The median is the most commonly used parameter to describe a survival curve, but its inability to take into account the final part of the curve is well known (namely, the portion of the curve when residual survival goes below 50%). Although this limitation can be managed through different analytical approaches, the restricted mean survival time (RMST) is recognized as the most efficient.1,2 The median takes into account only the first part of the survival curve, and, when the curve has declined to 50% of residual survival, the median is defined as the time elapsed to reach the event in half of the patients; thus, the median is not influenced by the survival pattern of the final 50% of the patients. In contrast, the RMST examines the entire shape of the survival curve (from time 0 to the last time-point of the follow up) and is determined by measuring the area under the survival curve; whereas several mathematical techniques can be employed for this purpose, these techniques differ only in the mathematical model and associated computational procedure.
In summary, the RMST is advantageous because it captures the presence of a long-term survival plateau, which of course reflects a better prognosis. An original method of calculation, drawn from the field of pharmacokinetics, has markedly simplified the otherwise complex estimation of RMST.3,4
In the present analysis, we assessed the RMST from the progression-free survival curve of the patients with non-Hodgkin lymphoma enrolled in the phase II one-arm ZUMA-1 trial in which axicabtagene ciloleucel was evaluated.5 Our objective was simply to compare the RMST with the median reported by the ZUMA-1 investigators and to determine the ratio between these two parameters with reference to this CAR-T therapy. Details about the software used for RMST calculation (WebPlotDigitizer by Automeris) can be found in our previous references.3,4
The patient group treated with axicabtagene ciloleucel consisted of 108 patients. Their inclusion criteria were diffuse large B-cell lymphoma (n = 84) or transformed follicular lymphoma/primary mediastinal B-cell lymphoma (n=24), refractory disease, or relapsed after autologous stem-cell transplantation; an Eastern Cooperative Oncology Group performance status of 0 or 1; and had previously received an anti-CD20 monoclonal antibody containing-regimen and an anthracycline-containing chemotherapy.
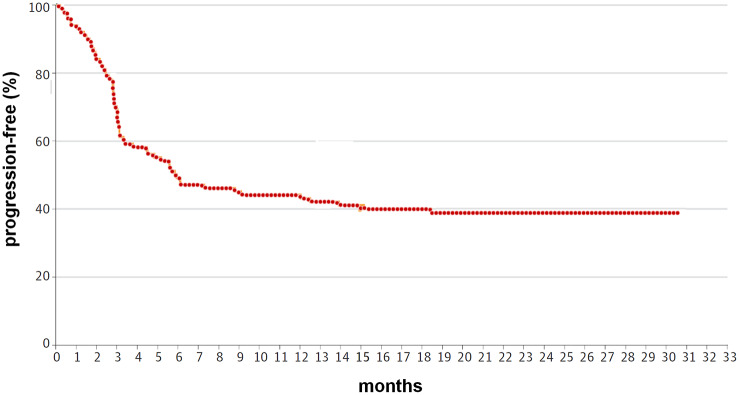
According to our analysis, the value of RMST in the progression-free survival curve (Figure 1) was 14.29 months (“milestone” set at 30 months of follow up). The corresponding median published in the ZUMA-1 trial was 5.9 months. Hence, the ratio RMST/median was 2.42. The milestone is the time-point in the follow up at which the area under the survival curve is truncated.
Figure 1.
Progression-free survival curve in the ZUMA-1 trial: the value of RMST was estimated by model independent methods.
RMST, restricted mean survival time.
The further follow up in the ZUMA-1 trial, which is eagerly awaited, will allow us to set a milestone at more than 30 months and to re-determine the RMST. The obvious hypothesis is that, with a longer follow up, the ratio of RMST/median could be increased to a greater degree. Likewise, the RMST method will hopefully be used once the ZUMA-7 (randomized clinical of axicabtagene ciloleucel versus salvage chemotherapy/transplant) is published or presented. The trial, based on PFS as primary end-point, has already completed enrolment [ClinicalTrials.gov identifier: NCT03391466].
In conclusion, the present analysis confirms the usefulness of the RMST in handling survival curves, particularly when the final portion of the curve shows a plateau. The main advantage consists of providing a numerical estimate of survival that captures the presence of the plateau. Hence, the RMST describes the progression-free survival curve of this CAR-T better than the median. On the other hand, the main disadvantage of the RMST is that the statistical techniques that handle this parameter still require a better standardization of the computational approach.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Andrea Messori, HTA Unit, Regional Health Service, Via San Salvi 12, Firenze, 50135, Italy.
Marco Chiumente, Scientific Direction, Italian Society for Clinical Pharmacy and Therapeutics, Milano, Italy.
Daniele Mengato, Department of Hospital Pharmacy, Bolzano Central Hospital, Bolzano, Italy.
References
- 1. Trinquart L, Jacot J, Conner SC, et al. Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials. J Clin Oncol 2016; 34: 1813–1819. [DOI] [PubMed] [Google Scholar]
- 2. McCaw ZR, Yin G, Wei LJ. Using the restricted mean survival time difference as an alternative to the hazard ratio for analyzing clinical cardiovascular studies. Circulation 2019; 140: 1366–1368. [DOI] [PubMed] [Google Scholar]
- 3. Damuzzo V, Agnoletto L, Leonardi L, et al. Analysis of survival curves: statistical methods accounting for the presence of long-term survivors. Front Oncol 2019; 9: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Messori A, Damuzzo V, Agnoletto L, et al. A model-independent method to determine restricted mean survival time in the analysis of survival curves. SN Compr Clin Med. Epub ahead of print 5 December 2019. DOI: 10.1007/s42399-019-00199-7. [DOI] [Google Scholar]
- 5. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]