Abstract
Background.
There are numerous medicinal plants including the leaves of Grewia ferruginea used as traditional medicine for the treatment of constipation. This study was conducted to evaluate the laxative activity of the leaves of G ferruginea.
Methods.
The laxative activity of the leaves of G ferruginea was tested using 3 models: laxative activity, gastrointestinal motility, and gastrointestinal secretion tests. The effect of the plant extract on mean number of feces, fecal water content, ratio of intestinal distance traveled by the charcoal meal and intestinal fluid accumulation were evaluated and analyzed.
Results.
Significant increase was observed in the mean weight of wet fecal matter at 200 (1.00 ± 0.03 g, P < .05) and 400 mg/kg (1.01 ± 0.02 g, P < .01), relative to loperamide constipated negative control group. Similarly, percent fecal water content was significantly improved in extract treated groups at 100 mg/kg (52.10% ± 2.04%, P < .05), 200 mg/kg (54.02% ± 2.15%, P < .01), and 400 mg/kg (54.25% ± 2.50%, P < .01) compared with the negative control group. The gastroinestinal transit ratio was also significantly increased with 200 mg/kg (P < .01) and 400 mg/kg (P < .001) of the extract relative to the constipated negative control. The crude extract showed significant increase in intestinal fluid accumulation at 200 mg/kg (0.48 ± 0.07 g, P < .05) and 400 mg/kg (0.51 ± 0.08 g, P < .01) compared with the negative control.
Conclusion.
The results of the present study indicated that 80% methanol extract of the leaves of G ferruginea possessed significant laxative activity. As such, this study corroborates the traditional claim of using G ferruginea in the treatment of constipation.
Keywords: laxative activity, constipation, Grewia ferruginea, mice
Constipation is a common bowel problem with symptoms, including difficulty or infrequent passage of stool, hardness of stool or a feeling of incomplete stool evacuation and often associated with pain.1,2 According to Rome III criteria, constipation is diagnosed if loose stools rarely present and 2 or more of the following symptoms are seen at least for 3 months: straining, lumpy or hard stool, sensation of incomplete evacuation, and anorectal obstruction or blockage of defecation and need of manual maneuvers to facilitate in 25% or more of defecation.3 Constipation can be classified into primary and secondary depending on the cause. Primary causes are intrinsic problems of colonic or anorectal function, whereas secondary causes are related to disease or medications use.4
Constipation affects people of all ages, but persons 65 years old or older are most affected. The occurrence of the disease becomes frequent with advancing age, but it should not be considered that it is necessarily a result of advancing age. A systematic review conducted in 2011 reported that worldwide prevalence rates of constipation ranging from 0.7% to 79% among the older population.4–6 Another systematic review and meta-analysis study showed that overall prevalence of chronic idiopathic constipation (CIC) was 14% and higher in women compared with men.7 A survey done in Canada indicated that the rate of constipation for women was close to twice of the rate among men.5 A study in Ethiopia reported that 56% of patients with acute abdominal pain showed symptom of constipation.8 Other similar studies in Ethiopia also revealed that the prevalence of constipation among chat chewers was found to be 48.6% to 80%.9,10
In most cases, constipation is a symptom of other underlying conditions, not a disease by itself. The possible causes of constipation could be disease related like gastrointestinal disorders, endocrine and metabolic disorders and neurological disorders. Drugs having anticholinergic effect, antidepressants, oral iron supplements, aluminum-containing compounds, clonidine, calcium antagonists, and ganglionic blockers, which reduce smooth muscle contractility, can also cause constipation.11–13
Constipation is a predisposing factor to colorectal disorders such as enlarged hemorrhoids and colorectal cancer.14 Chronic constipation significantly compromises health-related quality of life.15 Compared with the general population, patients of constipation are generally with impaired quality of life. The effect of constipation on quality of life is comparable with the more recognized conditions such as osteoarthritis, osteoporosis, and chronic allergies. So the physical consequences and impact of constipation on quality of life should be given due attention.6 The burden of chronic constipation also goes to a loss in work productivity and affects employment and social activities.16
Constipation can be managed by using nonpharmacologic approaches and/or pharmacologic agents. The common nonpharmacologic management includes dietary (fiber and adequate fluid intake), life style modification, and surgery.17 Pharmacologic agents used in the treatment of constipation include drugs such as laxatives, colonic secretagogues, opioid antagonists, and serotonin receptor agonists.18–24 However, studies showed that around 47% of patients are not satisfied with their current constipation treatments reflecting reasons for dissatisfaction such as efficacy related (less effective and inconsistent results), adverse effects and cost concerns.25 As a result, there are number of herbal alternative remedies used for the treatment of constipation around the world. Some of the medicinal plants that are traditionally used for the treatment of constipation include Grewia ferruginea, Plantago ovata (Psyllium), Agave americana, Medicago sativa, Aloe vera, Prunus mallus, Coffea arabica, Cucumis sativus, Cassia angustifolia, Linum usitatissimum, Rhamnus purshiana, and Rhamnus frangula.26–30
According to World Health Organization estimation, about 80% of Africans depend on plant based traditional medicine for their primary health care needs.31 A study showed that approximately 80% of the population in Ethiopia depends on traditional medicines to treat different diseases, including constipation.32 High percentages of useful drugs have been found from plants used in traditional medicine by scientific investigation; so it is a good approach to discover other useful drugs from plants.33
The plant G ferruginea (genus: Grewia, family: Tiliaceae), Lenquata in Amharic, is a wide spread shrub growing mainly in river line forests and near lakes within altitude of 1300 to 2700 m above sea level.34,35 Ethnobotanical and experimental studies on this plant indicated that it has many medicinal values for humans and animals. It has been reported to be used in the treatment of constipation, kidney infection, and intestinal parasite infestation, and for worm expulsion and evacuation of retained fetal membrane in traditional medicine. For instance, the leaf part of G ferruginea was reported to be used in the treatment of constipation.26,36–38 There are also other ethnobotanical documentations showing that the plant is used as soap for hair wash intending to treat dandruff and has antibacterial and antifungal properties.39,40 Therefore, this study was conducted to evaluate the laxative activity of the crude methanolic leaf extract of G ferruginea and to validate the claimed activity of the plant against constipation using scientific approaches. The finding of this study may pave the way for further investigation on the nature of phytochemical constituents responsible for the laxative activity of the plant and the possible mechanisms of action. The finding also can serve as scientific evidence to the traditional healers for the use of the plant in constipation.
Methods
Drugs and Chemicals
Methanol absolute (Loba Chemie), loperamide HCl (Macleods Pharmaceuticals Ltd), castor oil (Amman Pharmaceutical Industries Co), bisacodyl (Remedica), Tween 80 (Blulux Laboratories), and charcoal (Lab Reagents) were the drugs/chemicals used in the study. Additionally, the reagents used in phytochemical screening test included glacial acetic acid (Blulux Laboratories), Mayer’s reagent (Research Lab Fine Chemical Industries), ammonia (Loba Chemie), ferric chloride (Fisher Scientific), sulfuric acid (HiMedia Laboratories), and benzene (Blulux Laboratories).
The Plant Material
Fresh leaves and the aerial part of G ferruginea were collected from Maraki area, North Gondar Zone, northwest Ethiopia in December 2016. Identification and authentication of the plant specimen was done by a botanist at the Department of Biology, College of Natural Sciences, University of Gondar. A voucher number (MY001) has been deposited in Department of Biology, University of Gondar Herbarium Unit for future reference.
Extraction of Plant Material
The leaves of the plant were cleaned by rinsing with tap water and dried under shade at room temperature. After drying, the leaves were crushed to coarse powder using mortar and pestle. Then 900 g of the powder was macerated with 80% methanol in 100 g to 500 mL ratio. The macerate was kept for 72 hours with occasional shaking. Then the extract was filtered using Whatman filter paper (grade 3) and the marc was remacerated and filtered 2 times with the same volume of solvent in each round. The filtrate was concentrated using rotary evaporator and freeze drier to remove methanol and aqueous parts of the solvent, respectively. Then the dried extract was weighed and transferred into vials and kept in refrigerator until used. The extract was reconstituted with 2% Tween 80 suspending agent for oral administration during the experimental procedure.41
Experimental Animals
Swiss albino mice of either sex, weighing 20 to 30 g, were obtained from the Animal House Unit of Ethiopian Health and Nutrition Research Institute (EHNRI), Addis Ababa, Ethiopia. The mice were housed in plastic cages under standard environmental conditions with a 12-hour light and dark cycle. The animals were provided with the standard food and water ad libitum. They were acclimatized to the experimental environment for a period of 7 days before the beginning of the experiment.42
Preliminary Phytochemical Screening Test
The crude extract of the leaves of G ferruginea was tested for the presence of secondary metabolites such as alkaloids, glycosides, tannins, flavonoids, steroids, terpenoids, anthraquinones, saponins, and phenols using standard methods devised.43–46
Acute Oral Toxicity Test
An acute oral toxicity test was conducted, as per OECD 425 guideline, using 2000 mg/kg body weight limit dose of the crude extract in mice.47
Induction of Constipation
Loperamide, 5 mg/kg in 2% Tween 80 once daily, was given orally for 6 days to the mice to be included in constipated negative controls, test groups, or constipated positive controls. The normal control group was treated with 2% Tween 80, 10 mL/kg for the same duration. All animals were allowed to access food and water ad libitum.48,49
Laxative Activity Test in Loperamide Constipated Mice
One group containing 5 mice was used as normal control. The constipated mice were divided into 5 groups, each containing 5 mice and assigned group numbers 2 to 6. Then all groups were treated once daily for 5 days as follows.
Group 1 (normal control): received 10 mL/kg of 2% Tween 80
Group 2 (constipated negative control): received 10 mL/kg of 2% Tween 80
Groups 3, 4, and 5 (test groups): received 100, 200, and 400 mg/kg of crude extract, respectively
Group 6 (constipated positive control): treated with 0.25 mg/kg of bisacodyl
The mice were fasted overnight before they received the fifth dose. Following administration of the fifth dose they were kept in separate cages with nonabsorbable paper. Then fresh fecal pellets/wet feces from individual mice were collected in a 2-hour interval over a 12-hour period and counted and weighed. The fecal matter was then dried for 24 hours at room temperature and reweighed to determine dry fecal weight. Then percent fecal water content was calculated using the following formula.49
Gastrointestinal Motility Test in Loperamide Constipated Mice
The mice were grouped and treated as described above for laxative activity test in loperamide constipated mice. The mice were fasted for 12 hours prior to the fifth dose of Tween 80, the extract, or bisacodyl while free access to water was allowed. After 40 minutes of the fifth dose, each animal received 0.3 mL of freshly prepared 5% aqueous suspension of charcoal meal orally. Then the animals were sacrificed and the small intestine of each was dissected out from the pylorus to caecum after 30 minutes of charcoal meal administration. The ratio of distance traveled by the charcoal meal to the length of small intestine was calculated for each animal as follows.50
Gastrointestinal Secretion Test in Normal Mice
Twenty-five albino mice were fasted for 18 hours and grouped into 5 groups each containing 5 mice. Group 1 (negative control) was treated with 2% Tween 80, 10 mL/kg while group 2 (positive control) was given castor oil 0.5 mL per animal. Groups 3, 4, and 5 (test groups) were given 100, 200, and 400 mg/kg of the crude extract, respectively. After 1 hour of administration, all the animals were sacrificed, and the small intestine of each mouse was cutout from the pylorus to the cecum and weighed immediately. Thereafter, its content was removed by milking and reweighed. Then the weight difference of small intestine before and after milking was calculated for each animal.51
Statistical Analysis
SPSS version 20 was used for statistical analysis. The results were expressed as mean ± standard error of the mean (SEM). A difference between groups compared was determined by 1-way analysis of variance followed by post hoc Tukey’s multiple comparison test. At P < .05, the difference between means was considered as statistically significant.
Results
Phytochemical Screen Tests
The preliminary phytochemical screening test on the plant extract determined the presence of phenols, flavonoids, steroids, saponins, and tannins.
Acute Oral Toxicity
The animals given the limit dose, 2000 mg/kg, of the extract showed no sign of toxicity except hunched posture, erected furs and slight diarrhea during the first 4 hours. Furthermore, no animal died in the 14-day follow up.
Laxative Effect of the Crude Extract in Loperamide Constipated Mice
Loperamide was used to induce constipation and it clearly reduced number, weight, and water content of feces. G ferruginea leaf methanolic extract reduced constipation induced by loperamide and increased the number, weight, and water content of feces. The extract produced significant increase in the mean weight of wet fecal matter at 200 mg/kg (1.00 ± 0.03 g, P < .05) and 400 mg/kg (1.01 ± 0.02 g, P < .01) doses compared with the negative control. Fecal water content was also significantly improved in extract treated groups at 100 mg/kg (52.10% ± 2.04%, P < .05), 200 mg/kg (54.02% ± 2.15%, P < .01), and 400 mg/kg (54.25% ± 2.50%, P < .01) compared with the negative control group (41.77% ± 1.57%). The most significant increase in the wet fecal weight and fecal water content was produced by bisacodyl relative to the negative control. No significant change was produced in the weight of dry fecal matter between groups (Table 1).
Table 1.
Laxative Effect of Grewia ferruginea Methanol Extract in Loperamide-Induced Constipated Mice.*
| Group (treated with) | Dose | No. of feces in 12 h | Weight of wet feces | Weight of dry feces | % Fecal water content |
|---|---|---|---|---|---|
| Group 1 (2% Tween 80) | 10 mL/kg | 9.40 ± 1.29b1 | 0.97 ± 0.02b1 | 0.41 ± 0.02 | 58.18 ± 1.34b3 |
| Group 2 (2% Tween 80) | 10 mL/kg | 3.80 ± 0.80 | 0.76 ± 0.04 | 0.44 ± 0.03 | 41.77 ± 1.57 |
| Group 3 (extract) | 100 mg/kg | 7.80 ± 0.86 | 0.83 ± 0.04 | 0.40 ± 0.03 | 52.10 ± 2.04b |
| Group 4 (extract) | 200 mg/kg | 7.60 ± 1.99 | 1.00 ± 0.03b1 | 0.46 ± 0.02 | 54.02 ± 2.15b2 |
| Group 5 (extract) | 400 mg/kg | 7.00 ± 1.18 | 1.01 ± 0.02 b2 | 0.46 ± 0.02 | 54.25 ± 2.50b2 |
| Group 6 (bisacodyl) | 0.25 mg/kg | 9.00 ± 1.00 | 1.09 ± 0.09 b3c2 | 0.37 ± 0.04 | 66.10 ± 2.76b3c3d2e2 |
* Results expressed as mean ± SEM (n = 5). 1 P < .05, 2 P < .01, 3 P < .001, a: compared to group 1, b: compared to group 2, c: compared to group 3, d: compared to group 4, e: compared to group 5. Group 1 and group 2 animals are normal and constipated controls, respectively, and treated groups are all constipated.
The Effect of the Crude Extract on Gastrointestinal Motility in Loperamide Constipated Mice
The effect of G ferruginea leaf extract on gastrointestinal motility was evaluated by its effect on charcoal meal transit in the small intestine of mice. The plant extract significantly (47.4 ± 2.1 cm, P < .05) accelerated intestinal charcoal meal movement at the dose of 400 mg/kg compared with the negative control group. The gastrointestinal transit ratio was 65.01% ± 2.27%, 74.29% ± 1.31%, and 80.74% ± 3.10% with 100, 200, and 400 mg/kg doses of the extract, respectively, while this parameter was 59.98% ± 1.71% for constipated control group. The gastrointestinal transit ratio was significantly increased with 200 mg/kg (P < .01) and 400 mg/kg (P < .001) of the extract relative to the constipated control. The standard drug (bisacodyl 0.25 mg/kg) most significantly increased the distance traveled by the charcoal meal and gastrointestinal transit ratio relative to the constipated and normal controls and extract treated groups (Table 2).
Table 2.
Effect of Grewia ferruginea Methanol Extract on Gastrointestinal Transit in Loperamide-Induced Constipated Mice.*
| Group | Dose | Distance traveled by charcoal (cm) | Gastrointestinal transit ratio (%) |
|---|---|---|---|
| Group 1 (2% Tween 80) | 10 mL/kg | 46.0 ± 1.2 | 74.68 ± 1.31 |
| Group 2 (2% Tween 80) | 10 mL/kg | 38.1 ± 1.2 | 59.98 ± 1.71 |
| Group 3 (extract) | 100 mg/kg | 42.6 ± 2.3 | 65.01 ± 2.27 |
| Group 4 (extract) | 200 mg/kg | 44.6 ± 1.7 | 74.29 ± 1.31b2 |
| Group 5 (extract) | 400 mg/kg | 47.4 ± 2.1b1 | 80.74 ± 3.10b3c3 |
| Group 6 (bisacodyl) | 0.25 mg/kg | 60.1 ± 2.8a3b3c3d3e2 | 89.97 ± 3.07a3b3c3d3 |
* Results expressed as mean ± SEM (n = 5). 1 P < .05, 2 P < .01, 3 P < .001, a: compared to group 1, b: compared to group 2, c: compared to group 3, d: compared to group 4, e: compared to group 5. Group 1 and group 2 animals are normal and constipated controls, respectively, and treated groups are all constipated.
Gastrointestinal Secretion Effect of the Crude Extract in Normal Mice
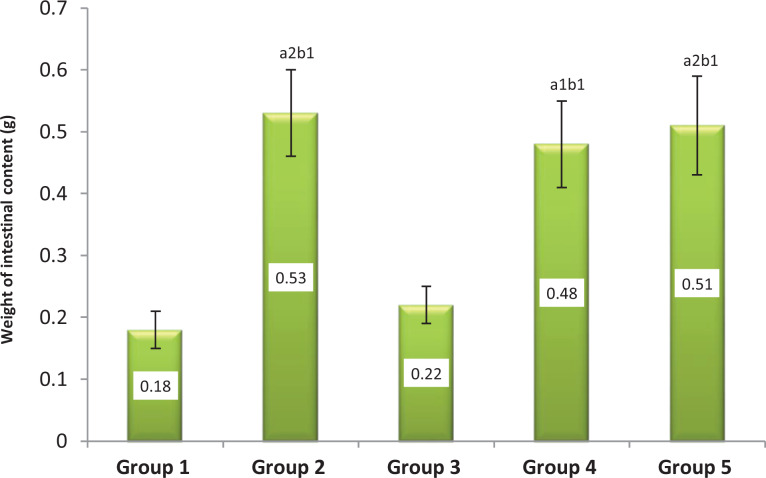
The crude extract showed significant increases in intestinal fluid accumulation at 200 mg/kg (0.48 ± 0.07 g, P < .05) and 400 mg/kg (0.51 ± 0.08 g, P < .01) compared with the negative control. These 2 doses of the extract produced more gastrointestinal fluid accumulation than 100 mg/kg (0.22 ± 0.03 g). Relatively highest mean gastrointestinal fluid accumulation (0.53 ± 0.07 g) was produced by castor oil and this was significant compared with the negative control (P < .01) and 100 mg/kg crude extract treated groups (P < .05) (Figure 1).
Figure 1.
Effect of Grewia ferruginea leaves crude extract on gastrointestinal secretion in normal mice. Results expressed as mean ± SEM (n = 5). 1 P < .05, 2 P < .01, 3 P < .001, a: compared to group 1, b: compared to group 3, c: compared to group 4, d: compared to group 5. Group 1 (10 mL/kg of 2% Tween 80) and group 2 (0.5 mL of castor oil per animal) animals are normal and positive controls, respectively. Group 3, group 4, and group 5 animals received 100, 200, and 400 mg/kg of the plant extract, respectively.
Discussion
A published ethnobotanical study conducted in Ethiopia reported that the leaf part of G ferruginea is traditionally used in the treatment of constipation.26 This report was used as a basis to carry out the present experimental work because there were no earlier studies to support this traditional claim. Accordingly, this study evaluated the laxative activity of 80% methanolic leaf extract of G ferruginea in Swiss albino mice using 3 models. The plant extract was evaluated for its effect on the number of defecations and stool water content, intestinal transit ratio, and intestinal fluid accumulation. The laxative activity of several traditionally used plants was scientifically validated through examining their activities in different animal models. Measuring of stool parameters, intestinal transit ratio, and intestinal fluid accumulation are the most frequently used methods in evaluating laxative activity of medicinal plants. The methods used in this study were in accordance with previously devised methods used in similar studies.52–54
In this study, loperamide was used for induction of constipation (except in intestinal fluid accumulation) because previous literatures indicated that the administration of 2 to 5 mg/kg body weight of loperamide through oral or subcutaneous route for 3 to 7 days can effectively induce constipation in rodents.48,49,55–57 The mice treated with loperamide showed decrease in frequency of defecation, weight of wet feces, and fecal moisture content compared with nonconstipated (normal control) group. These symptoms are manifestations of constipation.58 So increments in these parameters by the plant extract could be considered because of the laxative activity of the plant extract counteracting the constipation induced by loperamide.
In loperamide-induced constipation model, the plant extract produced a significant increase in the mean weight (at 200 and 400 mg/kg) and percent water content of feces (at all doses) compared with the constipated control group. This might indicate the laxative activity of the plant extract, that is, the reversal of constipation produced by loperamide as a result of the treatment by the plant extract. The most significant increase in the wet fecal weight and fecal water content was produced by bisacodyl. This may be due to lower concentrations of the active principles responsible for the laxative activity of the plant extract rather than efficacy problem. As can be seen in Table 1, slight or significant increase in mean fecal weight and fecal water content was observed with increasing dose of the plant extract.
In the gastrointestinal motility test model, the extract was able to increase intestinal motility as revealed by significant increase in the intestinal length traveled by charcoal meal in the highest dose of the extract. Furthermore, the results in this model showed an increasing tendency of the stimulatory effect on the gastrointestinal motility as the dose of the plant extract increased. The significant increase in the gastrointestinal transit ratio value by the middle (P < .01) and highest (P < .001) doses of the extract compared with the constipated negative controls could also indicate that the gastrointestinal motility was increased by the plant extract in dose-dependent manner. An increase in the intestinal motility decreases the stay of intestinal contents in the intestine and this might significantly decrease the time for absorption of water and electrolytes from the small intestine. This may increase the fecal moisture content. The significant increase in the fecal moisture content may in turn attribute to the increase in intestinal motility and hence the laxative activity of the plant extract.
The third model was done to investigate the effect of G ferruginea extract on the small intestinal fluid accumulation.51 The effects of the middle and highest doses of the plant extract on intestinal fluid accumulation were found to be comparable to the effect of the standard drug, castor oil. Castor oil releases active chemical, ricinoleic acid, on metabolism. Ricinoleic acid increases intestinal fluid accumulation through mechanisms such as inhibiting the enzyme Na+, K+ -ATPase, irritating intestinal wall leading to prostaglandin release. It also directly stimulates prostaglandin EP3 receptors. Through these activities, it increases gastrointestinal motility and electrolyte secretion while decreasing intestinal electrolyte absorption.59,60 All of these actions increase overall secretion of water and electrolytes into the bowel. Therefore, the significant increase in the intestinal fluid accumulation by the middle and highest doses of the plant extract may be via mechanisms mediating the effect of ricinoleic acid released from castor oil. In addition, laxative agents that increase water and electrolyte secretion in the intestinal lumen act through facilitating activation or expression of cystic fibrosis transmembrane conductance regulator or aquaporin.19,21 The positive effect of G ferruginea on intestinal fluid accumulation could be also through this mechanism.
In the present study, even though there was no significant difference seen in the frequency of defecation, the crude extract still could be considered having benefit in treating constipation as statistically significant increases in fecal mass and fecal water content were scored at least at the middle and the highest doses compared to constipated control. Increasing in the frequency of defecation alone cannot determine the laxative activity of a given plant extract unless accompanied by increasing fecal mass and moisture. This can be best explained by the fact that some types of constipations are characterized by hard and dry stool with a normal frequency of defecation or small and incomplete evacuation with frequent bowel movement. It has been also shown that constipation is more related to a condition of inability to evacuate stool completely and easily in every toilet rather than to decreasing in the number of bowel movements.4,61
The presence of phytochemicals like flavonoids, saponins, tannins, sterols, terpenoids, alkaloids, phenolic compounds in different plant extracts were reported to be responsible for stimulant, laxative, and gastrointestinal propulsive activities of the plants.62 Saponins have been reported to have smooth muscle contraction effect.63 Some types of flavonoids like naringenin and apigenin shown to increase Cl− secretion in colonic epithelia of loperamide induced constipated animals and have a good laxative profile.64 Qualitative phytochemical screening test on methanol extract of the leaves of G ferruginea exhibited the presence of phenols, flavonoids, steroids, saponins, and tannins. Therefore, the laxative activity of the plant extract may be attributed to the presence of some of these chemical constituents.
Conclusion
The findings of the present study indicated that 80% methanol extract of the leaves of G ferruginea possessed significant in vivo laxative activity. The extract showed increasing of stool weight and fecal water content, gastrointestinal motility, and intestinal fluid accumulation in mice. Even though the specific molecular mechanism of action remains undetermined, it could be concluded that the general mechanism of actions may be either by increasing gastrointestinal motility or secretion or both. As such, the current study corroborates the traditional claim of using G ferruginea in the treatment of constipation.
Supplemental Material
Supplemental Material, Manuscript_data for Laxative Activities of 80% Methanolic Extract of the Leaves of Grewia ferruginea Hochst Ex A Rich in Mice by Mulusew Yemiru Tessema, Zewdu Birhanu Wubneh and Assefa Belay Asrie in Journal of Evidence-Based Integrative Medicine
Acknowledgments
We would like to acknowledge Amhara Regional State Health Bureau for some financial support to undertake this study as a thesis in partial fulfillment of the requirements for MSc degree in pharmacology of the first author. We are also thankful to University of Gondar, Department of Pharmacology for allowing us to use the laboratory facility. The authors are also grateful to Mr Zemene Demelash for his technical support during the laboratory activities and Mrs Banchamlak Demamu for caring the laboratory animals used for this study.
Footnotes
Author Contributions: All the authors participated in proposing the title, proposal preparation and writing up of the final report. MYT conducted the laboratory activities, data compilation, and analysis. The manuscript was prepared by ABA. Finally, MYT and ZBW reviewed the manuscript before submission for publication. Moreover, all authors are responsible regarding the originality and correctness of this work.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Amhara Regional State Health Bureau. Some financial support to purchase laboratory chemicals and animals used was received from Amhara Regional State Health Bureau as this work was the thesis for partial fulfillment of requirements for MSc degree in pharmacology for the first author. Amhara Regional State Health Bureau was a sponsor of the MSc study to the first author.
ORCID iD: Zewdu Birhanu Wubneh  https://orcid.org/0000-0002-8187-6199
https://orcid.org/0000-0002-8187-6199
Assefa Belay Asrie  https://orcid.org/0000-0001-5935-643X
https://orcid.org/0000-0001-5935-643X
Ethical Approval: The animals used in this experiment were handled according to a guideline for the care and use of laboratory animals and the ethical clearance was obtained from the Experimental Animals Ethics Committee, Department of Pharmacology, University of Gondar prior to the study.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bharucha AE, Pemberton JH, Locke GR., 3rd American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Day MR. Constipation: epidemiology, assessment and treatment options. Promoting continence and management of incontinence conference Published April 19, 2017. Accessed April 30, 2020 http://www.muh.ie/images/CNE/ConstipationEpidemiology.pdf [Google Scholar]
- 3. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 4. Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol. 2011;25(suppl B):16B–21B. [PMC free article] [PubMed] [Google Scholar]
- 5. Battistella M, Alibhai SMH. Evaluation and treatment of constipation. Geriatr Aging. 2002;5:23–27. [Google Scholar]
- 6. Norgine. The Burden of Constipation in our Ageing Population. A report developed by the International Longevity Centre-UK (ILC-UK) and Norgine in consultation with a European expert working group. August 2013. Accessed April 30, 2020 https://ilcuk.org.uk/wp-content/uploads/2018/11/Burden-of-constipation-report.pdf
- 7. Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. [DOI] [PubMed] [Google Scholar]
- 8. Kotiso B, Abdurahman Z. Pattern of acute abdomen in adult patients in Tikur Anbesa Teaching Hospital, Addis Ababa, Ethiopia. ECAJS. 2007;12:47–52. [Google Scholar]
- 9. Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. [DOI] [PubMed] [Google Scholar]
- 10. Teni FS, Surur AS, Hailemariam A, et al. Prevalence, reasons, and perceived effects of Khat chewing among students of a college in Gondar Town, Northwestern Ethiopia: a cross-sectional study. Ann Med Health Sci Res. 2015;5:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Gastroenterological Association. Understanding constipation. A brochure produced by the AGA Institute and supported by grants from Forest Laboratories, Inc. and Ironwood Pharmaceuticals, Inc. https://gitract.mngastro.com.
- 12. Luca G, Domenico P, Caterina P, De Sarro G. Constipation treatment in neurological disorders. In: Catto-Smith A, ed. Constipation—Causes, Diagnosis and Treatment. InTech; 2012:99–116. Accessed April 30, 2020 https://pdfs.semanticscholar.org/b3c3/6203ba2380fe6ce8a1aee1cb1e0fb4be085f.pdf [Google Scholar]
- 13. Fosnes GS, Lydersen S, Farup PG. Drugs and constipation in elderly in nursing homes: What is the relation? Gastroenterol Res Pract. 2012; 2012:290231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Got a Gut Feeling Your Gut is Out of Whack. Is constipation dangerous for my health. Accessed April 30, 2020. https://www.gutsense.org/constipation/danger.html
- 15. Sun SX, DiBonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011; 56:2688–2695. [DOI] [PubMed] [Google Scholar]
- 16. Pinto Sanchez MI, Bercik P. Epidemiology and burden of chronic constipation. Can J Gastroenterol. 2011;25(suppl B):11B–15B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao SS. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastrointest Endosc Clin N Am. 2009;19:117–139. [DOI] [PubMed] [Google Scholar]
- 18. Mounsey A, Raleigh M, Wilson A. Management of constipation in older adults. Am Fam Phys. 2015;92:500–504. [PubMed] [Google Scholar]
- 19. Ikarashi N, Mochiduki T, Takasaki A, et al. A mechanism by which the osmotic laxative magnesium sulphate increases the intestinal aquaporin 3 expression in HT-29 cells. Life Sci. 2011;88:194–200. [DOI] [PubMed] [Google Scholar]
- 20. Beubler E, Juan H. Effect of ricinoleic acid and other laxatives on net water flux and prostaglandin E release by rat colon. J Pharm Pharmacol. 1979;31:681–685. [DOI] [PubMed] [Google Scholar]
- 21. Roque MV, Camilleri M. Linaclotide, a synthetic guanylate cyclase C agonist, for the treatment of functional gastrointestinal disorders associated with constipation. Expert Rev Gastroenterol Hepatol. 2011;5:301–310. [DOI] [PubMed] [Google Scholar]
- 22. Lee MJ, Choi S, Im W. 5-HT4 receptor agonists in the treatment of gastrointestinal motility disorders: current status and perspective. Int J Gastroenterol Disord Ther. 2014;1:1–8. [Google Scholar]
- 23. Nee J, Zakari M, Sugarman MA, et al. Efficacy of treatments for opioid-induced constipation: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1569–1584. [DOI] [PubMed] [Google Scholar]
- 24. Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. [DOI] [PubMed] [Google Scholar]
- 25. Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- 26. Gebeyehu G, Asfaw Z, Enyew A, Raja N. Ethnobotanical study of traditional medicinal plants and their conservation status in Mecha Woreda, West Gojjam Zone of Ethiopia. Int J Pharm Health Care Res. 2014;2:137–154. [Google Scholar]
- 27. Monograph. Plantagoovata (Psyllium). Altern Med Rev. 2002;7:155–159.11991795 [Google Scholar]
- 28. Rawat A, Srivastava S, Ojha SK. Herbal remedies for management of constipation and its ayurvedic perspectives. JIMSA. 2012;25:27–30. [Google Scholar]
- 29. University of Rochester Medical Center. Health Encyclopedia. Cascara Sagrada. https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=19&contented=CascaraSagrada. Accessed April 30, 2020.
- 30. European Medicines Agency. Evaluation of medicines for human use. Assessment report on Rhamnus frangula L. cortex. Accessed April 30, 2020 http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_HMPC_assessment_report/2009/12/WC500018613.pdf
- 31. WHO Regional Office for Africa. African Traditional Medicine Day. The African Health Monitor. Special Issue August, 2010.
- 32. Regassa R. Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. J Med Plants Res. 2013;7:517–535. [Google Scholar]
- 33. World Agroforestry Centre. Useful Trees and Shrubs of Ethiopia. Grewia ferruginea. Accessed April 30, 2020 http://www.worldagroforestry.org/usefultrees/pdflib/Grewia_ferruginea_ETH.pdf
- 34. Tesemma AB. Useful trees and shrubs of Ethiopia: identification, propagation, and management for 17 agroclimatic zones. Accessed April 30, 2020 http://www.worldagroforestry.org/publication/useful-trees-and-shrubs-ethiopia-identification-propagation-and-management-17
- 35. Ocho DL, Struik PC, Price LL, Kelbessa E, Kolo K. Assessing the levels of food shortage using the traffic light metaphor by analyzing the gathering and consumption of wild food plants, crop parts and crop residues in Konso, Ethiopia. J Ethnobiol Ethnomed. 2012;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belayneh A, Asfaw Z, Demissew S, Bussa NF. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J Ethnobiol Ethnomed. 2012;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wondimu T, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants around “Dheeraa” town, Arsi Zone, Ethiopia. J Ethnopharmacol. 2007;112:152–161. [DOI] [PubMed] [Google Scholar]
- 38. Firaol T, Waktole T, Ejigu K, Gizaw D, Rajeeb KR, Mekonnen S. Ethnoknowledge of plants used in veterinary practicesin Dabo Hana District, West Ethiopia. J Med Plants Res. 2013;7:2960–2971. [Google Scholar]
- 39. Megersa M, Asfaw Z, Kelbessa E, Beyene A, Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka District, East Welega Zone of Oromia Regional State, West Ethiopia. J Ethnobiol Ethnomed. 2013;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sileshi A, Gebre-Mariam T, Asres K. Antibacterial and antifungal activities of extracts of some medicinal plants of Ethiopia. Ethiop Pharmas J. 2007;25:111–120. [Google Scholar]
- 41. Tiwari P, Kumar B, Kaur M, et al. Phytochemical screening and extraction: a review. Int Pharma Sci. 2011;1:98–106. [Google Scholar]
- 42. Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th ed National Academies Press; 2011. [Google Scholar]
- 43. Dhivya R, Manimegalai K. Preliminary phytochemical screening and GC-MS profiling of ethanolic flower extract of Calotropis gigantea Linn. (Apocynaceae). J Pharmacog Phytochem. 2013;2:28–32. [Google Scholar]
- 44. Yadav R, Agarwal M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3:10–14. [Google Scholar]
- 45. Iqbal PJ. Phytochemical screening of certain plant species of Agra city. J Drug Delivery Therap. 2012;2:135–138. [Google Scholar]
- 46. Kumar RS, Venkateshwar C, Samuel G, Rao SG. Phytochemical screening of some compounds from plant leaf extracts of Holoptelea integrifolia (Planch.) and Celestrus emarginata (Grah.) used by Gondu tribes at Adilabad District, Andhra Pradesh, India. IJESI. 2013;2:65–77. http://www.ijesi.org/papers/Vol%202(8)/Version-2/L0282065070.pdf. Accessed April 30, 2020. [Google Scholar]
- 47. OECD. OECD guidelines for the testing of chemicals: acute oral toxicity: up and down procedures. Accessed April 30, 2020 https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf
- 48. Li C, Nie SP, Zhu KX, et al. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 2015;66:533–538. [DOI] [PubMed] [Google Scholar]
- 49. Choi JS, Kim JW, Kim KY, Lee JK, Sohn JH, Ku SK. Synergistic effect of fermented rice extracts on the probiotic and laxative properties of yoghurt in rats with loperamide-induced constipation. Evid Based Complement Alternat Med. 2014;2014:878503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guarize L, Costa JC, Dutra LB, Mendes RF, Lima IV, Scio E. Anti-inflammatory, laxative and intestinal motility effects of Senna macranthera leaves. Nat Prod Res. 2012;26:331–334. [DOI] [PubMed] [Google Scholar]
- 51. Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. Enteropooling assay: a test for diarrhoea produced by prostaglandins. Prostaglandins. 1976;11:809–828. [DOI] [PubMed] [Google Scholar]
- 52. Ajayi CO, Babarimis FF, Elujoba AA. Laxative activities of Cassia sieberiana and Senna obtusifolia . Afr J Tradit Complement Altern Med. 2014;11:44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou M, Jia P, Chen J, et al. Laxative effects of Salecan on normal and two models of experimental constipated mice. BMC Gastroenterol. 2013;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Méité S, Bahi C, Yéo D, Datté JY, Djaman JA, N’guessan DJ. Laxative activities of Mareya micrantha (Benth.) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats. BMC Complement Altern Med. 2010;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013;13:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seo JY, Kim SS, Kim HJ, Liu KH, Lee HY, Kim JS. Laxative effect of peanut sprout extract. Nutr Res Pract. 2013;7:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Steele SR, Mellgren A. Constipation and obstructed defecation. Clin Colon Rectal Surg. 2007;20:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrhoeal activity of 80% methanolic leaf extract of Justicia schimperiana . Evid Based Complement Alternat Med. 2018;2018:3037120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci U S A. 2012;109:9179–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gallegos-Orozco JF, Foxx-Orenstein AE, Sterler SM, Jean Stoa JM. Chronic constipation in the elderly. Am J Gastroenterol. 2012;107:18–25. [DOI] [PubMed] [Google Scholar]
- 62. Otshudi AL, Vercruysse A, Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhoea in Lomela area, Democratic Republic of Congo (DRC). J Ethnopharmacol. 2000;71:411–423. [DOI] [PubMed] [Google Scholar]
- 63. Gilani AH, Khan A, Khan AU, Bashir S, Rehman NU, Mandukhail SU. Pharmacological basis for the medicinal use of Holarrhena antidysenterica in gut motility disorders. Pharm Biol. 2010;48:1240–1246. [DOI] [PubMed] [Google Scholar]
- 64. Yang ZH, Yu HJ, Pan A, et al. Cellular mechanisms underlying the laxative effect of flavonol naringenin on rat constipation model. PLoS One. 2008;3:e3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Manuscript_data for Laxative Activities of 80% Methanolic Extract of the Leaves of Grewia ferruginea Hochst Ex A Rich in Mice by Mulusew Yemiru Tessema, Zewdu Birhanu Wubneh and Assefa Belay Asrie in Journal of Evidence-Based Integrative Medicine