Abstract
Platelet aggregate formation is a multistep process involving receptor-mediated, as well as biomechanical, signaling cascades, which are highly dependent on actin dynamics. We have previously shown that actin depolymerizing factor (ADF)/n-cofilin and Twinfilin 2a, members of the ADF homology (ADF-H) protein family, have distinct roles in platelet formation and function. Coactosin-like 1 (Cotl1) is another ADF-H protein that binds actin and was also shown to enhance biosynthesis of pro-inflammatory leukotrienes (LT) in granulocytes. Here, we generated mice lacking Cotl1 in the megakaryocyte lineage (Cotl1−/−) to investigate its role in platelet production and function. Absence of Cotl1 had no impact on platelet counts, platelet activation or cytoskeletal reorganization under static conditions in vitro. In contrast, Cotl1 deficiency markedly affected platelet aggregate formation on collagen and adhesion to immobilized von Willebrand factor at high shear rates in vitro, pointing to an impaired function of the platelet mechanoreceptor glycoprotein (GP) Ib. Furthermore, Cotl1−/−platelets exhibited increased deformability at high shear rates, indicating that the GPIb defect may be linked to altered biomechanical properties of the deficient cells. In addition, we found that Cotl1 deficiency markedly affected platelet LT biosynthesis. Strikingly, exogenous LT addition restored defective aggregate formation of Cotl1−/− platelets at high shear in vitro, indicating a critical role of platelet-derived LT in thrombus formation. In vivo, Cotl1 deficiency translated into prolonged tail bleeding times and protection from occlusive arterial thrombus formation. Together, our results show that Cotl1 in platelets is an integrator of biomechanical and LT signaling in hemostasis and thrombosis.
Introduction
Platelets are small anucleate cells that are essential for hemostasis and maintenance of vascular integrity, but are also implicated in thrombosis resulting in ischemia and infarction under pathological conditions.1 The classic, simplified model of platelet-dependent arterial thrombus formation comprises sequential steps, involving platelet deceleration on the injured vessel wall via interaction of the platelet mechanoreceptor glycoprotein (GP) Ib with von Willebrand factor (vWF) immobilized on the injured vessel wall. This is followed by cellular activation via the collagen receptor GPVI and G protein-coupled receptors (GPCR) stimulated by soluble agonists such as ADP, thromboxane A2 (TxA2) or thrombin. The final common pathway of these activatory events is the functional upregulation of integrins, which mediate firm platelet adhesion and aggregation.
Critical determinants of thrombus formation are the locally prevailing rheological conditions. With increasing shear rate, e.g. during the development of stenosis, platelet adhesion and aggregate formation become increasingly dependent on GPIb-vWF interactions. At sites of very high, pathological shear rates and disturbed flow, occlusive arterial thrombus formation can be mediated predominantly by the GPIb-vWF interaction, does not involve visible platelet shape change or activation, and may thus be mediated, at least in part, by the biomechanical interaction of platelets.2 In accordance with this, it was shown that vWF-mediated pulling at the GPIbα receptor under shear induces the unfolding of a juxtamembrane mechanosensitive domain, which might contribute to platelet mechanosensing under dynamic conditions and GPIb-induced intracellular signaling.3 Thus, it appears that platelet-mediated thrombus formation in vivo involves both agonist receptor and biomechanical signaling, the interplay of which is still poorly understood.
Platelets are produced by megakaryocytes (MK) in the bone marrow (BM) through a cytoskeleton-driven process. The critical role of the actin cytoskeleton for platelet production and function is illustrated by the association of mutations in genes encoding proteins that are involved in actin cytoskeletal organization, such as Diaphanous Related Formin 1 (DIAPH1),4 filamin A (FLNA),5 Wiskott Aldrich syndrom protein (WASP),6 actinin 1 (ACTN1),7 or tropomyosin 4 (TPM4)8 with platelet disorders in humans and mice. In circulating platelets, the actin cytoskeleton is essential to maintain cell morphology and to exert key functions upon activation, such as granule release, as well as the formation of filopodia and lamellipodia.9 However, the complex protein network orchestrating actin dynamics in platelets is not fully understood.
The ADF homology domain (ADF-H) is one of the best-characterized actin binding motifs. The ADF-H protein family comprises twinfilin (Twf), ADF/n-cofilin, Abp1/drebrin, and coactosin-like 1 (Cotl1/CLP). We have previously shown that Twf2a and ADF/n-cofilin play distinct, critical roles in platelet formation and function.10,11 Although sharing less than 20% sequence identity with the other ADF-H family members, Cotl1 is structurally highly homologous, suggesting a similar role for cytoskeletal dynamics.12 Indeed, Cotl1 binds F-actin, but does not interact with actin monomers.13 Furthermore, Cotl1 was shown to prevent n-cofilin-mediated depolymerization of actin filaments, thereby promoting lamellipodia formation at the immune synapse.14 Besides its interaction with F-actin, Cotl1 is implicated in the biosynthesis of leukotrienes (LT),15 lipid-derived pro-inflammatory mediators involved in a variety of inflammatory processes such as allergy or asthma. Cotl1 was shown to interact with 5-lipoxygenase (5-LO), a key enzyme in LT biosynthesis that catalyzes two of the initial steps, namely the oxygenation of arachidonic acid (AA) to 5-HPETE and the subsequent dehydration into the epoxide LTA4.15–17 Platelet-stored LT have been shown to contribute to inflammatory responses, e.g. during lung inflammation.18 However, the mechanism underlying this contribution, as well as the precise role of LT for platelet function, have not been defined.
Here, we generated conditional knockout mice lacking Cotl1 in the MK lineage. We found that Cotl1 is critically required for stable platelet thrombus formation under conditions of shear flow in vitro and in vivo by modulating the function of the mechanoreceptor GPIb, as well as platelet LT biosynthesis.
Methods
Animals
Cotl1+/− mice were generated by injection of embryonic stem cell clone Cotl1tm1a(EUCOMM)Hmgu into C57Bl/6 blastocysts. Germline transmission was confirmed by backcrossing of the chimeric mice with C57Bl/6 mice. Cotl1+/− mice were intercrossed with mice carrying Flp recombinase to generate Cotl1fl/+ mice, which were intercrossed with mice carrying Cre recombinase under control of the platelet factor 4 (Pf4) promoter to generate mice lacking Cotl1 specifically in MK and platelets.19 For all experiments, 12- to 16-week old Cotl1fl/fl;Pf4Cre and Cotl1fl/fl littermate controls, maintained on a C57Bl/6 background, were used. All mice were derived from the following breeding strategy: Cotl1fl/fl;Pf4Cre X Cotl1fl/fl..
Animal studies
Animal studies were approved by the district government of Lower Franconia (AZ15_14; Bezirksregierung Unterfranken).
Further details of reagents and experimental procedures are given in the Online Supplementary Appendix.
Results
Cotl1 deficiency does not affect platelet function under static conditions
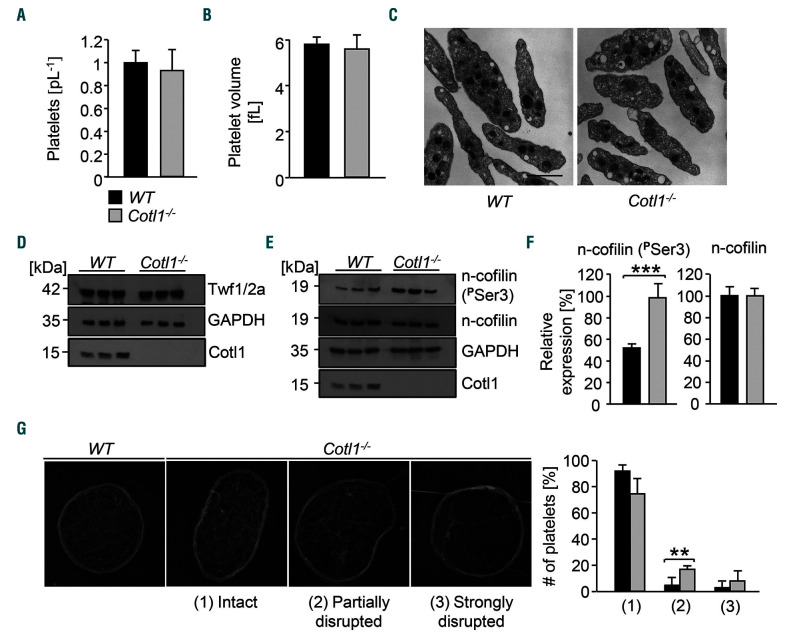
Cotl1fl/fl;Pf4Cre mice (hereon referred to as Cotl1−l−) were viable and born in the expected Mendelian ratios. The absence of Cotl1 in platelets was confirmed by western blotting (Online Supplementary Figure S1A). Cotl1 deficiency did not affect either peripheral platelet count, size or ultrastructure (Figure 1A–C) nor the expression of prominent platelet surface receptors (Online Supplementary Table S1). This is in contrast to deficiency in the ADF-H protein family members Twf2a11 or n-cofilin,10 which results in thrombocytopenia in mice. Thus, Cotl1−/− mice represent the first knockout mouse model of an ADF-H family member with apparently normal platelet biogenesis.
Figure 1.
Cotl1 is not essential for platelet formation and function under static conditions in vitro. (A and B) Platelet count (A) and size (B) were determined with an automated cell analyzer (ScilVet). (C) Visualization of platelet size and structure using transmission electron microscopy (n=4). Scale bar, 2 μm. (D–F) Platelets were left untreated, lysed, and processed for immunoblotting. Total twinfilin (D), phosphorylated n-cofilin and total n-cofilin (E) were probed with the respective antibodies and analyzed by densitometry (F). GAPDH served as loading control. Values are mean±standard deviation (SD) (n=3). (G) Images of the platelet cytoskeleton ultrastructure on poly-L-lysine. (Left) WT sample. (Right) Cotl1−/− sample. 0: intact, 1: partially disrupted, 2: strongly disrupted F-actin structures. Scale bar, 1 μm. At least 158 platelets per genotype were analyzed.
A common feature of ADF-H family members is their involvement in cytoskeletal dynamics. Consistently, n-cofilin deficiency resulted in impaired stimulus-dependent F-actin assembly, whereas Twf2a-deficient mice displayed increased actin dynamics.10,11 According to previous studies, n-cofilin and Cotl1 are highly abundant in both human and mouse platelets,20,21 while Twf2 levels are slightly lower. The results for Twf1 and drebrin, which was not listed in the mouse dataset, suggest that they are expressed at a lower level. Importantly, both datasets indicate that the expression of ADF-H proteins is similar in human and mouse platelets.
We determined expression levels of the ADF-H members Twf1/2a and n-cofilin by western blotting, but could not detect differences in total expression levels of either protein between WT and Cotl1−/− platelets (Figure 1D and E). Strikingly, we observed a strong (2-fold) increase in expression of the (inactive) phosphorylated form of n-cofilin (Ser3) in Cotl1−/− platelets (***P<0.001) (Figure 1E and F). Next, we analyzed the localization of Cotl1 in resting and spread platelets (Online Supplementary Figure S1B and C). In line with previous observations,22 we observed a cytoplasmic localization which partially overlapped with that of F-actin and tubulin. When visualizing the F-actin ultrastructure in resting platelets by transmission electron microscopy (TEM),23 we observed that the actin scaffold was disrupted in a significant proportion of Cotl1-deficient platelets compared to the WT: (1) partially disrupted: Cotl1−/− 17.1% versus WT 4.9%, **P>0.01; (2) strongly disrupted: Cotl1−/− 8.2% vs. WT 3% (Figure 1G). However, we could not detect changes in resting F-actin levels or agonist-induced F-actin polymerization in Cotl1−/−platelets (Online Supplementary Figure S2A and B). In addition, although Cotl1 was recently described as a regulator of T-cell spreading at the immune synapse,14 Cotl1 deficiency in platelets did not affect their ability to spread on fibrinogen (Online Supplementary Figure S2C), as shown by normal morphology and distribution of F-actin and tubulin in the spread platelets (Online Supplementary Figure S2D and E).
Together, these results indicated that Cotl1 is not essential for platelet production or platelet actin remodeling.
Cotl1 is required for platelet aggregate formation under flow conditions
We next studied the effect of Cotl1 deficiency on agonist-induced platelet activation. Flow cytometry was used to determine activation of the major platelet integrin αIIbβ3 as well as degranulation (P-selectin exposure) in response to a panel of standard agonists (Online Supplementary Figure S3A and B). In contrast to the hyper-reactivity of Twf2a-deficient platelets,11 Cotl1−/− platelets displayed unaltered responses to agonists acting on both GPCR (thrombin, ADP, TxA2 analog U46619) and (hem)ITAM signaling (collagen-related peptide (CRP), convulxin, rhodocytin) (Online Supplementary Figure S3A and B). Furthermore, washed Cotl1−/− platelets showed unaltered aggregation upon stimulation with thrombin, U46619, collagen and CRP as compared to the control (Online Supplementary Figure S4A). Similar results were obtained when using platelet-rich plasma (PRP) instead of washed platelets (Online Supplementary Figure S4B). These results demonstrated that Cotl1 is not required for platelet activation and aggregation responses under static conditions in vitro.
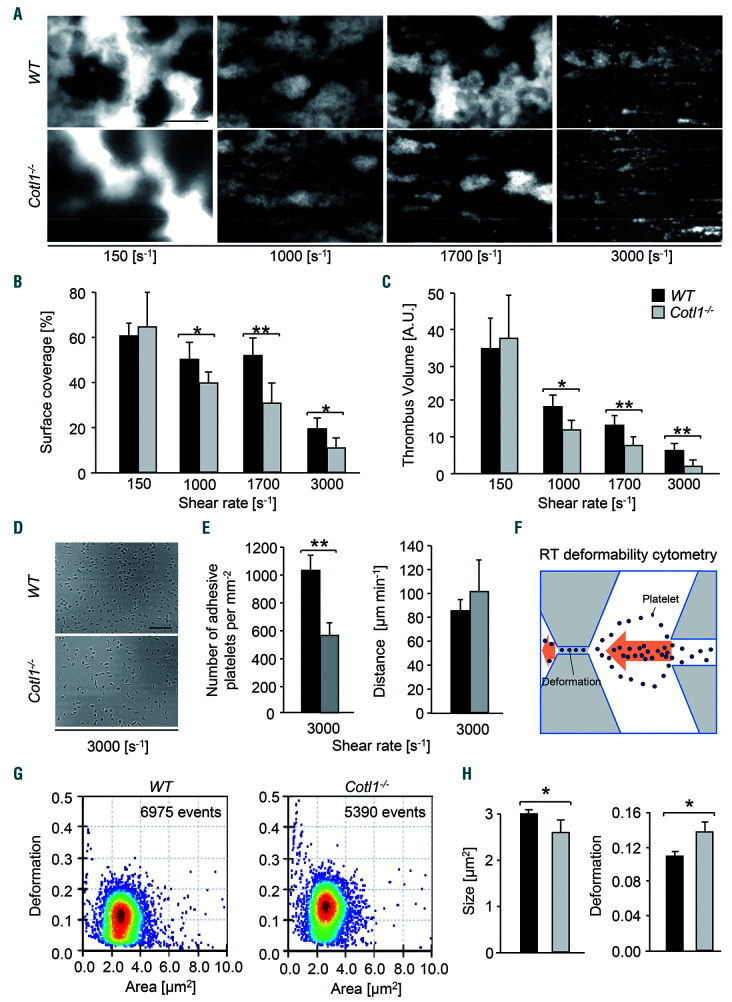
In sharp contrast, however, Cotl1 deficiency markedly affected platelet aggregation and thrombus formation under shear flow conditions in vitro. We used a flow adhesion assay where the perfusion of whole anticoagulated WT blood over a collagen-coated surface leads to rapid platelet adhesion, activation and three-dimensional aggregate formation. While aggregate formation at low shear (150 s-1) was comparable between Cotl1−/− and WT samples, we observed significantly reduced platelet adhesion, surface coverage and thrombus volume in blood from Cotl1−/−animals at medium and high shear rates (1 000 s-1, *P<0.05; 3 000 s-1, **P<0.01) (Figure 2A–C and Online Supplementary Figure S5). Strikingly, thrombus volume and platelet surface coverage were also significantly reduced in blood from Cotl1−/− mice when the flow adhesion assay was carried out in the presence of coagulation (Online Supplementary Figure S6).
Figure 2.
Cotl1 is required for thrombus formation and stability at high shear. (A–C) Assessment of platelet adhesion (A and B) and aggregate formation (A and C) on Horm collagen (70 μg mL−1) under flow (150-3 000 s−1) in heparinized whole blood of WT and Cotl1−/− mice. Values are mean±standard deviation (SD) (n=12). Scale bar, 50 μm. (D and E) Heparinized whole blood of WT and Cotl1−/− mice was perfused over a von Willebrand factor (vWF)-coated cover slip for 4 minutes (min) at a shear rate of 3,000 s−1. (D) Representative phase contrast images taken at the end of the perfusion time and (E) analysis of the number of adherent platelets per mm2 ±SD (left), as well as the rolling velocity calculated from the distance a platelet covered per minute in μm ±SD (right) was performed. Images were acquired with a Zeiss Axiovert 200 inverted microscope (40x/0.6 oil objective). Images are representative of at least 12 animals per group. Scale bar, 50 μm. Unpaired Student t-test: **P<0.01; *P<0.05. (F–H) Real-time deformability cytometry (RT-DC),27 a method for continuous mechanical characterization of cells which are deformed by deceleration at the stagnation point of fast extensional flow. (F) Scheme depicting the principle of real-time deformability cytometry (RT-DC.) (G) Representative dot plots showing the relative deformation, as well as the (H) the size of washed WT and Cotl1−/− platelets. Values are mean±SD (n=3). *P<0.1; **P<0.01. A.U. : arbitrary units.
Platelet adhesion and aggregate formation under medium and high shear rates is dependent on the interaction between the mechanoreceptor GPIb and immobilized vWF.24 To investigate a possible involvement of Cotl1 in GPIb-mediated tethering/adhesion, we perfused blood from WT and Cotl1−/− animals over a vWF-coated surface at high shear (3,000 s−1). The velocity of individual rolling Cotl1−/− platelets on immobilized vWF was comparable to the WT (Figure 2E, right); however, the number of adherent Cotl1−/− platelets was significantly reduced (**P<0.01) (Figure 2D and E). Our results thus indicated that Cotl1 is required to ensure GPIb function during platelet adhesion and aggregate formation under conditions of high shear.
Growing experimental evidence suggests that signaling induced by the GPIb-vWF interaction involves mechanotransduction and transmission of forces to the actin cytoskeleton.3,25,26 Therefore, to assess the impact of Cotl1 deficiency on platelet biomechanical properties more generally, we subjected Cotl1−/− platelets to the recently described real-time deformability cytometry (RT-DC),27 a method for continuous mechanical characterization of cells which are deformed by shear forces in a microfluidics chamber (Figure 2F). Strikingly, we found a significantly increased deformability of Cotl1−/− platelets (*P<0.05) as compared to WT (Figure 2G and H, right). Importantly, we could exclude the possibility that the increased deformability was due to an increased platelet size; by contrast, the measured size of Cotl1−/− platelets in this assay was even slightly decreased as compared to the control (Figure 2H, left). Together, these results indicate that Cotl1 supports GPIb function and thus the formation of stable platelet aggregates under shear conditions, and that this function may, in part, be mediated by the modulation of platelet biomechanical properties.
Cotl1 regulates leukotriene biosynthesis in platelets
Leukotrienes are pro-inflammatory lipid mediators mainly produced by immune competent cells such as leukocytes, e.g. mast cells, eosinophils, neutrophils, monocytes and basophils, and are implicated in a variety of inflammatory responses. Interestingly, besides its function as an actin-regulatory protein, Cotl1 was shown to bind and modulate the activity of the enzyme 5-lipoxygenase (5-LO).15,17,28 5-LO catalyzes the two initial steps of LT biosynthesis: (1) the oxygenation of AA to 5-HPETE; and (2) the subsequent dehydration into leukotriene A4 (LTA4) which is then further converted to LTB4 (Online Supplementary Figure S7).29,30 Besides serving as substrate for LT biosynthesis, AA is converted to thromboxanes (TxA/B2), prostacyclin (PGI2), and prostaglandines (PGE/F2) by cyclo-oxygenases in platelets (Online Supplementary Figure S4).31,32
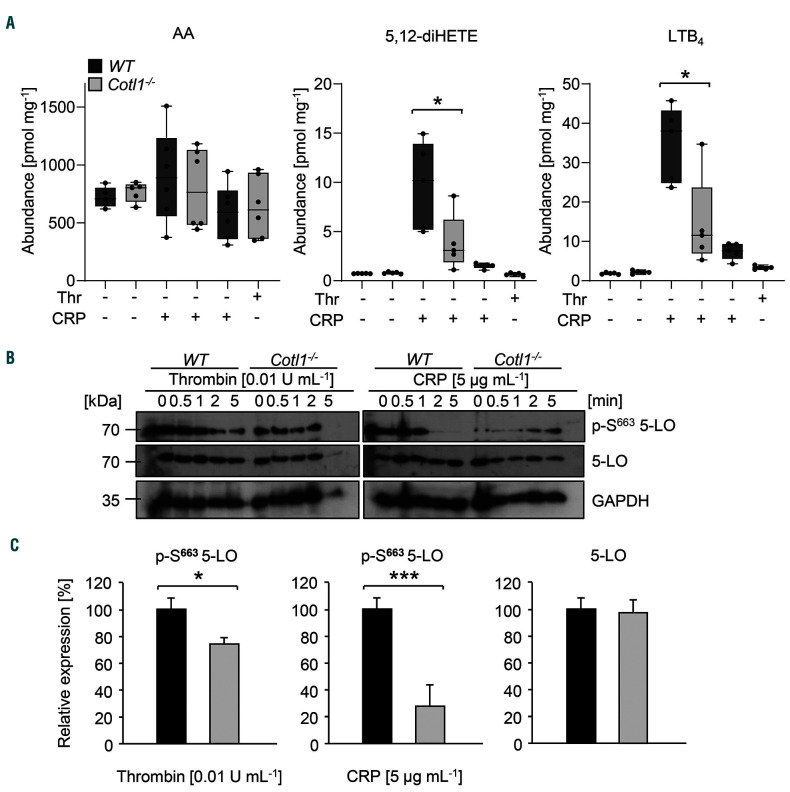
To investigate the effect of Cotl1 deficiency on LT production in platelets, we assessed the release of 5,12-diHETE and LTB4 in the supernatant of washed CRP- or thrombin-stimulated platelets. Strikingly, the secretion of both lipid mediators was significantly reduced in Cotl1−/−platelets upon CRP activation as compared to WT controls and a similar tendency was observed for thrombin-stimulated platelets (*P<0.05) (Figure 3A). Of note, the total AA amount was comparable to WT platelets, demonstrating that the abundance of this initial metabolite was not affected by Cotl1 deficiency (Figure 3A, left). To assess whether AA was consumed by other pathways, we analyzed TxB2 levels by ELISA. Strikingly, we found significantly increased TxB2 release in thrombin-stimulated and, to a lesser extent, CRP-stimulated Cotl1−/− platelets compared to the control (*P<0.05), indicating that the excess of available AA in Cotl1−/− platelets was consumed by an upregulation of prostaglandin biosynthesis (Online Supplementary Figure S8).
Figure 3.
Cotl1 is a regulator of leukotriene biosynthesis in platelets. (A) For lipid mediator analysis platelets were either left untreated or stimulated with CRP [5 μg mL−1] or thrombin [0.01 U mL−1] for 5 minutes (min). Subsequently, samples were spun down, pellet and supernatant were separately shock-frozen in liquid nitrogen. Lipid abundance was assessed using liquid crystal mass spectrometry. Values are mean±standard deviation (SD) (n=15). (B and C) Platelets were either left untreated or stimulated with CRP [5 μg mL−1] or thrombin [0.01 U mL−1] for 5 min, lysed, and processed for immunoblotting. Total 5-LO, phospho-5-LO (S663) and GAPDH (B) were probed with the respective antibodies and analyzed by densitometry (C). Values are mean±SD (n=4).
To assess whether, indeed, Cotl1 directly influences 5-LO activity, lysates of CRP- or thrombin-stimulated platelets were probed for active 5-LO (S663 phosphorylation) by western blotting (Figure 3B). Of note, basal levels of active 5-LO were comparable between Cotl1−/− and WT platelets. Strikingly, while activation induced pronounced S663 phosphorylation in WT platelets, this process was significantly reduced in Cotl1−/− platelets (thrombin: *P<0.05; CRP: **P<0.01) (Figure 3B and C). Together, these results demonstrated that Cotl1 directly influences 5-LO activity, ultimately resulting in reduced biosynthesis and, subsequently, release of LT from Cotl1−/− platelets.
Defective shear-dependent thrombus formation in Cotl1-deficient mice is rescued by exogenous addition of leukotriene B4
We next investigated whether the reduced release of lipid mediators in Cotl1−/− mice contributed to defective platelet aggregate formation of Cotl1−/− platelets under flow. We decided to focus on LTB4, one of the end products of the LT biosynthesis pathway downstream of LTA4, which was shown to stimulate neutrophil chemotaxis33 and activation.34 First, we tested whether LTB4 was able to directly induce platelet activation. As LTB4 was described to induce neutrophil aggregation and degranulation at concentrations of 0.1 μM35,36 and leukocyte aggregation, chemotaxis and chemokinesis at a subnanomolar range of 0.39 nM,35 we used concentrations of 0.025–250 nM LTB4 (Cayman Chemicals) in our assays. None of the tested LTB4 concentrations induced platelet activation under static conditions in vitro (Online Supplementary Figures S9A and S10A). Likewise, LTB4 addition did not further enhance integrin αIIbβ3 activation, degranulation or aggregation of WT or Cotl1−/− platelets (Online Supplementary Figures S9B and S10B).
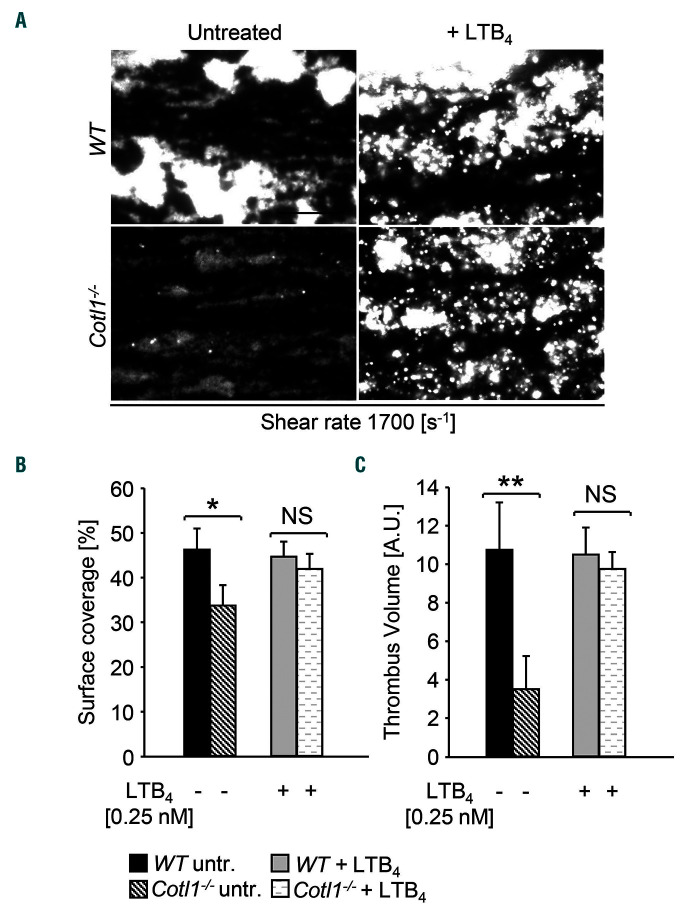
Using the in vitro flow adhesion assay (Figure 2A–C), we next investigated the effect of LTB4 on platelet aggregate formation under flow. Adding concentrations of 2.5 nM or higher interfered with aggregate formation (1,700s−1) in WT blood, whereas lower concentrations had no effect (Online Supplementary Figure S11A and B). Strikingly, pre-incubation with 0.25 nM LTB4 could fully restore aggregate formation of Cotl1−/− platelets to WT levels (Figure 4A and B), indicating that the defect observed in untreated Cotl1−/− samples was caused by impaired platelet-derived LTB4 production. To investigate whether LTB4 has a more general function in this context, we analyzed two additional knockout mouse lines with a described defect in the flow adhesion assay: Grb2fl/fl;Pf4Cre (Grb2−/−) mice have normal platelet counts but the platelets display a selective GPVI/ITAM activation defect.37 RhoAfl/fl;Pf4Cre (RhoA−/−) mice are thrombocytopenic and deficient platelets display impaired G protein coupled receptor (GPCR) signaling.38 Notably, the addition of LTB4 to anticoagulated blood from Grb2−/− mice resulted in moderately increased platelet surface coverage (P=0.06) as compared to untreated samples, but could not restore aggregate formation to WT levels (Online Supplementary Figure S12). Furthermore, LTB4 addition had no effect on aggregate formation in platelet-count adjusted blood of RhoA−/− mice (Online Supplementary Figure S13). Together, these findings demonstrated that addition of LTB4 cannot compensate for defective platelet aggregate formation under flow in the presence of prominent platelet activation/secretion defects. At the same time, our results emphasize that reduced LTB4 production in Cotl1−/− platelets, which display no obvious activation defect per se, significantly contributed to the impaired aggregate formation in the presence of shear.
Figure 4.
Defective shear-dependent thrombus formation in Cotl1-deficient mice can be rescued by exogenous addition of leukotriene B4. (A–C) Assessment of platelet adhesion (A and B) and aggregate formation in heparinized blood (A and C) on Horm collagen (70 μg mL−1) under flow (1700 s−1). WT and Cotl1−/− samples were either left untreated or were pre-incubated for 5 minutes (min) with LTB4 [0.25 nM]. Images are representatives of at least 12 mice per group. Values are mean±standard deviation. Scale bar, 50 μm. *P<0.1; **P<0.01.
Cotl1 modulates thrombosis and hemostasis
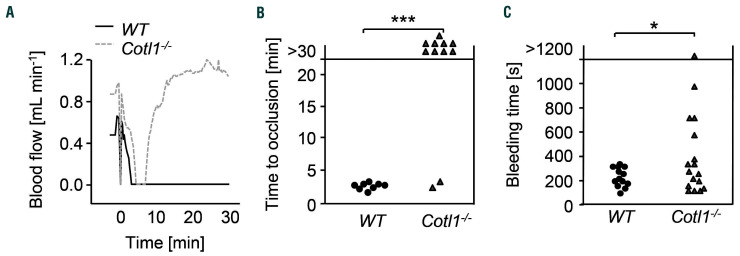
To investigate whether the impaired shear-dependent aggregate formation translated into a phenotype in vivo, we subjected Cotl1−/− mice to an experimental model of arterial thrombosis. Since it is well documented that collagen is a main driver of thrombus formation in bigger vessels, particularly in models of mechanical injury, we chose a model where the abdominal aorta is mechanically injured. This procedure triggers rapid platelet adhesion to the injured vessel wall, followed by the development of a large occlusive thrombus associated with dynamic changes in both shear and biomechanical forces acting on adhering platelets in the growing thrombus. Strikingly, Cotl1−/− mice were profoundly protected from occlusive thrombus formation in this model (Figure 5A and B). In WT mice, irreversible vessel occlusion was observed within 7 minutes (min) after injury (mean occlusion time 3.37±0.72 min). In sharp contrast, while a progressive reduction in blood flow occurred during the first minutes after injury in Cotl1−/− mice, indicative of beginning thrombus formation and increasing stenosis, blood flow afterwards normalized and 9 of 11 mice displayed normal blood flow through the injured vessel at the end of the observation period (30 min) (***P<0.001). These results demonstrated that Cotl1 is essential for occlusive arterial thrombus formation in vivo.
Figure 5.
Cotl1 modulates thrombosis and hemostasis. (A and B) Intravital thrombosis model. (A) Representative graph of blood flow of one WT and one Cotl1−/−mouse after mechanical injury of the abdominal aorta. (B) Occlusion times after mechanical injury of the abdominal aorta. Data are mean±standard deviation of at least eight mice per group. (C) Tail bleeding times in WT and Cotl1−/− mice (filter paper method). Each symbol represents one individual. Unpaired Student t-test: ***P<0.001; *P<0.05.
To assess the hemostatic function of Cotl1−/− platelets, we performed a tail bleeding assay. Notably, while tail bleeding times were overall significantly increased in Cotl1−/− mice (7.8±12.2 min in Cotl1−/− mice vs. 3.3±1.8 min in WT; *P<0.05) the hemostatic defect was rather mild given the profound protection in the arterial thrombosis model (Figure 5C), indicating that Cotl1 may be particularly important in settings of pathological thrombus formation.
Discussion
Here, we demonstrate for the first time that the small ADF-H-domain-containing actin-binding protein Cotl1 has entirely different functions compared to the other protein family members ADF/n-cofilin and Twf, at least in platelets. Cotl1 deficiency neither had an impact on thrombopoiesis or platelet function under static conditions in vitro, nor did it obviously affect actin reorganization. Strikingly, we could reveal a critical role of Cotl1 for stable thrombus formation under conditions of shear in vitro and in vivo. Our results point to two distinct and so far undescribed roles of Cotl1 in this process. On the one hand, the F-actin binding function of the protein is required for proper GPIb function and possibly shear-induced biomechanical signaling. On the other hand, the 5-LO enzyme-modulating function of Cotl1 promotes the biosynthesis of LT, which positively modulate thrombus formation.
The crucial role of actin cytoskeletal rearrangements for platelet formation and reactivity has been demonstrated in a number of studies.5,7,8,10,11,39 While we have previously shown that lack of either Twf2a or n-cofilin in the MK lineage results in thrombocytopenia and distinct platelet function defects,10,11 deficiency of Cotl1 did not affect circulating platelet counts. This may be explained by the distinct actin-binding properties and biological activities of each ADF-H member, which can be attributed to their different domain structure.12 Hence, n-cofilin deficiency decreased stimulus-dependent F-actin assembly, whereas on the contrary, Twf2a-deficient mice displayed enhanced actin dynamics.10,11 Notably, we observed strongly elevated levels of phosphorylated (inactive) n-cofilin in Cotl1-deficient platelets. This finding was unexpected given that, in T cells, Cotl1 was shown to be required for spreading at the immune synapse by protecting F-actin from n-cofilin-mediated severing,14 which would suggest enhanced rather than reduced n-cofilin activity in the absence of Cotl1. We still cannot explain this apparent discrepancy but can exclude the possibility that it was caused by a direct compensation by another ADF-H protein member since expression of Twf1/2a and n-cofilin were unaltered in Cotl1−/− compared to WT platelets.
Over the past few years, greater attention has been given to the critical influence of blood rheology and its dynamical changes on platelet adhesion and thrombus growth, including the relevance of mechanotransduction-based signaling in vivo. Best studied in this context is the platelet mechanoreceptor GPIb which plays a pivotal role for platelet adhesion, as well as thrombus formation at high shear.24 We observed that the reduced aggregate formation of Cotl1−/− platelets on collagen under flow in vitro was most pronounced at high shear rates, where GPIb becomes increasingly important. Consistently, GPIb-mediated adhesion of Cotl1−/− platelets to vWF was significantly reduced. Together, this indicates an involvement of Cotl1 in basic GPIb-mediated platelet responses.
The cytoplasmic domain of the GPIbα subunit is tightly linked to the actin cytoskeleton. This interaction is critical for the correct localization of GPIb in the plasma membrane40 and probably also enables mechanotransduction upon binding of GPIb to its ligand vWF at high shear rates. To study whether the actin-regulating function of Cotl1 in platelets may be specifically critical under shear conditions, we used a novel, quite general, approach to characterize platelet biomechanical properties by assessing their shear-induced deformability using RT-DC.27 This assay has the advantage that, in contrast to other experimental approaches, the biomechanical function of a high cell number can be readily analyzed, and this increases the reliability of the results. Despite not detecting defects in actin assembly under static conditions, strikingly, we observed higher deformability of Cotl1-deficient platelets in RT-DC measurements. Our results, therefore, clearly show that biomechanical properties are significantly altered in Cotl1-deficient platelets, which may have a substantial influence on their function in vivo, possibly also affecting signaling of the mechanoreceptor GPIb.
Besides its interaction with F-actin, Cotl1 is a binding partner of 5-LO, the key enzyme in LT biosynthesis,15 which is expressed in immune competent cells and platelets. LT are a group of inflammatory mediators derived from AA. Upon activation, intracellular Ca2+ levels increase, free AA is liberated from membrane phospho-lipids by phospholipases, and 5-LO is activated, leading to the generation of intermediate LTA4 and subsequently the production of the different LT types (Online Supplementary Figure S7).35 Besides the cysteinyl (cysLT) LT (LTC4, LTC4, LTE4), these also include LTB4, which stimulates neutrophil chemotaxis,33 enhances neutrophil-endothelial interactions,41 and stimulates neutrophil activation, leading to degranulation and the release of mediators, enzymes, and superoxides.34 LTB4 can also act on other cell types, e.g. by increasing interleukin (IL)-6 production by human monocytes.42 Platelet-derived LT were shown to contribute to inflammatory responses, e.g. during acute inflammation via activation of leukocytes,18,43 but only a few very early in vitro studies indicated an impact of LT directly on platelet aggregation.18,43
A recent comprehensive analysis of the platelet lipidome by Peng et al. revealed that the AA/5-LO/LT pathway is significantly induced by platelet activation.44 Therefore, to directly assess whether lack of Cotl1 downregulates 5-LO activity and hence LT biosynthesis, we characterized platelet lipid mediator levels using mass spectrometry.44 Our data confirm previous findings from other cell types that Cotl1 positively regulates 5-LO,17 as lack of Cotl1 induced a shift from LT to prostaglandin biosynthesis downstream from AA, leading to reduced levels of LTA4 and LTB4, but higher levels of TxB2 in the knock-out platelets. Interestingly, CRP (but not thrombin) was able to induce significant LTB4 release in WT platelets. This is in line with findings by Peng et al. who observed that CRP, but not thrombin alone, was able to induce significant changes in the platelet lipidome.44 Thus, our detailed study further shows that the AA/LTB4 pathway is induced by GPVI/ITAM rather than GPCR signaling in platelets.
Strikingly, our results indicate that exogenous addition of LTB4 could fully rescue the defective aggregate formation of Cotl1-deficient platelets on collagen under flow in vitro (Figure 4). While this finding indicates that the exogenous addition of LT can compensate for the GPIb function defect in Cotl1-deficient platelets, we cannot exclude that GPIb signaling itself is involved in LT biosynthesis. Notably, exogenous addition of LTB4 did not restore aggregate formation of RhoA- or Grb2-deficient platelets, which per se display significant activation/ secretion defects. These results show that LTB4 secretion is required to fine-tune platelet function under flow rather than being a strong positive regulator of thrombus formation. However, LTB4 addition could moderately increase aggregate formation of Grb2-deficient platelets, which display defective GPVI/ITAM signaling. This further suggests that LTB4 is particularly relevant for platelet aggregate formation induced through the GPIb/GPVI/ITAM axis. It will be important to dissect the detailed signaling mechanisms leading to LT generation, as well as the precise role of LT and their signaling pathways for platelet thrombus formation in vivo in future studies.
Taken together, our study reveals that Cotl1 modulates biomechanical properties of platelets and acts as a signaling integrator in thrombotic processes. Given that both GPIb and LT represent potential therapeutic targets for a number of thrombo-inflammatory and autoimmune diseases, our findings may contribute to a better understanding of the molecular pathways orchestrating these processes.
Supplementary Material
Acknowledgments
We thank Stefanie Hartmann for excellent technical assistance and the microscopy platform of the Bioimaging Center (Rudolf Virchow Centre) for providing technical infrastructure and support. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (NI 556/11-2 to BN and project number 374031971 – TRR 240). MB is supported by an Emmy Noether grant of the DFG (BE5084/3-1). OO gratefully acknowledges support from the German Ministry of Education and Research (ZIK grant to OO under grant agreement 03Z22CN11). RA received further support from the Federal Ministry of Education and Research (BMBF) in the German Network Bioinformatics Infrastructure (de.NBI) initiative (grants n. 031L0108A and n. 031 A 534B).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/6/1667
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1(7):1602–1612. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Deng W, Zhou L, et al. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood. 2015;125(3):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stritt S, Nurden P, Turro E, et al. A gain-of-function variant in <em>DIAPH1</em> causes dominant macrothrombocytopenia and hearing loss. Blood. 2016;127(23):2903–2914. [DOI] [PubMed] [Google Scholar]
- 5.Nurden P, Debili N, Coupry I, et al. Thrombocytopenia resulting from mutations in filamin A can be expressed as an isolated syndrome. Blood. 2011;118(22):5928–5937. [DOI] [PubMed] [Google Scholar]
- 6.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10(3):182–192. [DOI] [PubMed] [Google Scholar]
- 7.Kunishima S, Okuno Y, Yoshida K, et al. ACTN1 mutations cause congenital macrothrombocytopenia. Am J Hum Genet. 2013;92(3):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleines I, Woods J, Chappaz S, et al. Mutations in tropomyosin 4 underlie a rare form of human macrothrombocytopenia. J Cin Invest. 2017;127(3):814–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118(6):1421–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender M, Eckly A, Hartwig JH, et al. ADF/n-cofilin-dependent actin turnover determines platelet formation and sizing. Blood. 2010;116(10):1767–1775. [DOI] [PubMed] [Google Scholar]
- 11.Stritt S, Beck S, Becker IC, et al. Twinfilin 2a regulates platelet reactivity and turnover in mice. Blood. 2017;130(15):1746–1756. [DOI] [PubMed] [Google Scholar]
- 12.Hellman M, Paavilainen VO, Naumanen P, Lappalainen P, Annila A, Permi P. Solution structure of coactosin reveals structural homology to ADF/cofilin family proteins. FEBS Lett. 2004;576(1–2):91–96. [DOI] [PubMed] [Google Scholar]
- 13.Provost P, Doucet J, Stock A, Gerisch G, Samuelsson B, Rådmark O. Coactosin-like protein, a human F-actin-binding protein: critical role of lysine-75. Biochem J. 2001;359(Pt 2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Shapiro MJ, Bamidele AO, et al. Coactosin-Like 1 Antagonizes Cofilin to Promote Lamellipodial Protrusion at the Immune Synapse. PLoS One. 2014;9(1): e85090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provost P, Doucet J, Hammarberg T, Gerisch G, Samuelsson B, Rådmark O. 5-Lipoxygenase Interacts with Coactosin-like Protein. J Biol Chem. 2001;276(19):16520–16527. [DOI] [PubMed] [Google Scholar]
- 16.Esser J, Rakonjac M, Hofmann B, et al. Coactosin-like protein functions as a stabilizing chaperone for 5-lipoxygenase: role of tryptophan 102. Biochem J. 2010;425(1):265–274. [DOI] [PubMed] [Google Scholar]
- 17.Rakonjac M, Fischer L, Provost P, et al. Coactosin-like protein supports 5-lipoxyge-nase enzyme activity and up-regulates leukotriene A(4) production. Proc Natl Acad Sci U S A. 2006;103(35):13150–13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evangelista V, Celardo A, Dell’Elba G, et al. Platelet contribution to leukotriene production in inflammation: in vivo evidence in the rabbit. Thromb Haemost. 1999;81(3):442–448. [PubMed] [Google Scholar]
- 19.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. <em>Pf4-Cre</em> transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503–1506. [DOI] [PubMed] [Google Scholar]
- 20.Burkhart JM, Vaudel M, Gambaryan S, et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–82. [DOI] [PubMed] [Google Scholar]
- 21.Zeiler M, Moser M, Mann M. Copy number analysis of the murine platelet proteome spanning the complete abundance range. Mol Cell Proteomics. 2014;13(12):3435–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provost P, Doucet J, Stock A, Gerisch G, Samuelsson B, Rådmark O. Coactosin-like protein, a human F-actin-binding protein: critical role of lysine-75. Biochem J. 2001;359(Pt 2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spindler M, van Eeuwijk JMM, Schurr Y, et al. ADAP deficiency impairs megakaryocyte polarization with ectopic proplatelet release and causes microthrombocytopenia. Blood. 2018;132(6):635–646. [DOI] [PubMed] [Google Scholar]
- 24.Turitto VT, Weiss HJ, Baumgartner HR. The effect of shear rate on platelet interaction with subendothelium exposed to citrated human blood. Microvasc Res. 1980;19(3):352–365. [DOI] [PubMed] [Google Scholar]
- 25.Feghhi S, Munday AD, Tooley WW, et al. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophys J. 2016;111(3):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen CE, Qiu Y, McCarty OJT, Lam WA. Platelet Mechanotransduction. Ann Rev Biomed Eng. 2018;20(1):253–275. [DOI] [PubMed] [Google Scholar]
- 27.Otto O, Rosendahl P, Mietke A, et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat Met. 2015;12(3):199–202. [DOI] [PubMed] [Google Scholar]
- 28.Provost P, Samuelsson B, Rådmark O. Interaction of 5-lipoxygenase with cellular proteins. Proc Natl Acad Sci U S A. 1999;96(5):1881–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuelsson B, Dahlen S, Lindgren J, Rouzer C, Serhan C. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–1176. [DOI] [PubMed] [Google Scholar]
- 30.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220(4597):568–575. [DOI] [PubMed] [Google Scholar]
- 31.Hamberg M, Samuelsson B. Detection and Isolation of an Endoperoxide Intermediate in Prostaglandin Biosynthesis. Proc Natl Acad Sci U S A. 1973;70(3):899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nugteren DH, Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta Lipids Lipid Metab. 1973;326(3):448–461. [DOI] [PubMed] [Google Scholar]
- 33.Palmer RMJ, Stepney RJ, Higgs GA, Eakins KE. Chemokinetic activity of arachidonic acid lipoxygenase products on leuocyctes of different species. Prostaglandins. 1980;20(2):411–418. [DOI] [PubMed] [Google Scholar]
- 34.Sha’Afi RI, Naccache PH, Molski TFP, Borgeat P, Goetzl EJ. Cellular regulatory role of leukotriene B4: Its effects on cation homeostasis in rabbit neutrophils. J Cell Physiol. 1981;108(3):401–408. [DOI] [PubMed] [Google Scholar]
- 35.Ford-Hutchinson AW. Leukotriene B4 in inflammation. Crit Rev Immunol. 1990; 10(1):1–12. [PubMed] [Google Scholar]
- 36.McMillan RM, Foster SJ. Leukotriene B4 and inflammatory disease. Agents Actions. 1988;24(1):114–119. [DOI] [PubMed] [Google Scholar]
- 37.Dutting S, Vogtle T, Morowski M, et al. Growth factor receptor-bound protein 2 contributes to (hem)immunoreceptor tyrosine-based activation motif-mediated signaling in platelets. Circ Res. 2014;114(3):444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pleines I, Hagedorn I, Gupta S, et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2012;119(4):1054–1063. [DOI] [PubMed] [Google Scholar]
- 39.Bender M, Stritt S, Nurden P, et al. Megakaryocyte-specific Profilin1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nat Commun. 2014;5(4746. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura F, Pudas R, Heikkinen O, et al. The structure of the GPIb–filamin A complex. Blood. 2006;107(5):1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoover RL, Karnovsky MJ, Austen KF, Corey EJ, Lewis RA. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984;81(7):2191–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brach MA, De Vos S, Arnold C, Gruß H-J, Mertelsmann R, Herrmann F. Leukotriene B4 transcriptionally activates interleukin-6 expression involving NK-xB and NF-IL6. European J Immunol. 1992;22(10):2705–2711. [DOI] [PubMed] [Google Scholar]
- 43.Maclouf J, de Laclos BF, Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982;79(19):6042–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng B, Geue S, Coman C, et al. Identification of key lipids critical for platelet activation by comprehensive analysis of the platelet lipidome. Blood. 2018;81(7):e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.