Abstract
Background
Population-based studies suggest increasing rates of concurrent use of vaping products that contain either nicotine or cannabinoids. The aim of this pilot study was to test in vitro the acute inhalation toxicity of vaporized flavored and unflavored nicotine solutions co-administered with cannabidiol (CBD).
Methods
Bronchial epithelial cells (H292) were exposed directly to aerosol generated from electronic cigarettes refilled with propylene glycol only, unflavored nicotine solutions in propylene glycol with and without CBD, as well as to solutions containing only CBD. Cells were also exposed to a commercially available flavored solution containing nicotine and CBD. The in vitro toxicological effects were assessed after exposure using the following methods: 1) a trypan blue exclusion assay (cell viability), 2) neutral red uptake assay (metabolic activity) and 3) ELISA (concentrations of inflammatory mediators).
Results
Unflavored solution containing only CBD was significantly more cytotoxic than unflavored solution containing only nicotine. Unflavored solution containing both CBD and nicotine was significantly more cytotoxic than unflavored solutions with only nicotine. Levels of released cytokines were significantly higher when cells were co-exposed to nicotine and CBD as compared to cells exposed to only nicotine or only CBD. Overall, flavored products showed increased toxicity as compared to unflavored solutions.
Conclusions
This pilot in vitro study suggests independent and additive toxic effects of vaporized nicotine and CBD. Observed toxic effects are accentuated by flavorings. Future studies are needed to determine the potential long-term health consequences of concurrent use of vaporized nicotine and cannabis products.
Keywords: Electronic cigarettes, E-cigarettes, Electronic nicotine delivery systems (ENDS), Inhalation, Toxicity, Cannabinoids
Background
Electronic cigarettes (e-cigarettes), or electronic nicotine delivery systems (ENDS), were developed as potentially less-harmful nicotine delivery products than combustible tobacco cigarettes. While ENDS have become highly effective in delivering nicotine, population based studies have shown that these products have also been used to vaporize other psychoactive substances, including cannabinoids [1, 2]. Population-based studies have shown that a significant proportion of tobacco smokers also use cannabis [3, 4]. Although cannabis-derived products are becoming de-criminalized throughout individual states in the United States [5], products containing a mixture of cannabinoids are still classified as Schedule 1 substances under the United States Drug Enforcement Agency Controlled Substances Act. However, products that only contain cannabidiol (CBD) are promoted and marketed without restrictions based on a claim that CBD-only products are derived from hemp, and not from cannabis. As marijuana smoking remains the most popular way for delivering cannabinoids to the body, very little research has been performed to examine delivery and health effects of vaporized cannabinoids, including CBD.
Cannabidiol was discovered in the early 1930’s and has been found to have anti-convulsive [6–8], anti-psychotic [9, 10], anti-inflammatory [11, 12] and sedative effects [13, 14] in vitro and in vivo. While these studies showed positive effects of CBD when administered orally, topically or via intraperitoneal injection, few studies to date have examined health effects of CBD when inhaled using ENDS devices.
Potential respiratory effects associated with co-use of nicotine and CBD have not been studied. In this pilot study, we used a physiologically relevant in vitro model to examine respiratory effects of inhaling aerosols containing nicotine with and without CBD, as well as to determine if there are any additive effects associated with combined used of nicotine, CBD with and without flavorings.
Methods
Commercially purchased ENDS device and refill solutions
A puff activated eGO tank (SmokeTek), was purchased online for this study. This product had a fixed battery output voltage of 3.8 V and the coil in the CE4 tanks had an average resistance of 4.0 Ω resulting in 3.6 W of power. CBD-containing liquid of a single flavor labeled “Easy Rider” and a labeled CBD concentration of 50 mg/30 ml (1.7 mg/ml) was purchased online. While the flavor classification of this liquid was unknown, we speculate it has a fruity flavor based on the smell and GC/MS profile of detected flavoring chemicals. We also purchased one unflavored CBD liquid labeled “pure” CBD 1000 mg/30 ml (33.3 mg/ml).
Lab-made and lab-modified refill solutions
Refill solutions containing propylene glycol only (PG, solvent control, 99 + % Acros Organics), PG with nicotine only (1.7 mg/ml, NIC), PG with CBD only (1.7 mg/ml; CBD), PG with nicotine and CBD (1.7 mg/ml each; NIC + CBD) as well as flavored liquid (Easy Rider) with PG, nicotine and CBD (1.7 mg/ml each; NIC + CBD + Flavor) were tested (Fig. 1). PG with CBD only and PG with NIC + CBD was made using commercial liquid containing a listed 33.3 mg/ml CBD concentration. This product was diluted with PG to create a solution with CBD concentration of 1.7 mg/ml. Nicotine (99 + %, Acros Organics) was added to the commercially purchased flavored CBD liquid to create NIC + CBD + Flavor solution with the equal CBD and nicotine concentrations of 1.7 mg/ml. Nicotine was also added to PG to create a 1.7 mg/ml solution (NIC).
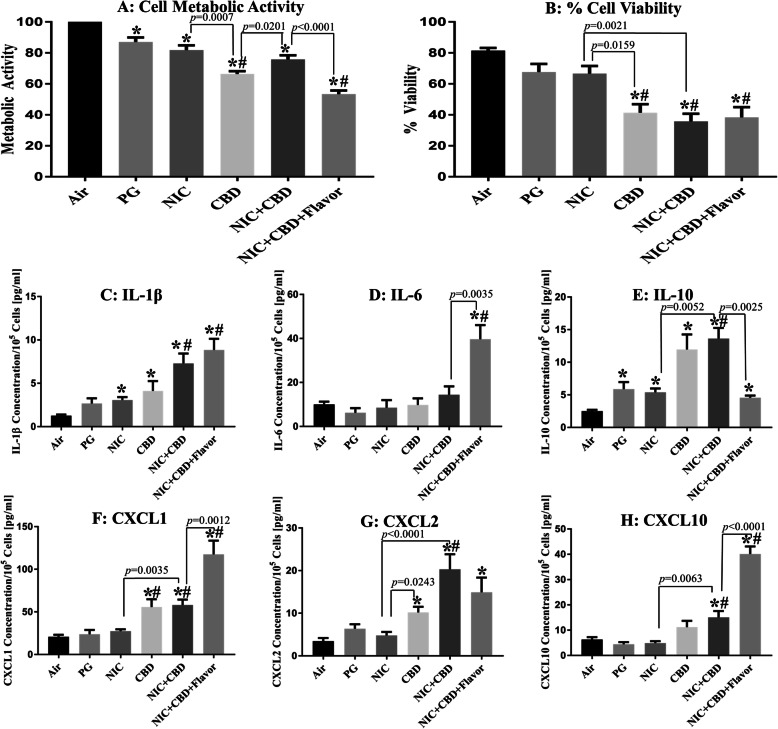
Fig. 1.
Comparison of cellular toxicity and levels of released inflammatory mediators (cytokines) from H292 bronchial epithelial cells directly exposed at the air-liquid interface to 55 puffs of nicotine and CBD aerosols. All aerosol was generated from an eGO tank system, with battery output voltage set to 3.8 V and refilled with PG-only solution with the same nicotine and CBD concentrations (1.7 mg/mL). * indicates significant difference from the air control and # indicates significant difference from the PG solvent control
GC/MS analysis of refill solutions
Flavoring chemicals were identified in each liquid using a gas chromatography/mass spectrometry (GC/MS) method, as described previously [15]. CBD concentrations were compared with the same peak area of analyzed samples. All commercially purchased CBD liquids were listed as industrial hemp derived and contained no delta-9 tetrahydrocannabinol (THC) as confirmed by GC/MS analysis.
Generation of ENDS aerosol
Aerosol from the eGO ENDS was generated using a Borgwaldt LX-1 (Richmond, VA) single-port piston-operated smoking machine. The Health Canada Intense (HCI) puffing protocol was utilized with the following conditions: 2 s puff duration, every 30 s, with a 55-mL puff volume. The puffing protocol was used continuously for 55 puffs or 30 min following protocol described previously [15]. Air-exposures (air control) were run during each experiment.
Cell exposure conditions
The NCI-H292 cell line (ATCC) was used for all experimentation. Cells were exposed directly to freshly generated aerosol in an air liquid interface (ALI) as described previously [15]. During cell exposure to air or ENDS aerosol, fresh media was cycled over the basal side of the permeable support at a flow rate of 5 mL/min. After exposure, 1 ml of culture media was added to the apical side of the cells grown on permeable supports. Then we waited 2.5 h before we examined endpoints, see toxicity assays below. While this system, like any ALI system, does have its limitations we tried to overcome these by exposing cells to an air control and PG control for each experimental day to ensure equal exposure.
Metabolic activity
Metabolic activity of exposed H292 cells was measured by Neutral Red Uptake Assay [16, 17] as described previously [15]. Briefly, the top and bottom surface of cells was covered with a diluted neutral red dye. After 2.5 h each permeable support was washed with PBS, then a de-stain solution was added to top and bottom of the permeable support then rocked for 10 min. This de-stain solution was added to a 96-well plate and measured with a BioTek Epoch spectrophotometer at 540 nm in triplicate.
Cell viability
Cell viability was measured by Trypan Blue Assay as described previously [15]. Briefly, after exposure, the top and bottom surface of cells were covered with complete media. After 2.5 h the media in the top of the permeable support (contains detached/dead cells) was transferred to a 1.5-mL tube and centrifuged. A portion of the supernatant was transferred to a clean 1.5-mL tube and stored at − 80 °C for ELISA assay. To detach adherent/live cells from the permeable support, 0.25% trypsin (Corning) was added to the top and bottom of each well. After 10 min, complete media was added to the top of each permeable support and this media was mixed with the remaining supernatant and pellet. The media was then mixed with trypan blue dye (Corning), pipetted into a hemocytometer (Invitrogen) and measured in triplicate using a Countess cell counter (Invitrogen).
Elisa
Six cytokines (IL-1β, IL-6, IL-10, CXCL1, CXCL2, and CXCL10) were measured as markers of cell inflammatory response using commercially available ELISA kits (CXCL2 Abcam, all others R&D Systems). For all assays, the manufacturer’s protocols were followed. Cytokine concentrations were adjusted for the number of live cells observed in the corresponding trypan blue assay.
Statistical analysis
Statistical analysis was performed using Prism version 7.05 (GraphPad). Kruskal-Wallis non-parametric tests were performed for each study outcome to compare: 1) the mean rank of liquids vs. air controls 2) the mean rank of liquids vs. PG controls 3) the mean rank of NIC, CBD and NIC + CBD vs NIC, CBD and NIC + CBD. A Mann-Whitney t-test was performed to compare the statistical difference between NIC + CBD and NIC + CBD + Flavor. All experiments were performed in at least triplicate, with each outcome measured three times per experiment.
Results
GC/MS analysis of refill solutions
GCMS analysis showed that the primary cannabinoid in our products was CBD as listed on the packaging. Additionally, we found 2,3-butanediol, acetoin, acetone alcohol, benzaldehyde, and propylene glycol in the flavored commercial liquid, Supplemental Table1.
Effect of nicotine and CBD with and without flavor
PG only (PG, solvent control) exposure
Aerosols generated from various solutions (PG, NIC, CBD, NIC + CBD and NIC + CBD + Flavor) differed significantly in their toxicity on bronchial epithelial cells (Fig. 1). Metabolic activity decreased significantly compared to the air controls when cells were exposed to aerosols containing PG (p = 0.0101, Fig. 1a). When examining cytokine levels released after exposure to PG aerosols, we found a significant increase in IL-10 (p = 0.0081, Fig. 1e) compared to the air control.
PG + nicotine (NIC) exposure
Metabolic activity decreased significantly compared to the air controls when cells were exposed to aerosols containing NIC (p = 0.0009, Fig. 1a). When examining cytokine levels released after exposure to NIC aerosols, we found a significant increase in IL-1β (p = 0.0016, Fig. 1c) and IL-10 (p = 0.0005, Fig. 1e) compared to the air control.
PG + CBD (CBD) exposure
After exposure to aerosols containing CBD, metabolic activity and cell viability were significantly decreased compared to the air control (both assays p < 0.0001, Fig. 1a, b), as well as to the PG control (p < 0.0001 and p = 0.0088 respectively, Fig. 1a, b). Aerosol containing NIC were found to be significantly different from aerosol containing CBD in both assays (p = 0.0007 and p = 0.0159 respectively, Fig. 1a, b). Additionally, exposure to CBD aerosol resulted in a significant increase in IL-1β, IL-10, CXCL1 and CXCL2 release compared to the air control (p < 0.0109, Fig. 1c-g), as well as compared to the PG control for CXCL1 (p = 0.0022, Fig. 1f). Finally, exposure to aerosol containing NIC resulted in significantly decreased release of cytokine CXCL2 compared to CBD aerosol (p = 0.0243, Fig. 1g).
PG + nicotine+CBD (NIC + CBD) exposure
When examining the effects of exposure to aerosol containing both NIC + CBD, we found a significant decrease in metabolic activity and cell viability compared to the air control (both assays p < 0.0001, Fig. 1a, b). In addition, we found a significant decrease in cell viability compared to PG control (p = 0.0012, Fig. 1b). Additionally, aerosol with NIC + CBD negatively affected cell viability compared to NIC condition (p = 0.0021, Fig. 1b). Metabolic activity of cells exposed to aerosol with CBD was also found to be significantly decreased compared to NIC + CBD condition (p = 0.0201, Fig. 1a). When examining the effect of exposure to NIC + CBD on inflammation, we found that IL-1β, IL-10, CXCL1, CXCL2 and CXCL10 were significantly increased compared to the air control (p < 0.0014, Fig. 1c-h). Similar differences were observed for PG control (p < 0.0103, Fig. 1c-h). Finally, exposure to aerosol containing NIC + CBD resulted in significant increase of cytokine release compared to NIC for IL-10, CXCL1, CXCL2 and CXCL10 (p < 0.0063, Fig. 1e-h).
PG + nicotine+CBD + flavor (NIC + CBD + Flavor) exposure
Cell viability and metabolic activity of H292 cells decreased significantly after exposure to aerosols from all liquids that contained NIC + CBD + Flavor compared to air (both assays p < 0.0001, Fig. 1a, b) and PG controls (p < 0.0001 and p = 0.0119, respectively, Fig. 1a, b). NIC + CBD + Flavor aerosol was found to have a significantly more deleterious effect on metabolic activity than unflavored NIC + CBD aerosol (p < 0.0001, Fig. 1a). Exposure to NIC + CBD + Flavor aerosol resulted in a significant increase in IL-1β, IL-6, IL-10, CXCL1, CXCL2 and CXCL10 levels compared to the air control (p < 0.0263, Fig. 1c-h). IL-1β, IL-6, CXCL1 and CXCL10 release was also significantly increased after exposure to NIC + CBD + Flavor aerosols compared to the solvent-only control (p < 0.0015, Fig. 1c-h). Exposure to NIC + CBD + Flavor aerosol resulted in increased production of IL-6, CXCL1 and CXCL10 (p < 0.0035, Fig. 1d-h) as well as a decrease in production of IL-10 (p = 0.0025, Fig. 1e) compared to unflavored NIC + CBD aerosol.
Discussion
This pilot study used an in vitro model to examine potential respiratory effects of nicotine and CBD when co-administered together. Our data show that exposure to NIC containing liquids result in significant cytotoxic and inflammatory effects on the H292 bronchial epithelial cell line similarly to the effects observed after exposure to pure solvent (PG). These results are consistent with past in vitro studies that utilized a similar ALI exposure system [15, 18]. A novel finding is that exposure to CBD resulted in stronger cytotoxic and inflammatory effects compared to NIC.
Another novel finding is that co-exposure to nicotine and CBD (NIC + CBD) resulted in an additive cytotoxic effect on bronchial epithelial cells. This finding suggests that vapers who co-use nicotine and cannabinoid products may have increased risk of respiratory symptoms as compared to vapers who only use a single substance. Additionally, co-exposure of NIC + CBD aerosol resulted in an additive pro-inflammatory (IL-1β and chemokines, Fig. 1c, f-h) as well as additive anti-inflammatory (IL-10, Fig. 1e) response as compared to NIC or CBD aerosol. These results merit additional mechanistic studies to examine the effects of aerosolized CBD products on the inflammatory pathway. However, an important limitation of our study is that only one concentration of nicotine and CDB was utilized and we did not estimate dose-response effects. Further studies are needed to test these effects using varying nicotine and CBD concentrations to determine if these results are affected by drug concentration. Although we used a physiologically relevant ALI system, we did not measure any clinically relevant health outcome in ENDS users. Future in vivo studies are needed to determine if the effects of this study are applicable to human subjects.
Our study confirmed that addition of flavoring additives to liquid results in increased cytotoxic and inflammatory effects compared to unflavored products. These results reaffirm findings from previous studies that reported cytotoxic effects of various flavorings used in ENDS products [15, 19]. Additionally, the use of flavored e-cigarette liquids with NIC + CBD resulted in a significantly increased pro-inflammatory response as compared to the air and PG controls as well as compared to the NIC + CBD liquid without flavoring (IL-1β. IL-6 and Chemokines, Fig. 1c, d, f, h). This also resulted in a lowered anti-inflammatory response as compared to all other e-cigarette liquids in this study (IL-10, Fig. 1e). These results suggest that while CBD containing aerosol may produce an elevated pro-inflammatory response as compared to the NIC and PG solutions, that the largest factor that may result in e-cigarette use related inflammation is associated with co-exposure with flavoring agents. A limitation of this study is that only one flavor was utilized; thus, future studies are needed to test cytotoxic effects of products with different flavors. Another limitation of our study is that we did not examine whether decreased viability of cells had been a result of apoptosis or necrosis. Since we observed a significant increase in the pro-inflammatory cytokines/chemokines IL-1b, CXLC1, CXCL2 and CXCL10 and a significant increase in the anti-inflammatory cytokines (IL-10), we hypothesized that CBD aerosol was causing necrosis. However, it is also possible that CBD aerosol has caused apoptosis of these cells similar to the observations of Yu et al. 2016 [20]. Those alternative hypotheses should be tested in more comprehensive mechanistic studies in future.
Conclusion
Our pilot in vitro study suggests a cumulative respiratory effect of inhaled nicotine and CBD. As co-use of nicotine and cannabis is increasing, studies are urgently needed to evaluate potential health consequences in users of both substances. This in vitro study suggests independent and additive toxic effects of vaporized nicotine and CBD further amplified by flavorings. With increased popularity of vaporized products, potential long-term respiratory effects need to be evaluated in those who vape nicotine and cannabinoids.
Supplementary information
Additional file 1: Supplemental Table 1. Qualitative comparison of commercially purchased flavored e-cigarette liquids with and without CBD using gas chromatography. Liquid 1 (Flavor) contained propylene glycol and “Easy Rider” flavoring, while liquid 2 (CBD + Flavor) contained propylene glycol, 1.7 mg/ml CBD and “Easy Rider” flavoring. Qualitative detection of a compound is indicated with an X when identified in both National Institute of Standards and Technology (NIST) and Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds (FFNSC) mass spectrometry libraries.
Acknowledgements
The authors thank C Hall, A Huang, K Powers, A Russillo, P Tran and E Yang for their assistance running pilot ALI experiments. The authors also thank AK Leigh, KP Leigh and JD Fusani for the conception of the work.
Abbreviations
- CBD
Cannabidiol
- e-cigarette(s)
Electronic cigarettes
- ENDS
Electronic nicotine delivery systems
- PG
Propylene glycol only
- NIC
Nicotine
- NIC + CBD
Nicotine with cannabidiol
- NIC + CBD + Flavor
Nicotine, cannabidiol and flavoring
- GC/MS
Gas chromatography/mass spectrometry
- ALI
Air liquid interface
Authors’ contributions
MLG and NJL contributed to the conception of the work. MLG and NJL contributed to data analysis. MLG and NJL drafted the manuscript. NJL ran experiments. All authors approved the final version of the manuscript. MLG has full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA 016056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MLG reports grants from Pfizer and served as a scientific advisory board member to Johnson & Johnson, pharmaceutical companies that manufacture smoking cessation drugs. NJL declares no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40360-020-00418-1.
References
- 1.Blundell M, Dargan P, Wood D. A cloud on the horizon–a survey into the use of electronic vaping devices for recreational drug and new psychoactive substance (NPS) administration. QJM. 2017;111:9–14. doi: 10.1093/qjmed/hcx178. [DOI] [PubMed] [Google Scholar]
- 2.Lee DC, Crosier BS, Borodovsky JT, Sargent JD, Budney AJ. Online survey characterizing vaporizer use among cannabis users. Drug Alcohol Depend. 2016;159:227–233. doi: 10.1016/j.drugalcdep.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramo DE, Liu H, Prochaska JJ. Tobacco and marijuana use among adolescents and young adults: a systematic review of their co-use. Clin Psychol Rev. 2012;32:105–121. doi: 10.1016/j.cpr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Assessing the overlap between tobacco and marijuana: trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict Behav. 2015;49:26–32. doi: 10.1016/j.addbeh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Peace MR, Butler KE, Wolf CE, Poklis JL, Poklis A. Evaluation of two commercially available cannabidiol formulations for use in electronic cigarettes. Front Pharmacol. 2016;7:279. doi: 10.3389/fphar.2016.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlini E, Leite J, Tannhauser M, Berardi A. Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol. 1973;25:664–665. doi: 10.1111/j.2042-7158.1973.tb10660.x. [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo I, Orsingher OA, Berardi AC. Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia. 1973;28:95–102. doi: 10.1007/BF00413961. [DOI] [PubMed] [Google Scholar]
- 8.Karler R, Cely W, Turkanis SA. Anticonvulsant properties of Δ9-tetrahydrocannabinol and other cannabinoids. Life Sci. 1974;15:931–947. doi: 10.1016/0024-3205(74)90009-5. [DOI] [PubMed] [Google Scholar]
- 9.Zuardi AW, Rodrigues JA, Cunha J. Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology. 1991;104:260–264. doi: 10.1007/BF02244189. [DOI] [PubMed] [Google Scholar]
- 10.Zuardi AW, Morais S, Guimarães FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- 11.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava MD, Srivastava B, Brouhard B. Δ9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40:179–185. doi: 10.1016/S0162-3109(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 13.Pickens JT. Sedative activity of cannabis in relation to its Δ ‘-trans-tetrahydrocannabinol and cannabidiol content. Br J Pharmacol. 1981;72:649–656. doi: 10.1111/j.1476-5381.1981.tb09145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuardi AW, Cosme R, Graeff FG, Guimarães F. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–88. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 15.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS) Tob Control. 2016;25:ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ates G, Vanhaecke T, Rogiers V, Rodrigues RM. Assaying cellular viability using the neutral red uptake assay. Cell Viability Assays Springer. 2017:19–26. [DOI] [PubMed]
- 17.Putnam K, Bombick D, Doolittle D. Evaluation of eight in vitro assays for assessing the cytotoxicity of cigarette smoke condensate. Toxicol in Vitro. 2002;16:599–607. doi: 10.1016/S0887-2333(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 18.Scheffler S, Dieken H, Krischenowski O, Förster C, Branscheid D, Aufderheide M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health. 2015;12:3915–3925. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behar R, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol in Vitro. 2014;28:198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Alexander LEC. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Qualitative comparison of commercially purchased flavored e-cigarette liquids with and without CBD using gas chromatography. Liquid 1 (Flavor) contained propylene glycol and “Easy Rider” flavoring, while liquid 2 (CBD + Flavor) contained propylene glycol, 1.7 mg/ml CBD and “Easy Rider” flavoring. Qualitative detection of a compound is indicated with an X when identified in both National Institute of Standards and Technology (NIST) and Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds (FFNSC) mass spectrometry libraries.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.