Abstract
Continued improvements in cancer therapies have increased the number of long-term cancer survivors. Radiation therapy remains one of the primary treatment modalities with about 60% of newly diagnosed cancer patients receiving radiation during the course of their disease. While radiation therapy has dramatically improved patient survival in a number of cancer types, the late effects remain a significant factor affecting the quality of life particularly in pediatric patients. Radiation-induced brain injury can result in cognitive dysfunction, including hippocampal-related learning and memory dysfunction that can escalate to dementia. In this article, we review the current understanding of the mechanisms behind radiation-induced brain injury focusing on the role of neuroinflammation and reduced hippocampal neurogenesis. Approaches to prevent or ameliorate treatment-induced side effects are also discussed along with remaining challenges in the field.
Keywords: astrocytes, cognitive dysfunction, neurotoxicity, radiation-induced brain injury, senescence
Radiation therapy is widely used to effectively treat primary and metastatic brain tumors in adult and pediatric patients.1,2 During standard approaches such as fractionated partial- and whole-brain radiation treatment (PBRT and WBRT, respectively), healthy brain tissue is inevitably exposed to radiation. As a result many patients experience side effects associated with damage to healthy brain tissue including hippocampal-related learning and memory dysfunction,3 focal neurological deficits, increased intracranial pressure,4 and rarely secondary epilepsy5 and progressive dementia.6 Cognitive domains affected include learning, processing speed, memory, executive function, and attention.7 Despite the advent of modern radiotherapy techniques, radiation-induced brain injury remains an important complication where cognitive impairment can range from mild to severe and more rarely progressive and debilitating.8–10
The frequency of cognitive impairments following brain radiotherapy varies widely by study and is influenced by a number of factors including variability in time to assessment, definition of neurocognitive impairment, tumor type, patient age, baseline neurocognitive function, disease progression, radiotherapy modality (WBRT, PBRT, stereotactic), radiation dose, and the use of multimodal treatments including concurrent chemotherapy. Thus, determining the precise frequency of cognitive decline in the clinical setting remains challenging and it may be underestimated due to a number of factors including (1) a long-term follow-up is required to detect late posttreatment changes, (2) attrition bias favoring those with higher cognitive functioning and not counting those with lower cognitive functioning,7 and (3) a paucity of clinical studies examining histological confirmed cases of radiation-induced injury.11 Nevertheless, studies have sought to identify risk factors associated with more severe cognitive dysfunction after radiation and include advanced age,12 smoking history,7 WBRT rather than PBRT,13,14 higher radiation dose,15 and concurrent chemotherapy.16,17
Traditionally, radiation-induced brain injury is classified into acute, early-delayed, and late-delayed based on the time between the start of radiotherapy and the onset of side effects.18–23 While acute and early-delayed injuries are generally transient and occur days to months following treatment, late-delayed injury occurs at least 6 months after radiation is considered irreversible and progressive. Acute injury is characterized by edema, headaches, drowsiness and is rare with modern radiation therapy techniques and is generally improved by dexamethasone. The early-delayed reaction is characterized by transient demyelination, somnolence, attention deficits, and short-term memory loss. Late-delayed injury involves white matter necrosis, vascular abnormalities, and more permanent demyelination, gliosis, and lasting cognitive impairment.
Decades ago, oncologists recognized these secondary neurological dysfunctions and risk of therapy-induced cancers in their patients. Therefore, the National Cancer Institute established the Late Effects of Cancer Therapy Program so that patients could be followed up for decades following successful therapy of their primary cancer.24 This initiative and historical patient cohort studies have led to improved therapies with fewer and less harmful delayed neurological and oncogenic effects.25 Additionally, these studies led to the evaluation of remedial interventions to ameliorate these adverse effects.
The mechanism of radiation-induced injury that corresponds to the clinical findings are not completely understood; however, recognized neuropathological sequelae and several new hypotheses exist which are detailed in the sections that follow. Given the increasing population of long-term cancer survivors, it is critical to understand the causes of radiation-induced brain injury and to develop strategies to prevent them.
Mechanisms of Radiation-Induced Brain Injury
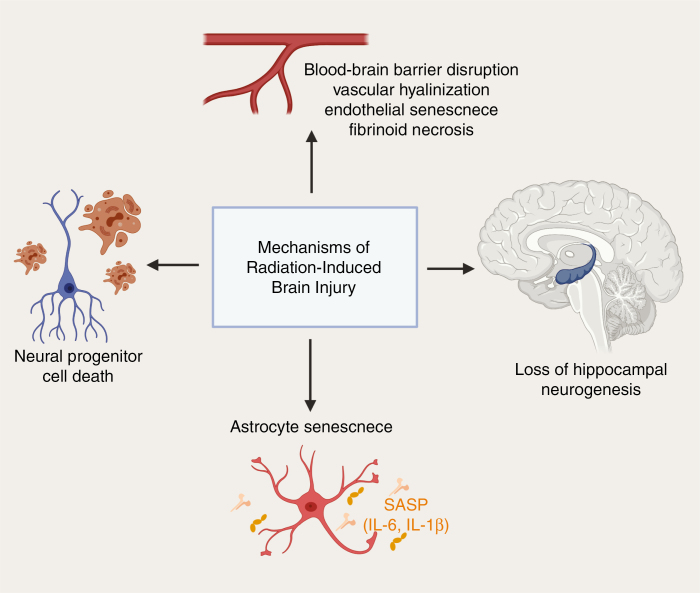
Over the past 20 years, research into the late effects of radiation revealed that it arises from dynamic interactions between multiple cell types and not simply delayed mitotic death of vascular and parenchymal cells of the target organ. It is now known that the cellular response to radiation injury in the brain involves multiple cell types including astrocytes, microglia, oligodendrocytes, endothelial cells, and neurons that initiate and respond to inflammatory cascades and contribute to progressive neurological damage.18,26 Animal models have greatly aided research into the potential mechanism of radiation-induced brain injury and have shed light on the roles of neuroinflammation. In terms of factors leading to a neuroinflammatory cascade, multiple processes are thought to occur concomitantly including damage to the neurovascular unit leading to blood–brain barrier (BBB) damage, neural progenitor cell (NPC) death, inhibition of neurogenesis in the hippocampus, and direct activation of glia resulting in the senescence-associated secretory phenotype (SASP) (Figure 1). Evidence for each of these mechanisms is detailed in the sections below.
Figure 1.
Mechanisms of radiation-induced brain injury. Vascular changes including blood-brain barrier disruption, vascular hyalinization, endothelial senescence, and fibrinoid necrosis. Other proposed mechanisms include loss of hippocampal neurogenesis, astrocyte senescence resulting in the release of senescence-associated secretory phenotype (SASP) cytokines, and neural progenitor cell death that result in cognitive decline following brain irradiation.
BBB Disruption
The BBB functions to restrict the passage of most soluble molecules found in the systemic circulation into the CNS. Therefore, disruption BBB results in a pathway for systemic immune and inflammatory cells to enter the brain and propel neuroinflammation. The BBB is composed of endothelial cells, pericytes, and astrocyte end-feet that form tight junctions and aid endothelial vesicular transport. In the acute setting, radiation results in the destabilization of the plasma membrane of vascular endothelial cells of the BBB27 and changes in endothelial morphology are observed including basal lamina thickening, cytoplasmic vacuolization, and cell swelling.28 Additionally, decreased endothelial cell density is apparent29 as cells undergo apoptosis within the first 24 h following radiation.30 Finally, studies have demonstrated a direct link between endothelial cell apoptosis and an increase in BBB permeability, which is significantly reduced in acid sphingomyelinase (ASMase) knockout mice, suggesting that endothelial cell apoptosis is mediated by the ASMase pathway.27
In terms of mechanisms of late endothelial damage, inadequate repair of damaged endothelial cells and BBB disruption contribute to tissue hypoxia and the upregulation of hypoxia-responsive genes. Gene expression changes that were persistent for weeks following radiation include induction of vascular endothelial growth factor (VEGF), which is thought to be triggered by hypoxia,31 leading to a further increase in BBB permeability. Upregulation of tumor necrosis factor alpha, IL-1β, and NF-kB contributes to an inflammatory microenvironment and in turn upregulates intercellular adhesion molecule 1 (ICAM-1). Increased ICAM-1 is associated with BBB disruption in multiple injury and disease models.32,33 While ICAM-1 is predominantly expressed by vascular endothelium following radiation, astrocytes also expressed the protein, and it is hypothesized that it mediates the release of proinflammatory cytokines in glia contributing to the toxic microenvironment of the irradiated CNS.34
Loss of Hippocampal Neurogenesis
The hippocampus is essential for learning and memory function. Adult neurogenesis occurs primarily in the dentate gyrus and subgranular zone (SGZ) of the hippocampus35 and the subventricular zone (SVZ) of the lateral ventricles.36 Radiation impairs neurogenesis in these areas and suppresses the differentiation of NPCs into mature neurons in animal models.37–39 In one study, mice exhibiting reduced neurogenesis following 10 Gray (Gy) of intracranial radiation also had reduced cognitive performance on the maze test.38 Further evidence supporting the role of NPC loss in cognitive dysfunction following radiation comes from studies showing that cognitive function can be partially rescued by neural stem cell transplantation to replace the lost hippocampal NPCs following the whole-brain irradiation in mice.40
Numerous studies seek to elucidate the mechanism by which radiation depletes NPCs in the hippocampus. One of the major hypotheses is that radiation induces inflammation and microvascular damage to the hippocampal SGZ and SVZ thereby altering the progenitor cell microenvironment in a manner that suppresses differentiation to the neuronal phenotype. Dysregulated signaling of hippocampal neurons, including downregulation of hippocampal glutamate receptor 1 and protein kinase C-gamma via Homer1a, is known to reduce long-term potentiation, working memory, and synaptic plasticity.41 Furthermore changes to hippocampal neuron signaling may cause NPCs in this region to preference glial rather than neuronal differentiation.42,43 Several clinical trials suggest that the findings from animal models are also applicable to humans. In an prospective observational study, Gondi et al.44 enrolled adults with benign or low-grade brain tumors treated with fractionated stereotactic radiotherapy and correlated hippocampal dose-volume histogram data with cognitive impairment. The study concluded that bilateral hippocampal doses greater than 7.3 Gy are associated with long-term cognitive impairment and thus serves as a rationale for hippocampal avoidance strategies. In a phase II trial (RTOG 0933), Gondi et al.3 enrolled patients with brain metastases treated with intensity-modulated radiotherapy (IMRT), which allowed avoidance of the hippocampus. Cognitive function was assessed using the Hopkins Verbal Learning Test before and at 2-month intervals following treatment up to 6 months. The patients receiving hippocampal avoidance radiotherapy were compared to historical controls of patients receiving whole-brain radiation without hippocampal avoidance. The historical control demonstrated a 30% mean relative decline in cognitive function from baseline in 4 months, while hippocampal avoidance resulted in a 7% mean relative decline in cognitive function. These results demonstrate that cognitive function is preserved with hippocampal avoidance.
Radiation-Induced Senescence and the SASP
Following radiation exposure cells adopt one of many cellular responses including DNA damage, which occurs either as a direct response to radiation or a secondary effect of free radicals and reactive oxygen species (ROS). If DNA double-strand breaks are not repaired cells undergo one of several fates: apoptosis, cellular senescence, mutation, or genomic instability. Multiple pathways are responsible for inducing cellular senescence in irradiated cells.45 Although senescent cells do not replicate, they may avoid clearance and persist in tissues while continuing to produce inflammatory factors that contribute to tissue injury.46 Therefore, radiation-induced cellular senescence is an important mediator of tissue dysfunction promoting chronic inflammation and contributing to radiation-induced side effects have been observed in multiple organs including the brain, lung, and heart.47,48
In the CNS, it has long been known that glia play many supportive roles for neurons, endothelial cells, and the neurovascular unit. Astrocytes protect against oxidative injury49 and maintain the function of the BBB.50 In response to various exogenous injuries astrocytes release a host of proinflammatory cytokines. In animal models, endothelial cell senescence has also been observed in response to brain radiation and is thought to be a consequence of proinflammatory cytokine release from activated astrocytes,51,52 which in turn release further proinflammatory molecules, upregulate adhesion molecules, and increase ROS production.48,53 Recently, the role of IL-6 as a key proinflammatory cytokine in the SASP54 was demonstrated in response to radiation in astrocytes.55 Induction of senescence in response to radiation has previously been demonstrated in a variety of other cell types including fibroblasts,56 endothelial cells,57 and chondrocytes.58 Clinically, pulmonary59 and myocardial fibrosis60 are considered a response to radiation therapy involving cellular senescence.
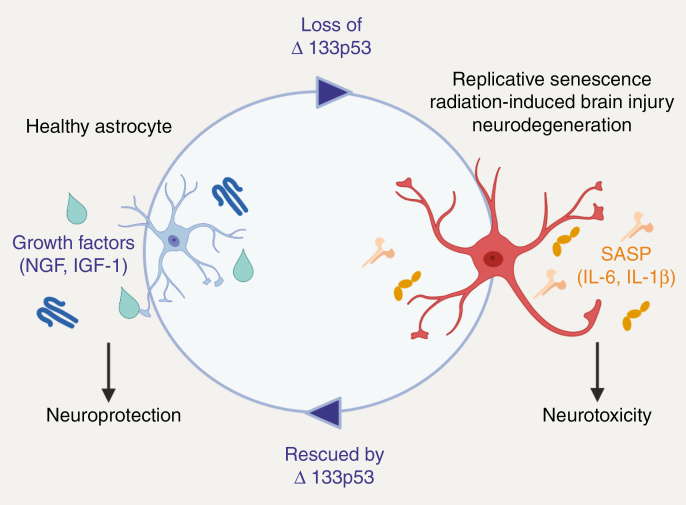
Recently our group has demonstrated that p53 isoforms, Δ133p53α and p53β, regulate cellular senescence in a variety of cell types including human fibroblasts,61 CD8+ T cells,62 and astrocytes.55,63 Decreased Δ133p53α and increased p53β expression exists in senescent cells undergoing replicative as well as radiation-induced senescence. Furthermore, overexpression of Δ133p53α extends the replicative lifespan of cells by dominant negative inhibition of senescence inducing p53 target genes including: p21, miR-34a, PAI-1, and IGFBP7 as demonstrated in human fibroblasts.61 Restored expression of Δ133p53α also rescues astrocytes from senescence in the setting of replicative and radiation-induced senescence.55,63 Furthermore, senescent astrocytes are characterized by their expression of the SASP proinflammatory cytokines including IL-6 and IL-1β. Restored expression of Δ133p53α in near-senescent astrocytes reduces expression of these cytokines and increases expression of neuroprotective factors, Nerve growth factor (NGF) and Insulin-like growth factor 1 (IGF-1). When senescent astrocytes are co-cultured with neurons, neuronal apoptosis is observed. However, upon overexpression of Δ133p53α in the astrocyte population following radiation or replicative senescence, there is a reduction in neuronal apoptosis indicating that overexpression of Δ133p53α is neuroprotective. Thus, p53 isoforms, Δ133p53α and p53β, are important regulators of cellular senescence that can be manipulated for potential therapeutic effect (Figure 2).
Figure 2.
Schematic of Δ133p53α regulation of astrocyte-mediated neuroprotection and neurotoxicity. The transition from healthy to senescent astrocytes is evidenced in the setting of replicative senescence, radiation-induced brain injury, and neurodegenerative disease accompanied by the loss of Δ133p53α. In the senescent state, astrocytes release SASP cytokines and result in neurotoxicity. Senescent astrocytes can be rescued to a healthy astrocyte phenotype by overexpression of Δ133p53α which results in neuroprotection.
Histopathological Changes in the CNS After Radiation
The histopathological changes in the brain after radiation therapy are variable from person to person and dependent on multiple factors including brain location treated, age, diagnosis, and dose/technique of the therapy. These have been described as acute, early delayed, and late delayed.64,65 Treatment-induced necrosis of the neoplasm is desirable, while necrosis of surrounding tissues is an ongoing and serious clinical challenge.66 Recognition of radiation-induced tissue necrosis is a diagnostic challenge for the radiologist, as there is a lack of optimal advanced MRI modality or imaging biomarkers. In addition, other non-radiation therapies such as glucocorticoids, antiangiogenics, or immune/targeted therapies can make radiographic interpretations difficult.
If a decision is made to re-biopsy or resect an area suspicious for recurrence of tumor in the radiation field the pathologist is then tasked with interpretation of the tissue for tumor and/or treatment-related changes. No established histopathological classification system has been established for this, and the experience and training of the pathologist are key to obtaining an accurate diagnosis. In some cases, only necrotic tissue is present for the pathologist to evaluate. When viable, non-neoplastic tissue is present the following changes can be (at least in part) attributed to radiation therapy: astrogliosis, vascular changes, tissue rarefaction, chronic inflammation, and glial/neuronal cytomorphologic atypia.67,68 The vascular changes seem to predominate and can range from thrombosis, hemorrhage, hyalinization, to fibrinoid necrosis which can further exacerbate the hypoxic/ischemic necrosis in the area. Distinguishing residual and recurrent glial tumor cells within these areas can also be very difficult. Immunohistochemical studies for mIDH1, GFAP, KI67, and p53 may help to highlight actively proliferating tumor cells in some cases, as proliferating cells are not as prominent from radiation change. Judging whether necrosis is related to disease progression or from radiation therapy can be crucial to subsequent treatment decisions and prognosis.
Approaches to Targeting the Neuroinflammatory Microenvironment
Enhancing neuronal survival, promoting hippocampal neurogenesis, and dampening the neurotoxic microenvironment are all strategies that have been proposed to ameliorate cognitive dysfunction in patients receiving brain irradiation. Drugs that are routinely used in other neurological conditions have recently been repurposed to treat or prevent radiation-induced brain injury and several clinical trials are ongoing. Studies elucidating the role of the neurotoxic microenvironment have led to a series of approaches to dampen inflammation and reverse NPC loss.
Lithium
As a pretreatment option, lithium increases NPC proliferation and rescues radiation-induced cell cycle arrest in animal models.69 Both in vivo and in vitro studies have shown that lithium induces neurogenesis, which is otherwise decreased following radiation.70,71 In lithium-treated animals, hippocampal neurons were protected from radiation-induced apoptosis and performed better on learning and memory tests.71 One mechanism by which lithium reduces neuronal apoptosis may be due to a reduction in ROS via the glutathione pathway.72
Memantine
Glutamate N-methyl-d-aspartate (NMDA) receptors are involved in learning and memory and NMDA receptor agonist, memantine, is used to treat moderate to severe vascular dementia and Alzheimer’s disease. As the same cognitive domains are also involved in radiation-induced brain injury, memantine has been investigated in several phase III trials. In one study (RTOG 0614),73 patients receiving memantine had delayed time to cognitive decline and reduced the rate of memory decline, executive function, and processing speed compared to the control group. The follow-up study, NRG CC001,73 a phase II/III trial that evaluated the combined neuroprotective effects of hippocampal avoidance (discussed in the section to follow) in addition to memantine during WBRT for brain metastases. Patients receiving WBRT for brain metastases were randomized to receive placebo or memantine within 3 days of initiating radiotherapy for 24 weeks and then cognitive function tests were performed. Patient receiving memantine had a significant delay in cognitive decline (hazard ratio 0.78, 95% confidence interval 0.62–0.99, P = .01), superior executive function at 8 and 16 weeks (P = .0137), and superior processing speed (P = .0149) at 24 weeks. While there was an improvement in recall in the memantine arm, it was not statistically significant (P = .059). This study is limited by a small number of patients (n = 149) who were analyzable by 24 weeks due to significant patient loss, which amounted to only a 35% statistical power.
Antioxidants
During radiation, the production of ROS leads to DNA, protein, and lipid membrane damage. Neurons are particularly susceptible to ROS due to their enhanced unsaturated fatty acid contents and higher levels of lipid peroxidation in response to radiation. Several preclinical studies have demonstrated some effect of antioxidant drugs or agents in reducing radiation-induced brain injury. For instance, the flavonoid quercetin has been shown to have neuroprotective properties and was investigated in an animal model exposed to 20 Gy of whole-brain irradiation and found to protect against some histopathological features of brain injury and neuroinflammation.74 Specifically, astrocyte hypertrophy decreased along with vascular dilatation and endothelial damage. Proinflammatory cytokine release and neuronal survival were not evaluated.
Renin–Angiotensin System Blockage
Preclinical studies demonstrate that renin–angiotensin system (RAS) blockage may ameliorate late-delayed radiation induced organ injury, including kidney, lung, and brain.75 Animal studies have shown that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers effectively reduce inflammatory pathway cascades including NF-kB and AP-1 in the brain. Furthermore, these RAS blockers prevent cognitive impairment in rodent models if they are administered before, during, or after fractionated whole-brain irradiation.76 The angiotensin-converting enzyme inhibitor, ramipril, was administered to rats 2 weeks after 30 Gy radiation exposure and was associated with decreased optic neuropathy 6 months following irradiation.76 It is hypothesized that RAS blockers may attenuate radiation-induced brain injury by decreasing Ang II activity and also increasing the generation of anti-inflammatory peptide, Ang-(1–7). In one study primary rat astrocytes pretreated with Ang-(1–7) had decreased expression of cytokines IL-6 and IL-1β.77 It is uncertain whether the acute changes in cytokine release in vitro will translate to late-delayed cognitive changes in vivo. A phase II trial (NCT03475186) is currently underway for ramipril in patients with brain tumors. Another drug affecting the RAS pathway under investigation for radiation-induced brain injury is angiotensin type 1 receptor antagonist. In murine models, administration of the drug before, during, and after fractionated whole-brain irradiation prevented or reduced cognitive impairment at 26 and 52 weeks after irradiation.78
Small Molecule Compounds Targeting p53 Isoform, Δ133p53α
As the SASP adopted by healthy neighboring cells plays an important role in chronic inflammation and cellular damage in a number of target organs, one approach is to target cells which release IL-6 and other damaging proinflammatory factors. Recently, we have shown that SASP can be diminished through the overexpression of p53 isoform, Δ133p53α.55 Small molecule compounds that increase expression of Δ133p53α could therefore be used to prevent or ameliorate tissue damage due to radiation.
Exercise
It has long been known that hippocampal neurogenesis and memory are enhanced by exercise. Several studies have examined whether exercise ameliorates cognitive decline following brain radiation in animal models. Results of these studies produced conflicting findings with some showing that exercise improved79,80 or had no effect81 on behavioral deficits following radiation. Few studies investigate the mechanism by which exercise may modulate cognition post-irradiation. Wong-Goodrich et al.82 investigated whether voluntary wheel running improved spatial learning and memory and whether this might be due to increased hippocampal neurogenesis in mice exposed to exercise after WBRT.83 The WBRT group had significantly decreased spatial learning ability and this was rescued by wheel running up to 4 months following radiation exposure. These mice also had restored hippocampal neurogenesis and increased brain-derived neurotrophic factor (BDNF), IGF-1, and VEGF. To determine the mechanism, another study demonstrates that DNA 5-hydroxymethylation modification (5 hmC) and ten-eleven translocation (Tet) proteins decrease in the hippocampus post-radiation and that forced running increased levels of these factors along BDNF while increasing neurogenesis and improving cognitive dysfunction.84 Tet inhibitor, SC1, was found to partially reverse such changes demonstrating that these effects may be Tet-dependent. The authors propose that Tet-mediated 5 hmC modification could represent a diagnostic biomarker of radiation-induced brain injury, although this has yet to be investigated in human studies.
Human studies examining the effects of exercise on radiation-induced brain injury are limited. Gehring et al. examined a cognitive rehabilitation program in patients with glioma among these patients 61% had received prior radiation therapy and 39% had not.85 However, the study stratified in minimization for the effect of irradiation, so it is uncertain what effect exercise had on radiation-induced cognitive changes. Nevertheless, the authors concluded that there was a moderate improvement in attention and verbal memory in the 6-month follow-up after the exercise program, so those with previous radiotherapy did benefit. A subgroup analysis comparing whether patients receiving radiation differed from those that did not in terms of improvement in cognitive function would be required to determine the magnitude of the effect of exercise on radiation-induced injury.
Peroxisomal Proliferator-Activated Receptor Agonists
Peroxisomal proliferator-activated receptors (PPARs) are a nuclear hormone receptor family that can activate neuroprotective and anti-inflammatory pathways in the CNS.86,87 PPAR agonists inhibit proinflammatory cytokine release in both microglia and astrocytes88 and enhance neuroprotection in animal models of neurodegenerative diseases and stroke.89 In animal models, the use of PPAR agonists has decreased late cognitive effects of radiation. Two such agents that have been used in animal models are pioglitazone, a PPARγ agonist, and fenofibrate, a PPARα agonist. PPARγ was administered 3 days before, during, and for 4 weeks after 40 Gy fractionated whole-brain irradiation and prevented the radiation-induced decline in cognitive function.90 Additionally, in a study administering fenofibrate to mice receiving 10 Gy of whole-brain irradiation, animals receiving the drug had an increased number of hippocampal neurons and less microglial activation.91 Administration of PPARγ agonist during whole-brain irradiation raises the issue of whether the approach enhances tumor cell survival against radiation. However, multiple studies demonstrated that in fact antiproliferative signaling pathways and tumor cell cytotoxicity are preserved in both in vitro studies using a variety of cancer cell lines as well as animal models and clinical trials.92 In a phase I trial (NCT01151670) the repurposed antidiabetic drug, pioglitazone, was found to be well tolerated by brain tumor patients undergoing radiotherapy and established a safe dose to be applied to future efficacy trials.93
Hippocampal Avoidance Strategies
The hippocampus plays an essential role in memory formation. NPCs of the dentate gyrus of the hippocampus are highly susceptible to radiation injury. Decreased hippocampal neurogenesis is thought to contribute to a decline in memory function following brain radiotherapy.42 In animal studies, the extent of radiation-induced damage to NPCs predicts the duration of neurogenic and cognitive dysfunction suggesting that this group of cells plays a key role.37,94 Hence strategies to avoid damage to the hippocampus have emerged as a strategy to reduce radiation-induced brain injury. Current strategies to avoid hippocampal radiation are detailed below.
Stereotactic Conformal Radiotherapy
High-precision conformal techniques have been proposed for brain tumor radiotherapy due to superior dosimetric delivery, long-term tumor control, and the potential for reduced radiation-induced cognitive dysfunction. In a prospective randomized trial comparing stereotactic conformal radiotherapy (SCRT) to conventional radiotherapy, SCRT demonstrated improved functional outcomes in several neurocognitive domains evaluated longitudinally over 5 years.95 Mean full-scale or global intelligence quotient (IQ) and performance IQ scores were significantly higher in the group that received SCRT. Mean full-scale IQ scores of patients in the SCRT group were either stable or improved over 5 years while those receiving conventional radiotherapy initially improved but then gradually declined. Neurocognitive domains of performance quotient and memory were particularly enhanced in the SCRT group over the conventional group. Thus, SCRT may represent a superior modality to preserve cognitive functioning in patients vulnerable to radiation-induced brain injury.
Intensity-Modulated Radiotherapy
A phase II trial (NRG/RTOG 0933) demonstrated enhanced memory preservation following WBRT with hippocampal avoidance using IMRT.3 This study is discussed in detail in the prior section on hippocampal neurogenesis. A phase III trial (NRG-CC001) enrolling 518 patients with brain metastases compared WBRT (30 Gy in 10 fractions) and memantine with or without hippocampal avoidance. In terms of radiation dose, a per protocol dose to 100% of the hippocampus did not exceed 9 Gy and maximal hippocampal dose did not exceed 16 Gy. The primary endpoint was time to neurocognitive function failure as defined by a decrease in one of the neurocognitive tests (Hopkins Verbal Learning Test—Revised, Controlled Oral Word Association, or Trial Making Test). The primary outcome measure was time to neurocognitive function decline, which was significantly longer with hippocampal avoidance while achieving similar intracranial tumor control and survival.
Proton Beam Therapy
Previous studies have shown that increased radiation dose to the temporal lobes is associated with worse neurocognitive outcome and this is particularly severe in adult survivors of childhood brain tumors.13,96 In a recent study,97 3 different treatment modalities were compared to determine if one might have a superior radiation dose sparing to normal tissues. The three modalities compared were: (1) double scattering proton therapy (DSPT), (2) proton beam therapy (PBT) via pencil beam scanning using a temporal lobe sparing field configuration, and (3) volumetric modulated arch therapy (VMAT), which is conventional photon radiotherapy with a temporal lobe sparing field configuration. These modalities were compared in 10 children with craniopharyngioma. The aim was to reduce the dose to the temporal lobe and hippocampus, while delivering the same dose to the tumors consistent with the DSPT plans. PBT consistently had the lowest volume fractions of temporal lobes across all investigated dose levels, leading to better estimated memory outcomes.
Another recent study98 followed up 62 brain tumor patients treated with PBT and assessed neurocognition and quality of life at baseline and every 3 months following therapy. They found that the neurocognitive parameters tested remained largely stable during recurrence-free follow-up for an average of 22.5 months. Larger clinical studies are underway, including NCT02824731 and NCT03180502, and will be important for validating these findings and also allowing direct comparison to patients treated with photon radiotherapy.
Future Directions
Cognitive dysfunction following radiotherapy is a challenging and multifaceted adverse effect that limits treatment options for many patients while impairing quality of life for those who receive this treatment. An increased understanding of the mechanisms behind radiation-induced brain injury, including disruption of the BBB, decreased hippocampal neurogenesis, and increased neurotoxic SASP, has enhanced our ability to target the neuroinflammatory microenvironment. While currently no standard of care has been established, several preclinical studies demonstrate promising pharmacological approaches to ameliorate brain injury and several key clinical trials are currently underway. Advancing our understanding of radiation-induced brain injury remains challenging for several reasons, including the mechanistic uncertainty behind the molecular and cellular changes that occur after radiation to the brain. Furthermore, it is difficult to evaluate and quantify the severity of cognitive dysfunction in affected patients. In terms of the use of brain imaging, the severity of cognitive dysfunction is inconsistently correlated to brain imaging studies99,100 and currently no biomarkers exist that predict poor outcome following therapy.
Beyond the adverse effects of radiation, other main cancer treatment modalities, including chemotherapy and immunotherapies, have also demonstrated therapy-related cognitive decline that is thought to be related to similar neuroinflammatory effects. For instance, cytokine release syndrome has been reported following CAR T-cell therapy and associated with release of SASP proinflammatory cytokines.101 As combination therapy (including surgery, chemo and/or immunotherapy, and radiation therapy) is the standard of practice for treating CNS neoplasms, a better understanding of the pathophysiologic mechanisms surrounding cellular/tissue injury and long-term sequelae is essential. Hence, further research into prevention or amelioration of the neurotoxic microenvironment in the setting of radiation could present a promising approach applicable to a range of anticancer treatments. Translation of the preclinical findings to patient treatments presents the opportunity to significantly improve quality of life and dose limitation barriers for patients with brain tumors receiving radiation.
Funding
This article was not funded.
Acknowledgments
We would like to thank Izumi Horikawa and Jessica Beck for reviewing the manuscript. Figures were created with BioRender.com.
Conflict of interest statement. None declared.
Authorship Statement. C.T., B.T.H., and C.C.H. wrote the manuscript.
References
- 1. Fisher BJ, Bauman GS, Leighton CE, Stitt L, Cairncross JG, Macdonald DR. Low-grade gliomas in children: tumor volume response to radiation. J Neurosurg. 1998;88(6):969–974. [DOI] [PubMed] [Google Scholar]
- 2. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. [DOI] [PubMed] [Google Scholar]
- 3. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang Y, Li Y, Luo D, Rong X, Ye J, Peng Y. Epilepsy related to radiotherapy in patients with nasopharyngeal carcinoma. Epilepsy Res. 2011;96(1–2):24–28. [DOI] [PubMed] [Google Scholar]
- 6. Vigliani MC, Duyckaerts C, Hauw JJ, Poisson M, Magdelenat H, Delattre JY. Dementia following treatment of brain tumors with radiotherapy administered alone or in combination with nitrosourea-based chemotherapy: a clinical and pathological study. J Neurooncol. 1999;41(2):137–149. [DOI] [PubMed] [Google Scholar]
- 7. McDuff SG, Taich ZJ, Lawson JD, et al. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry. 2013;84(12):1384–1391. [DOI] [PubMed] [Google Scholar]
- 8. Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19(9):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haldbo-Classen L, Amidi A, Wu LM, et al. Long-term cognitive dysfunction after radiation therapy for primary brain tumors. Acta Oncol. 2019;58(5):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michaelidesová A, Konířová J, Bartůněk P, Zíková M. Effects of radiation therapy on neural stem cells. Genes (Basel). 2019;10(9). doi: 10.3390/genes10090640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee DS, Yu M, Jang HS, et al. Radiation-induced brain injury: retrospective analysis of twelve pathologically proven cases. Radiat Oncol J. 2011;29(3):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nolan CP, DeAngelis LM. Neurologic complications of chemotherapy and radiation therapy. Continuum (Minneap Minn). 2015;21(2 Neuro-oncology):429–451. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12(11):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Surma-aho O, Niemelä M, Vilkki J, et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56(10):1285–1290. [DOI] [PubMed] [Google Scholar]
- 15. Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. [DOI] [PubMed] [Google Scholar]
- 16. Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. [DOI] [PubMed] [Google Scholar]
- 17. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. [DOI] [PubMed] [Google Scholar]
- 18. Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153(4):357–370. [DOI] [PubMed] [Google Scholar]
- 19. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hopewell JW, Wright EA. The nature of latent cerebral irradiation damage and its modification by hypertension. Br J Radiol. 1970;43(507):161–167. [DOI] [PubMed] [Google Scholar]
- 21. Walker AJ, Ruzevick J, Malayeri AA, et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol. 2014;10(7):1277–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–1228. [DOI] [PubMed] [Google Scholar]
- 23. Schultheiss TE, Stephens LC. Permanent radiation myelopathy. Br J Radiol. 1992;65(777):737–753. [DOI] [PubMed] [Google Scholar]
- 24. Meadows AT, D’Angio GJ, Evans AE, Harris CC, Miller RW, Mike V. Oncogenesis and other late effects of cancer treatment in children. Radiology. 1975;114(1):175–180. [DOI] [PubMed] [Google Scholar]
- 25. Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17(2):141–148. [DOI] [PubMed] [Google Scholar]
- 27. Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63(18):5950–5956. [PubMed] [Google Scholar]
- 28. Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989;7(3):287–294. [DOI] [PubMed] [Google Scholar]
- 29. Ljubimova NV, Levitman MK, Plotnikova ED, Eidus LKh. Endothelial cell population dynamics in rat brain after local irradiation. Br J Radiol. 1991;64(766):934–940. [DOI] [PubMed] [Google Scholar]
- 30. Shinohara C, Gobbel GT, Lamborn KR, Tada E, Fike JR. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res. 1997;57(13):2694–2702. [PubMed] [Google Scholar]
- 31. Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–3353. [DOI] [PubMed] [Google Scholar]
- 32. Lossinsky AS, Buttle KF, Pluta R, Mossakowski MJ, Wiśniewski HM. Immunoultrastructural expression of intercellular adhesion molecule-1 in endothelial cell vesiculotubular structures and vesiculovacuolar organelles in blood-brain barrier development and injury. Cell Tissue Res. 1999;295(1):77–88. [DOI] [PubMed] [Google Scholar]
- 33. Lossinsky AS, Mossakowski MJ, Pluta R, Wisniewski HM. Intercellular adhesion molecule-1 (ICAM-1) upregulation in human brain tumors as an expression of increased blood-brain barrier permeability. Brain Pathol. 1995;5(4):339–344. [DOI] [PubMed] [Google Scholar]
- 34. Greenwood J, Etienne-Manneville S, Adamson P, Couraud PO. Lymphocyte migration into the central nervous system: implication of ICAM-1 signalling at the blood-brain barrier. Vascul Pharmacol. 2002;38(6):315–322. [DOI] [PubMed] [Google Scholar]
- 35. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. [DOI] [PubMed] [Google Scholar]
- 38. Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. [DOI] [PubMed] [Google Scholar]
- 39. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 40. Acharya MM, Christie LA, Lan ML, et al. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71(14):4834–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore ED, Kooshki M, Wheeler KT, Metheny-Barlow LJ, Robbins ME. Differential expression of Homer1a in the hippocampus and cortex likely plays a role in radiation-induced brain injury. Radiat Res. 2014;181(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 43. Schneider L, Pellegatta S, Favaro R, et al. DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem Cell Reports. 2013;1(2):123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3462659/. Accessed April 4, 2020. [DOI] [PMC free article] [PubMed]
- 45. Santivasi WL, Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. 2014;21(2):251–259. [DOI] [PubMed] [Google Scholar]
- 46. Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. [DOI] [PubMed] [Google Scholar]
- 47. Citrin DE, Shankavaram U, Horton JA, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105(19):1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Boerma M, Zhou D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat Res. 2016;186(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75(10–11):1149–1163. [PubMed] [Google Scholar]
- 50. Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. [DOI] [PubMed] [Google Scholar]
- 51. Khan SY, Awad EM, Oszwald A, et al. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci Rep. 2017;7:39501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rochfort KD, Cummins PM. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans. 2015;43(4):702–706. [DOI] [PubMed] [Google Scholar]
- 53. Ungvari Z, Podlutsky A, Sosnowska D, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68(12):1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5(1):99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turnquist C, Beck JA, Horikawa I, et al. Radiation-induced astrocyte senescence is rescued by Δ133p53. Neuro Oncol. 2019;21(4):474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Korpela E, Liu SK. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat Oncol. 2014;9:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hong EH, Lee SJ, Kim JS, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285(2):1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beach TA, Johnston CJ, Groves AM, Williams JP, Finkelstein JN. Radiation induced pulmonary fibrosis as a model of progressive fibrosis: contributions of DNA damage, inflammatory response and cellular senescence genes. Exp Lung Res. 2017;43(3):134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu LK, Ouyang W, Zhao X, et al. Pathogenesis and prevention of radiation-induced myocardial fibrosis. Asian Pac J Cancer Prev. 2017;18(3):583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fujita K, Mondal AM, Horikawa I, et al. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11(9):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mondal AM, Horikawa I, Pine SR, et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest. 2013;123(12):5247–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Turnquist C, Horikawa I, Foran E, et al. p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ. 2016;23(9):1515–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sheline GE. Radiation therapy of brain tumors.1977. https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/1097-0142(197702)39:2+%3C873::AID-CNCR2820390725%3E3.0.CO;2-Y. Accessed April 3, 2020.
- 65. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):1118–1130. [DOI] [PubMed] [Google Scholar]
- 66. Winter SF, Loebel F, Loeffler J, et al. Treatment-induced brain tissue necrosis: a clinical challenge in neuro-oncology. Neuro Oncol. 2019;21(9):1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. [DOI] [PubMed] [Google Scholar]
- 68. Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity–molecular and cellular mechanisms. Br J Cancer. 2001;85(9):1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zanni G, Di Martino E, Omelyanenko A, et al. Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro. Oncotarget. 2015;6(35):37083–37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huo K, Sun Y, Li H, et al. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol Cell Neurosci. 2012;51(1–2):32–42. [DOI] [PubMed] [Google Scholar]
- 71. Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66(23):11179–11186. [DOI] [PubMed] [Google Scholar]
- 72. Machado-Vieira R, Andreazza AC, Viale CI, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett. 2007;421(1):33–36. [DOI] [PubMed] [Google Scholar]
- 73. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kale A, Piskin Ö, Bas Y, et al. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J Radiat Res. 2018;59(4):404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Robbins ME, Zhao W, Garcia-Espinosa MA, Diz DI. Renin-angiotensin system blockers and modulation of radiation-induced brain injury. Curr Drug Targets. 2010;11(11):1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee TC, Greene-Schloesser D, Payne V, et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moore ED, Kooshki M, Metheny-Barlow LJ, Gallagher PE, Robbins ME. Angiotensin-(1-7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP kinase signaling. Free Radic Biol Med. 2013;65:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Robbins ME, Payne V, Tommasi E, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73(2):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Naylor AS, Bull C, Nilsson MK, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105(38):14632–14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–1058. [DOI] [PubMed] [Google Scholar]
- 81. Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27(6):1494–1502. [DOI] [PubMed] [Google Scholar]
- 82. Wong-Goodrich SJ, Pfau ML, Flores CT, Fraser JA, Williams CL, Jones LW. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res. 2010;70(22):9329–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kitamura T, Saitoh Y, Takashima N, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–827. [DOI] [PubMed] [Google Scholar]
- 84. Zhang J, Li J, Zhu Y, Miao Z, Tian Y. Forced running exercise mitigates radiation-induced cognitive deficits via regulated DNA hydroxymethylation. Epigenomics. 2020;12(5):385–396. [DOI] [PubMed] [Google Scholar]
- 85. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 86. Bright JJ, Kanakasabai S, Chearwae W, Chakraborty S. PPAR regulation of inflammatory signaling in CNS diseases. PPAR Res. 2008;2008:658520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771(8):1031–1045. [DOI] [PubMed] [Google Scholar]
- 88. Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49(2):183–189. [DOI] [PubMed] [Google Scholar]
- 89. Bordet R, Ouk T, Petrault O, et al. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34(Pt 6):1341–1346. [DOI] [PubMed] [Google Scholar]
- 90. Zhao W, Payne V, Tommasi E, Diz DI, Hsu FC, Robbins ME. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67(1):6–9. [DOI] [PubMed] [Google Scholar]
- 91. Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75(3):870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5(7):419–429. [DOI] [PubMed] [Google Scholar]
- 93. Cramer CK, Alphonse-Sullivan N, Isom S, et al. Safety of pioglitazone during and after radiation therapy in patients with brain tumors: a phase I clinical trial. J Cancer Res Clin Oncol. 2019;145(2):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Achanta P, Fuss M, Martinez JL Jr. Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci. 2009;123(5):1036–1045. [DOI] [PubMed] [Google Scholar]
- 95. Jalali R, Gupta T, Goda JS, et al. Efficacy of stereotactic conformal radiotherapy vs conventional radiotherapy on benign and low-grade brain tumors: a randomized clinical trial. JAMA Oncol. 2017;3(10):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Merchant TE, Kiehna EN, Kun LE, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(2 Suppl):94–102. [DOI] [PubMed] [Google Scholar]
- 97. Toussaint L, Indelicato DJ, Muren LP, et al. Temporal lobe sparing radiotherapy with photons or protons for cognitive function preservation in paediatric craniopharyngioma. Radiother Oncol. 2019;142:140–146. [DOI] [PubMed] [Google Scholar]
- 98. Dutz A, Agolli L, Bütof R, et al. Neurocognitive function and quality of life after proton beam therapy for brain tumour patients. Radiother Oncol. 2020;143:108–116. [DOI] [PubMed] [Google Scholar]
- 99. Atwood T, Payne VS, Zhao W, et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat Res. 2007;168(5):574–581. [DOI] [PubMed] [Google Scholar]
- 100. Saykin AJ, de Ruiter MB, McDonald BC, Deprez S, Silverman DH. Neuroimaging biomarkers and cognitive function in non-CNS cancer and its treatment: current status and recommendations for future research. Brain Imaging Behav. 2013;7(4):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Joly F, Castel H, Tron L, Lange M, Vardy J. Potential effect of immunotherapy agents on cognitive function in cancer patients. J Natl Cancer Inst. 2020;112(2):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]