ± 0.4 | 0.011 | 4.9 ± 0.6 | 5.0 ± 0.9 | 0.618 | 0.31 ± 0.05 | 0.37 ± 0.07 | 0.026 |
| 90% | 3.8 ± 0.2 | 3.4 ± 0.3 | 0.004 | 6.5 ± 1.4 | 7.1 ± 1.3 | 0.300 | 0.34 ± 0.08 | 0.41 ± 0.05 | 0.012 |
| 95% | 5.1 ± 0.1 | 4.7 ± 0.5 | 0.004 | 8.6 ± 0.5 | 9.0 ± 1.4 | 0.377 | 0.34 ± 0.02 | 0.39 ± 0.05 | 0.032 |
Statistically significant defferences between groups are presented in bold (p<0.05, independent t-test). Abbreviations: VAF, variance accounted for threshold; TD, typical development; CP, cerebral palsy
The higher normalized cluster number in children with CP shows that their synergies were distributed into more clusters relative to the number of muscle synergies they produced during walking (Fig. 2) and is indicative of higher stride-to-stride variability. In total, 10 distinct clusters, constituting 10 different muscle synergy structures, were found across the 28 participants (8 TD and 20 CP). Several synergy structures were observed mostly (Clusters 8 and 9) or only (Cluster 10) in those with CP (Fig. 2). Whereas no cluster contained only synergies from children with TD, clusters 1 and 6 contained synergies from all children with TD.
Fig. 2.
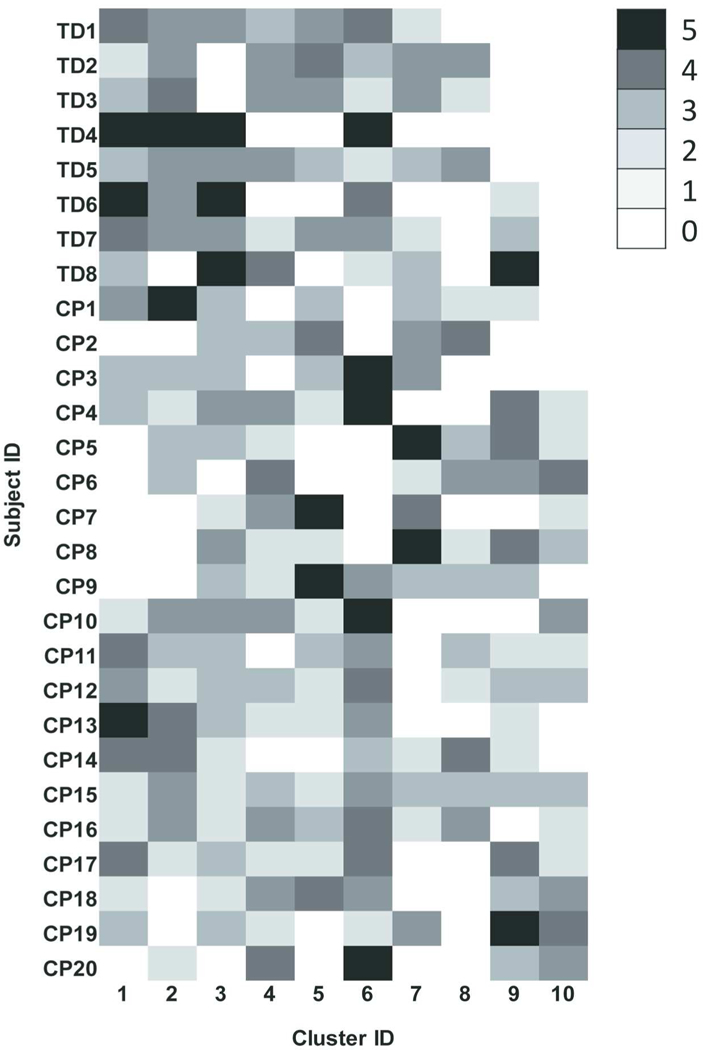
Distribution of similar synergies across individual participants. Muscle synergies in children with typical development (TD) and cerebral palsy (CP) were extracted from individual five strides, and similar synergies were assigned into the same clusters. Row and column are identification (ID) of subjects and clusters, respectively. Note that the same cluster ID between subjects indicates similar synergies. Cluster ID is ordered by most prevalent assignment of synergies in children with TD such that no synergies for children with TD were assigned to cluster 10. Brightness scale presents the number of synergies in a cluster.67×97mm (600 × 600 DPI)
The mean synergy activation and structure from each cluster are shown in Fig. 3. Cluster 10, which contains only synergies from children with CP, demonstrated a broad level of muscle activation, including co-activation of TA and RF in the non-dominant (more affected) side and TA, RF, and MH in the dominant (less affected) side. Cluster 6, which contained muscle synergies from all children with TD, and Cluster 2 were active around double stance and reflected similar structures across the limbs. Cluster 6, active around non-dominant heel strike, contained nondominant TA and MH and dominant MG, while Cluster 2, active around dominant heel strike, consisted of dominant TA and MH and non-dominant MG. Cluster 1, also containing synergies from all children with TD, was active during non-dominant swing phase and loading response, and mainly consisted of non-dominant TA and RF. The mean ICC values of muscle synergy structures across the 10 clusters were 0.64 ± 0.07, with a range of 0.53–0.70.
Fig. 3.
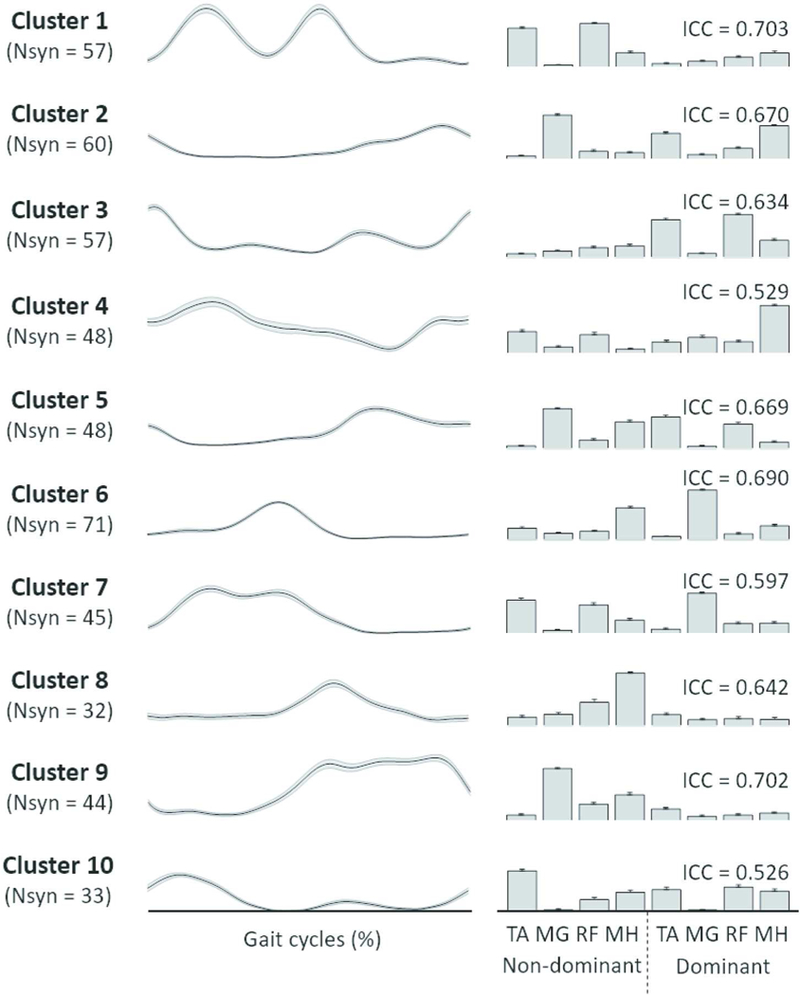
Muscle synergies assigned to individual clusters. The gait cycle is defined based on heel strike of dominant leg. Line (left) and bar (right) plots present synergy activations and structures, respectively. Each cluster number is corresponding to the cluster ID in Fig. 2. Abbreviations: Nsyn, the number of synergies assigned to each cluster; ICC, intraclass correlation coefficient (3,1) of synergy structures within each cluster; TA, tibialis anterior; MG, medial gastrocnemius; RF, rectus femoris; MH, medial hamstring.85×107mm (600 × 600 DPI)
Sample synergies from one participant with TD and one participant with CP are shown in Fig. 4. The TD participant was very consistent, with the same four synergies present in all 5 gait cycles. In contrast, participant CP 15 demonstrated variability in both the number of synergies extracted across gait cycles and the clusters to which those synergies were assigned, i.e. muscle synergies for each of 5 strides were assigned to clusters 1–4-6–9, 2–6-9–10, 2–6-8–10, 2–3-7–8, and 4–5-7, respectively.
Fig. 4.
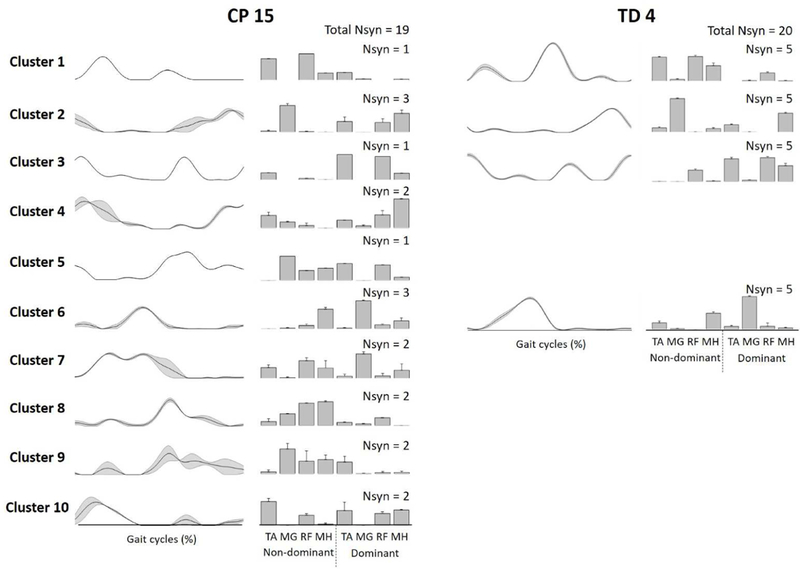
Samples of muscle synergies extracted from 5 individual strides in CP 15 and TD 4 (refer to Figure 3). Line and bar plots present synergy activations and structures, respectively. Synergies in TD 4 were assigned into four clusters indicating low stride-to-stride variability while those of CP 15 were assigned into ten clusters indicating high variability. Data are presented with means and standard errors. Abbreviations: Nsyn, number of synergies; TD, typical development; CP, cerebral palsy; TA, tibialis anterior; MG, medial gastrocnemius; RF, rectus femoris; MH, medial hamstring.150×106mm (600 × 600 DPI)
Inter-stride variability of EMG channels and joint angle data across the five strides (Fig. 5) showed no significant differences between CP and TD groups (EMG: p = 0.84; joint angle: p = 0.70). ICC values for EMG and joint angles were also not significantly different between groups (p = 0.48 and p = 0.69, respectively).
Fig. 5.
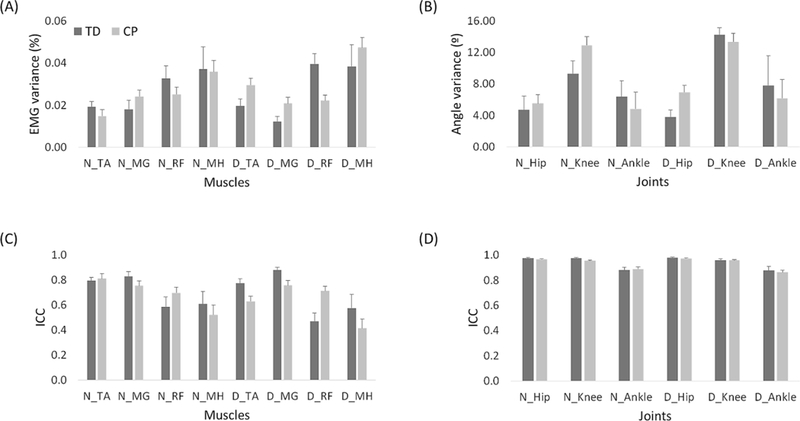
Variance (A, B) and ICC (C, D) values of EMG (A, C) and kinematic variables (B, D) across five strides in children with TD (dark gray) and CP (light gray). Data are presented with means and standard errors. Abbreviations: TD, typical development; CP, cerebral palsy; EMG, electromyography; ICC, intraclass correlation coefficient; N, non-dominant side; D, dominant side; TA, tibialis anterior; MG, medial gastrocnemius; RF, rectus femoris; MH, medial hamstring. 311×168mm (300 × 300 DPI)
Mean VAF by 1 synergy was significantly higher (p < 0.001) in children with CP (0.71 ± .04; range: 0.36–0.78) compared to TD (0.61 ± 0.03; range: 0.57–0.65). Similarly, walk-DMC values in children with CP (65.0 ± 14.2; range: 40.2–91.3) were significantly lower (p < 0.001) than in TD (100 ± 10; range: 85.10–113.0) suggesting less complexity. In children with CP, GMFCS level was correlated only with normalized cluster number (r = 0.54, p = 0.01), with no significant correlation with total number of synergies, number of clusters, or complexity measures.
Discussion
Our results show that children with CP exhibit more variability in muscle synergies deployed during walking compared to those with TD. Interestingly, we found that children with CP utilize the same synergies as those with TD in some strides while at other times exhibiting distinct synergies not present in those with TD. Our results confirm previous findings that the number of muscle synergies required to account for a set threshold of variance varies across strides in both normal and pathological gait.28 We extracted between 3 and 4 synergies from each gait cycle for those with TD and between 2 and 4 synergies for those with CP at a VAF threshold of 90%.
Across the five gait cycles, we extracted more synergies on average from children with TD than those with CP, a result in line with previous studies.18,39 However, unlike previous studies which have extracted synergies from a random single stride18 or concatenated strides13,23, or averaged synergies across strides,16 we extracted synergies required to meet the 90% VAF threshold from each stride. The variable number of synergies for each stride makes conventional correlation analysis for repeatability difficult. Previous studies have evaluated variability between synergy structures across strides and visits by varying the number of synergies extracted from each stride (e.g., n = 1–5) and then computing the correlation between the mean structure and each stride for a given value of n.20 Some studies have divided walking into small groups of strides and then extracted the same number of synergies from each subgroup, all with VAF greater than 90%.40 One drawback of these approaches is that they do not identify the correct synergy level (value of n) for each individual or stride, which we contend is an important step given that spurious synergies can be included if the number of synergies from each stride is not properly determined.28 To overcome these limitations, we extracted synergies on a stride-tostride basis and then used clustering and discriminant analyses to group similar synergy structures across the entire set of synergies extracted from all strides and participants into the same clusters.
The number of clusters to which a participant had a synergy assigned provides a measure of stride-to-stride variability of the muscle synergies underpinning their gait. However, the number of clusters is also impacted by the total number of synergies extracted from an individual. Thus, we used the normalized cluster number to represent the stride-to-stride variability of muscle synergies for each participant, with a larger normalized cluster number indicating increased variability. We found that while children with CP generated fewer total number of synergies across the five strides, they did so with higher stride-to-stride variability. This higher variability may be indicative of a more immature gait pattern because mature gait is characterized by lower variability between strides.41 Our results are similar to a study demonstrating that synergy structures for walking on a narrow beam were more consistent in experts compared with novices.15 Given that walking may be more challenging in children with CP than in those with TD,42 this may explain their higher stride-to-stride variability of muscle synergies. This notion is consistent with earlier studies that reported an increase in variability of motor coordination patterns across multiple trials associated with more difficult tasks,43 insufficient practice,44 or brain injury.45
Despite the increased variability, children with CP generated the same synergies as those with TD in many strides, while in other strides they did not (Fig. 2). As a group, the children with CP exhibited 9 synergy patterns in common with children with TD, while only 1 synergy was not seen in any child with TD. This information could be useful in developing individualized rehabilitation strategies for gait training. For an individual who utilizes typical synergies in some strides, e.g. CP 15 (Fig. 4), the focus could be on making the typical synergies more reliable and repeatable during training. There are several ways in which this could be implemented. Muscle synergies could be computed for each gait cycle in real time, and feedback on recruited synergies provided to reinforce successful motor execution. Another method would be to utilize modelling tools to predict the limb motion created by the participant’s execution of the more typical synergy, and then to train the individual by having them repetitively perform and reinforce that movement. With practice, they may recalibrate their walking pattern to deploy the trained synergy more frequently. The absence of more typical synergies may also be useful information for rehabilitation. For instance, many participants with CP did not exhibit synergies in Cluster 1, which is present in all children with TD. This synergy is responsible for non-dominant hip flexion and ankle dorsiflexion in swing and leg stabilization during loading. The most expedient course of therapy for these children may be to optimize their limb motion during these phases given their learned synergy rather than attempting to train the pattern of children with TD. Our results may also provide insight into why some children may respond better to training. If the available synergy repertoire of a child with CP is similar to that of TD prior to training, he/she may have more capacity to improve by increasing the frequency of their occurrence rather than having to develop a specific synergy de novo, which may not even be possible in some given the brain injury.
Stride-to-stride variability of individual muscle EMG was not significantly different between groups, indicating that it is the pattern of how muscles are recruited or activated together that is more variable in children with CP. Our results do not address the question of whether synergies reflect the underlying neuromotor control modules or are instead reflective of biomechanical and/or task constraints.46 Thus, the more variable structures extracted across strides in our CP cohort may reflect variable patterns of activation due to spasticity, muscle tightness or weakness, all of which are common impairments in CP. Several studies have shown similar stride-to-stride
variability in gait parameters,47 joint angular displacements,48,49 and ground reaction forces50 in CP versus peers or between more and less affected legs. Increased muscle co-activation, and abnormal timing of activation have also been demonstrated in CP,1,51,52 the consequence of altered supraspinal control or maladaptive plasticity throughout the motor system, including spinal interneurons, in response to brain injury during development.53 Nevertheless, children with CP demonstrated considerable similarity in synergy structures with children with TD, with only one truly aberrant pattern (cluster 10) common in our cohort with CP but not seen in TD.
A majority of previous studies have extracted synergies from aggregated EMG data (e.g., averaged or concatenated strides), a process which filters the extracted synergies to remove stride-specific EMG features but may discard potentially meaningful stride-to-stride variability.28 Variability may arise from physiological or nonphysiological sources, and the challenge is to retain only the former. We utilized a clustering and discriminant analysis across all individuals to identify reliable synergy structures based on ICC, suggesting these were not due to nonphysiological noise. In line with studies that aggregate EMG across strides for synergy extraction,18,19,39 our results show the mean number of synergies within strides is less in CP than TD, and VAF for a single synergy is larger on average. Given previous results of similar synergy structures during walking in those with CP and TD,23,25 it is not surprising that we found children with CP may exhibit the same structures as those with TD. It was surprising that deployment of these structures was less consistent stride-to-stride and that, relative to the number of synergies per stride, this group of children with CP exhibited more structures than those with TD. Taken together these results provide a more nuanced view of motor control in those with CP. That is, while motor control of walking in those with CP may be diminished compared to TD on average, it is not necessarily less complex in terms of available synergy structures.
While our cluster analysis was performed using synergy structures, the resultant activation profiles did not overlap within a stride, indicating timing may be preserved across strides despite modularity in muscle grouping. For example, in CP 15 (Fig. 4) synergies from cluster 8 and 9 have similar activation profiles but distinct muscles patterns, particularly inclusion (or lack) of dominant TA. Both synergies were expressed in 2/5 strides examined but they were mutually exclusive, i.e., they were never deployed in the same stride. While it is possible that this could be interpreted as a noisy controller attempting to implement the same synergy in all four strides, by definition the additional TA activity constitutes a different synergy structure. Their repeated and exclusive emergence across multiple strides suggests that these variants reflect modular complexity in this individual; additional studies examining activation profiles across more strides should be undertaken to confirm this finding.
It is also possible that greater stride-to-stride synergy variability in CP, and in other neurological disorders, may explain the lower number of synergies identified when applying the same VAF threshold and the greater amount of variance explained by one synergy when these measures are computed across single, averaged or concatenated strides. While performing muscle synergy analysis at these broad scales may have value at the group level our results demonstrate the potential clinical utility of stride-to-stride analysis of muscle synergies, which highlights individual motor control capabilities, in children with CP. Regardless of the scale of synergy analysis, it should be noted that methodological and experimental choices, in particular the VAF threshold value28 and the number of muscles54 can influence the number of synergies extracted during walking. To evaluate the sensitivity of our methods to choice of VAF threshold, we varied the value from 75–95% in 5% increments. We found that VAF threshold had little effect on mean number of synergies per stride and normalized cluster number for the range of 80–95% (Table 1), supporting the sensitivity of our stride-to-stride variability outcomes. At VAF threshold of 75%, significant differences in stride-to-stride variability were not found between groups, which is not surprising given that only 1–2 synergies were extracted at this level for both TD and CP groups.
A limitation of our study is the relatively small number (eight) of bilateral EMG channels. Yet our finding of between 2–4 synergies required to meet the 90% VAF criteria agrees with recent studies of gait in children with TD, and hemiplegic and diplegic CP utilizing 22 channels of EMG which found the same range was sufficient to account for 80% of EMG variance of the larger muscle set.23 The same study utilized cluster analysis of synergies extracted from concatenated strides to identify four basic structures across all strides, with the remaining structures combined into an additional cluster. Although their modules were extracted based on limb specific gait cycles, whereas we extracted synergies across limbs within the same gait cycle (dominant heelstrike to dominant heelstrike), there were similarities in the structures and activations of their four identified synergies and those we identified from a more limited muscle set. For example, both studies report synergies that featured hamstrings that were active during late swing and early stance in dominant and nondominant limbs, respectively (synergies 2 and 6 in Fig. 3). Both studies also report synergies involving plantarflexors for forward propulsion (synergies 7 and 9 in Fig. 3). These similarities combined with the high repeatability of the synergies extracted across our cohort as measured by ICC suggest that the additional synergies observed in the CP group have a physiological basis. The fact that increased synergy structure variability is present despite no difference in kinematic variability suggests that motor control complexity, as measured by synergy repertoire, may be increased or at least similar in some individuals with CP. However, given the possibility that a reduced muscle set may overestimate VAF,54 future studies are necessary with larger muscle sets to confirm our findings of enhanced stride-to-stride variability of synergy structures utilized during walking in children with CP. Also, as previous studies have shown that the choice of EMG processing parameters, e.g. normalization procedures, filter type, and cutoff frequencies, may impact muscle synergy extraction,55 future work should examine the effects of these on stride-to-stride variability of muscle synergies.
Other limitations in this study include the possibility that fatigue, common in CP,56 may have influenced stride-to-stride variability results despite our decision to extract synergies from five random gait cycles to reduce this effect. A greater number of gait cycles may improve the robustness of stride-to-stride variability analysis. Although we found that variability measured by normalized cluster number was correlated with GMFCS level, the complexity measures were not, a result which differs from previous work.18,27 However, correlation analyses can be sensitive to range and sample size, thus our relatively small cohort and lack of children with GMFCS III and above may explain this discrepancy. Nevertheless, translating our results to less functional individuals should be done with caution. Our CP cohort contained 20 individuals, similar to previous observational synergy studies in CP19,23,25,39 although notably less than some retrospective studies.18 Given the heterogeneity of the CP population, the impact of cohort size and functional level should be carefully considered. It is possible that a larger group with a broader representation of age, CP sub-type, and functional mobility would identify more individuals who expressed fewer synergies because lower synergy number has been shown to be associated with higher (less functional) GMFCS levels.18
Acknowledgments
This work was support by the intramural research program of the NIH Clinical Center. Y.K. was also supported by a grant of the Korea Health Technology R&D Project through the Korea
Health Industry Development Institute (HI14C1155), funded by the Ministry of Health & Welfare, Republic of Korea.
References
- 1.Crenna P Spasticity and ‘spastic’ gait in children with cerebral palsy. Neurosci Biobehav Rev. 1998;22:571–578. [DOI] [PubMed] [Google Scholar]
- 2.Ostensjo S, Carlberg EB, Vollestad NK. Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities. Dev Med Child Neurol. 2004;46:580–589. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer B, Ashby P. Altered corticospinal projections to lower limb motoneurons in subjects with cerebral palsy. Brain. 1991;114 ( Pt 3):1395–1407. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DC, Damiano DL, Abel MF. The evolution of gait in childhood and adolescent cerebral palsy. J Pediatr Orthop. 1997;17:392–396. [PubMed] [Google Scholar]
- 5.Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2003;45:786–790. [DOI] [PubMed] [Google Scholar]
- 6.Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol. 2012;590:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting LH, Chiel HJ, Trumbower RD, et al. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron. 2015;86:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung VCK, d’Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci. 2005;25:6419–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominici N, Ivanenko YP, Cappellini G, et al. Locomotor Primitives in Newborn Babies and Their Development. Science. 2011;334:997–999. [DOI] [PubMed] [Google Scholar]
- 11.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol. 2006;95:3426–3437. [DOI] [PubMed] [Google Scholar]
- 12.Allen JL, Neptune RR. Three-dimensional modular control of human walking. J Biomech. 2012;45:2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci. 2012;32:12237–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozumalski A, Steele KM, Schwartz MH. Muscle synergies are similar when typically developing children walk on a treadmill at different speeds and slopes. J Biomech. 2017;64:112–119. [DOI] [PubMed] [Google Scholar]
- 15.Sawers A, Allen JL, Ting LH. Long-term training modifies the modular structure and organization of walking balance control. J Neurophysiol. 2015;114:3359–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden MG, Clark DJ, Kautz SA. Evaluation of Abnormal Synergy Patterns Poststroke: Relationship of the Fugl-Meyer Assessment to Hemiparetic Locomotion. Neurorehabil Neural Repair. 2010;24:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele KM, Rozumalski A, Schwartz MH. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev Med Child Neurol. 2015;57:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashiguchi Y, Ohata K, Osako S, et al. Number of Synergies Is Dependent on Spasticity and Gait Kinetics in Children With Cerebral Palsy. Pediatr Phys Ther. 2018;30:34–38. [DOI] [PubMed] [Google Scholar]
- 20.Shuman B, Goudriaan M, Bar-On L, Schwartz MH, Desloovere K, Steele KM. Repeatability of muscle synergies within and between days for typically developing children and children with cerebral palsy. Gait Posture. 2016;45:127–132. [DOI] [PubMed] [Google Scholar]
- 21.Allen JL, Kautz SA, Neptune RR. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech. 2013;28:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung VCK, Turolla A, Agostini M, et al. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A. 2012;109:14652–14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappellini G, Ivanenko YP, Martino G, et al. Immature Spinal Locomotor Output in Children with Cerebral Palsy. Front Physiol. 2016;7:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gizzi L, Nielsen JF, Felici F, Ivanenko YP, Farina D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J Neurophysiol. 2011;106:202–210. [DOI] [PubMed] [Google Scholar]
- 25.Cappellini G, Sylos-Labini F, MacLellan MJ, et al. Backward walking highlights gait asymmetries in children with cerebral palsy. J Neurophysiol. 2018;119:1153–1165. [DOI] [PubMed] [Google Scholar]
- 26.Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture. 2013;38:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz MH, Rozumalski A, Steele KM. Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev Med Child Neurol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Bulea TC, Damiano DL. Novel Methods to Enhance Precision and Reliability in Muscle Synergy Identification during Walking. Front Hum Neurosci. 2016;10:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolze H, Kuhtz-Buschbeck JP, Mondwurf C, Johnk K, Friege L. Retest reliability of spatiotemporal gait parameters in children and adults. Gait Posture. 1998;7:125–130. [DOI] [PubMed] [Google Scholar]
- 30.Prosser LA, Lee SCK, Barbe MF, VanSant AF, Lauer RT. Trunk and hip muscle activity in early walkers with and without cerebral palsy - A frequency analysis. J Electromyogr Kinesiol. 2010;20:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gormley Jr ME,Treatment of neuromuscular and musculoskeletal problems in cerebral palsy. Pediatr Rehabil. 2001;4:5–16. [DOI] [PubMed] [Google Scholar]
- 32.Butler AJ, Kahn S, Wolf SL, Weiss P. Finger extensor variability in TMS parameters among chronic stroke patients. J Neuroeng Rehabil. 2005;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401:788–791. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira AS, Gizzi L, Farina D, Kersting UG. Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Front Hum Neurosci. 2014;8:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon SJ, Brereton RG. Comparison of performance of five common classifiers represented as boundary methods: Euclidean Distance to Centroids, Linear Discriminant Analysis, Quadratic Discriminant Analysis, Learning Vector Quantization and Support Vector Machines, as dependent on data structure. Chemometr Intell Lab Syst. 2009;95:1–17. [Google Scholar]
- 36.Schott JR. Some tests for the equality of covariance matrices. J Stat Plan Inference. 2001;94:25–36. [Google Scholar]
- 37.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- 38.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. [DOI] [PubMed] [Google Scholar]
- 39.Tang L, Li F, Cao S, Zhang X, Wu D, Chen X. Muscle synergy analysis in children with cerebral palsy. J Neural Eng. 2015;12:046017. [DOI] [PubMed] [Google Scholar]
- 40.Rimini D, Agostini V, Knaflitz M. Intra-subject consistency during locomotion: similarity in shared and subject-specific muscle synergies. Front Hum Neurosci. 2017;11:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausdorff JM, Zemany L, Peng C, Goldberger AL. Maturation of gait dynamics: strideto-stride variability and its temporal organization in children. J Appl Physiol (1985). 1999;86:1040–1047. [DOI] [PubMed] [Google Scholar]
- 42.Bell KJ, Ounpuu S, DeLuca PA, Romness MJ. Natural progression of gait in children with cerebral palsy. J Pediatr Orthop. 2002;22:677–682. [PubMed] [Google Scholar]
- 43.Scholz JP, Reisman D, Schoner G. Effects of varying task constraints on solutions to joint coordination in a sit-to-stand task. Exp Brain Res. 2001;141:485–500. [DOI] [PubMed] [Google Scholar]
- 44.Latash ML, Scholz JP, Schoner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev. 2002;30:26–31. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y, Kim WS, Koh K, Yoon B, Damiano DL, Shim JK. Deficits in motor abilities for multi-finger force control in hemiparetic stroke survivors. Exp Brain Res. 2016;234:2391–2402. [DOI] [PubMed] [Google Scholar]
- 46.Kutch JJ, Valero-Cuevas FJ. Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLoS Comput Biol. 2012;8:e1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorsdahl AB, Moe-Nilssen R, Strand LI. Test-retest reliability of spatial and temporal gait parameters in children with cerebral palsy as measured by an electronic walkway. Gait Posture. 2008;27:43–50. [DOI] [PubMed] [Google Scholar]
- 48.Mackey AH, Walt SE, Lobb GA, Stott NS. Reliability of upper and lower limb threedimensional kinematics in children with hemiplegia. Gait Posture. 2005;22:1–9. [DOI] [PubMed] [Google Scholar]
- 49.Miller F, Castagno P, Richards J, Lennon N, Quigley E, Njiler T. Reliability of kinematics during clinical gait analysis: a comparison between normal and children with cerebral palsy. Gait Posture. 1996;4:169–170. [Google Scholar]
- 50.White R, Agouris I, Selbie RD, Kirkpatrick M. The variability of force platform data in normal and cerebral palsy gait. Clin Biomech. 1999;14:185–192. [DOI] [PubMed] [Google Scholar]
- 51.Berger W, Quintern J, Dietz V. Pathophysiology of gait in children with cerebral palsy. Electroencephalogr Clin Neurophysiol. 1982;53:538–548. [DOI] [PubMed] [Google Scholar]
- 52.Leonard CT, Hirschfeld H, Forssberg H. The Development of Independent Walking in Children with Cerebral-Palsy. Dev Med Child Neurol. 1991;33:567–577. [DOI] [PubMed] [Google Scholar]
- 53.Berger W Characteristics of locomotor control in children with cerebral palsy. Neurosci Biobehav Rev. 1998;22:579–582. [DOI] [PubMed] [Google Scholar]
- 54.Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci. 2013;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shuman BR, Schwartz MH, Steele KM. Electromyography Data Processing Impacts Muscle Synergies during Gait for Unimpaired Children and Children with Cerebral Palsy. Front Comput Neurosci. 2017;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Opheim A, Jahnsen R, Olsson E, Stanghelle JK. Walking function, pain, and fatigue in adults with cerebral palsy: a 7-year follow-up study. Dev Med Child Neurol. 2009;51:381–388. [DOI] [PubMed] [Google Scholar]


