Abstract
Background
Myocardial ischemia-reperfusion injury (IRI) is an important injury mechanism of myocardial infarction. The purpose of this study was to explore the effects of L-carnitine (LC) on myocardial IRI and its mechanism.
Material/Methods
The IRI model was made by ligating the left anterior descending coronary artery. Then, we injected LC intraperitoneally into the rats of the experimental group to assess the effect of LC on IRI rats by use of serum markers, Western blot, and qRT-PCR. H9c2 cells were cultured and then treated with hypoxia-reoxygenation. The effect of LC on oxidative stress, apoptosis, and nuclear transcription-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling pathway of H9c2 cells were detected by Western blot, RT-PCR, and flow cytometry.
Results
LC significantly reduced malondialdehyde (MDA), creatine kinase (CK), and lactate dehydrogenase (LDH) levels in rat myocardial tissue and increased superoxide dismutase (SOD) expression. LC also increased the expression of SOD1/2 and Bcl-2 in rat myocardial tissue and H9c2 cells and decreased the expression of caspase3/8 and Bax. In addition, LC increased the expression of Nrf2/HO-1 signaling pathway-related molecules in H9c2 cells and increased the activity of the Nrf2/HO-1 signaling pathway. Moreover, inhibition of the Nrf2/HO-1 signaling pathway attenuated the protective effect of LC on H9c2 cells.
Conclusions
LC can activate the Nrf2/HO-1 signaling pathway and reduce oxidative stress and apoptosis in cardiomyocytes, thereby reducing myocardial IRI.
MeSH Keywords: Carnitine, Myocardial Ischemia, Oxidative Stress, Reperfusion Injury
Background
The mortality and disability rates of acute myocardial infarction (AMI) are very high, and its global incidence rate is also increasing year by year [1]. At present, the treatment of AMI mainly focuses on rapid opening of the infarcted artery and rapid recovery of myocardial tissue perfusion, thereby saving the dying cardiomyocytes and reducing the infarct size and the incidence of adverse cardiovascular events [2]. However, even if the blood supply is restored in the shortest time possible, since the myocardium undergoes the process of ischemia-reperfusion, the function of the heart cannot be fully recovered, and the dysfunction and damage of the heart will increase, which will cause myocardial ischemia-reperfusion injury (IRI) [3]. The pathological mechanism of IRI is complex, involving oxygen free radicals, calcium overload, neutrophil activation, vascular endothelial cell injury, and apoptosis [4]. Myocardial IRI during cardiac recovery increases myocardial damage and arrhythmia and seriously affects the recovery of cardiac function after ischemia [5]. Therefore, it is very important to find an appropriate treatment for myocardial IRI.
L-carnitine (LC) is a natural substance essential for mammalian energy metabolism and is a kind of physiologically active vitamin. Its main function is to promote lipid metabolism and promote the entry of fatty acids into the mitochondria through β oxidation into the tricarboxylic acid cycle. Lack of LC in the body can cause disorders of fat metabolism and dysfunction of tissues such as heart muscle and skeletal muscle [6]. A study found that LC can increase the activity of reduced nicotinamide adenine dinucleotide (NADH), cytochrome oxidase, and cytochrome C reductase, promote protein degradation, and protect cell membranes [7]. In addition, LC also has an anti-oxidative effect. Mitochondrial membrane damaged by oxygen free radicals interfere with timely repair and anti-oxidation [8]. In addition, some studies have found that LC is highly expressed in the serum of AMI patients and has diagnostic value in determining the extent of AMI [9,10]. However, whether LC plays a protective role in myocardial IRI has not been studied.
Therefore, we used a rat model of myocardial IRI to study the protective effect of LC on rat myocardium and used the H9c2 cardiomyocyte cell line to study the mechanism of LC in cardiomyocytes. Our aim was to improve clinical treatment of myocardial ischemia disease.
Material and Methods
Animals and grouping
A total of 30 male Sprague-Dawley (SD) rats were used. A myocardial ischemia-reperfusion model was established in rats in the IRI group and LC group. Rats in the LC group were given a daily intraperitoneal injection of LC (150 mg/kg, Selleck, Shanghai, China) [11] for 1 month before constructing the model. At the same time, the rats in the IRI group were intraperitoneally injected with the same amount of normal saline daily before constructing the model. This study was approved by the Animal Ethics Committee of Jiamusi University Animal Center.
Operative procedure and treatment
After rats were weighed, they were intraperitoneally injected with anesthesia (10% chloral hydrate, 0.3 mg/100 g). We fixed the rats in supine position on an anatomical plate and made a 1-cm longitudinal incision in the middle of the neck. After blunt dissection of the neck muscles, we cut the trachea between the 3 to 4 annular cartilage of the rat, inserted the tracheal tube, and connected the animal ventilator. The parameters were set to a tidal volume of 5 mL/kg and a respiratory rate of approximately 70–100 breaths/min. We used a fine metal needle to puncture the rat’s limbs and connect the lead wires, and then used the Pclab-530c biomedical signal acquisition and processing system to observe the heart rate rhythm of lead II. After disinfecting the chest with alcohol, we cut the skin about 2–3 cm longitudinally 0.5 cm from the left side of the sternum, cut the 4th and 5th ribs, and gently pierced the pleura. An assistant then used 2 hooks to gently pull the chest tissue away from both sides. After the heart was exposed, we used sutures to ligate the left anterior descending coronary artery. After 30 min of ischemia, we untied the suture and restored blood flow to the left anterior descending coronary artery for 4 h. The ST-segment elevation of the lead II indicates successful modeling. Four hours after the recovery of blood flow, we sacrificed the rats by bleeding the abdominal aorta.
Cell culture and treatment
The rat cardiomyocyte cell line H9c2 was purchased from Shanghai Saibaikang Biotechnology Co. (Shanghai, China). We cultured cells in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and 1% penicillin plus streptomycin (Gibco, Rockville, MD, USA). The cells were cultured in an incubator at 37°C and 5% CO2. ML385 (Selleck, Shanghai, China), a nuclear transcription-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling pathway inhibitor, was used to inhibit the Nrf2/HO-1 signaling pathway in H9c2 cells. The H9c2 cell hypoxia-reoxygenation model was constructed as follows: After the H9c2 cell density reached 90%, we replaced the cell medium with phosphate-buffered saline (PBS). After filling the incubator with 95% N2, we placed the cells in an incubator for 4 h under hypoxia. Then, we removed the PBS buffer and replaced it with DMEM complete medium [12].
Western blot
The total protein of rat myocardial tissue or H9c2 cells was extracted. An appropriate amount of protein buffer was added to the well of electrophoresis gel, followed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein was then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After we blocked the PVDF membranes with 5% skim milk, the PVDF membranes were incubated overnight with a primary antibody (SOD1, 1: 3000, Rabbit; SOD2, 1: 3000, Rabbit; caspase3, 1: 2000, Rabbit; caspase8, 1: 1000, Rabbit; Bcl-2, 1: 2000, Rabbit; Bax, 1: 1000, Rabbit; Nrf2, 1: 3000, Rabbit; HO-1, 1: 5000, Rabbit; β-actin, 1: 3000, Rabbit, Abcam, Cambridge, MA, USA) at 4°C. After washing the next day, the PVDF membranes were incubated with secondary antibody (Goat anti-rabbit, 1: 3000, Abcam, Cambridge, MA, USA) for 2 h at room temperature. Finally, we used chemiluminescence to acquire the bands and used Image J software (NIH, Bethesda, MD, USA) to analyze the band gray values.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
We used TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) to extract total RNA from rat myocardial tissue and H9c2 cells. The RNA was reverse transcribed into complementary deoxyribose nucleic acid (cDNA) using the 5×PrimeScriptRT Master Mix kit (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions, and amplified using the Promega kit (Thermo Fisher Scientific, Waltham, MA, USA). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as endogenous control. The 2−ΔΔCt method was used to calculate relative expression level. The primer sequences of mRNA are shown in Table 1.
Table 1.
RT-PCR primer sequences.
| Name | Sense/anti-sense | Sequence (5′-3′) |
|---|---|---|
| SOD1 | Sense | GGTGAACCAGTTGTGTTGTC |
| Anti-sense | CCGTCCTTTCCAGCAGTC | |
| SOD2 | Sense | CAGACCTGCCTTACGACTATGG |
| Anti-sense | CTCGGTGGCGTTGAGATTGTT | |
| Caspase3 | Sense | TGGAACAAATGGACCTGTTGACC |
| Anti-sense | AGGACTCAAATTCTGTTGCCACC | |
| Caspase8 | Sense | TTCGAGCTACGAGCGCATAGGC |
| Anti-sense | ATCGATGCTACGAGCTACGTACGT | |
| Bcl-2 | Sense | GACTGAGTACCTGAACCGGCATC |
| Anti-sense | CTGAGCAGCGTCTTCAGAGACA | |
| Bax | Sense | CAGTTGAAGTTGCCATCAGC |
| Anti-sense | CAGTTGAAGTTACCATCAGC | |
| Nrf2 | Sense | TATGTCGACTGACTGACGTAC |
| Anti-sense | GACGGACCTATATGCGATTT | |
| HO-1 | Sense | GGCTATATCGGCGATTTGCGC |
| Anti-sense | ACGGTTATTGGGATTACGACA | |
| GAPDH | Sense | ACAACTTTGGTATCGTGGAAGG |
| Anti-sense | GCCATCACGCCACAGTTTC |
Malondialdehyde (MDA), superoxide dismutase (SOD), creatine kinase (CK), and lactate dehydrogenase (LDH) activity assay
After removing the appropriate amount of myocardial tissue, we used lysate to lyse the tissue. Then, we used an MDA kit (Lianke, Hangzhou, China) and SOD kit (Lianke, Hangzhou, China) to detect the content of MDA and SOD in myocardial tissue according to the manufacturer’s instructions. In addition, we isolated the serum of rats and used a CK kit (Lianke, Hangzhou, China) and LDH kit (Lianke, Hangzhou, China) to detect the levels of CK and LDH in the blood of rats according to the manufacturer’s instructions.
Na+-K+-ATPase and Ca2+-Mg2+-ATPase activity assay
The apical tissue was removed and ground to powder and dissolved in PBS buffer. We used enzymatic colorimetric assay to detect Na+-K+-ATPase and Ca2+-Mg2+-ATPase activity. ATPase can be broken down into ADP and inorganic phosphorus. The amount of inorganic phosphorus can be used to determine the enzyme activity, expressed as the amount of inorganic phosphorus produced by the ATPase per milligram of total protein in the decomposition of ATP per hour.
Immunocytofluorescence (IF) staining
H9c2 cells were cultured on 12-well plates. After treatment of the cells, we took out the 12-well plates and fixed the cells with 4% paraformaldehyde. We then soaked the cells in 0.5% Triton-PBS for 20 min. After blocking the cells with 10% goat serum, we used a primary antibody (SOD1, 1: 500, rabbit, Abcam, Cambridge, MA, USA) to incubate cells overnight at 4°C. After washing the next day, we incubated the cells with fluorescent secondary antibodies and then mounted them with a sealer containing 4′,6-diamidino-2-phenylindole (DAPI). Finally, we observed and recorded the results by fluorescence microscopy.
Cell Counting Kit-8 (CCK8) assay
H9c2 cells were cultured in 96-well plates at 5000 cells per well. After treatment of the cells, we added 10 μL of CCK8 reagent (Dojindo, Kumamoto, Japan) to each well. The cells were then incubated for 2 h in an incubator. Finally, we used a microplate reader to measure the absorbance at 450 nm of each well.
Flow cytometry
The levels of reactive oxygen species (ROS) and apoptosis levels were determined by flow cytometry. The DCFH-DA kit (Keygen, Nanjing, China) was used to detect ROS levels and the Annexin V-FITC kit (Keygen, Nanjing, China) was used to detect apoptosis levels according to the manufacturer’s instructions.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 21.0 software was used for statistical analysis, and Graphpad Prism 7 (La Jolla, CA, USA) was used to make charts. Quantitative data are presented as mean±standard deviation. One-way ANOVA followed by least significant difference post hoc test was used to compare data between multiple groups. All experiments were repeated 3 times. P<0.05 was considered statistically significant.
Results
LC attenuated myocardial IRI in rats
We intraperitoneally injected LC into model rats and then examined the relevant indicators of myocardial injury. We found that the level of MDA in the IRI group increased and the level of SOD decreased. However, MDA level of rats in the LC group was lower than that in the IRI group, while SOD level was higher than that in the IRI group, indicating that LC effectively reduced the level of oxidative stress in cardiomyocytes and increased the ability to scavenge free radicals (Figure 1A, 1B). CK and LDH, which are indicators of myocardial damage, were significantly elevated in the serum of rats in the IRI group, while LC reduced their expression (Figure 1C, 1D). In addition, in the IRI group of rat cardiomyocytes, Na+-K+-ATPase (Figure 1E) and Ca2+-Mg2+-ATPase (Figure 1F) activities were significantly reduced and LC increased their activity. These results indicate that LC effectively reduces myocardial damage in rats.
Figure 1.
LC attenuated myocardial IRI in rats. (A) MDA activity assay; (B) SOD activity assay; (C) CK activity assay; (D) LDH activity assay; (E) Na+-K+-ATPase activity assay; (F) Ca2+-Mg2+-ATPase activity. (“*” means statistical difference vs. control group and “#” means statistical difference vs. IRI group).
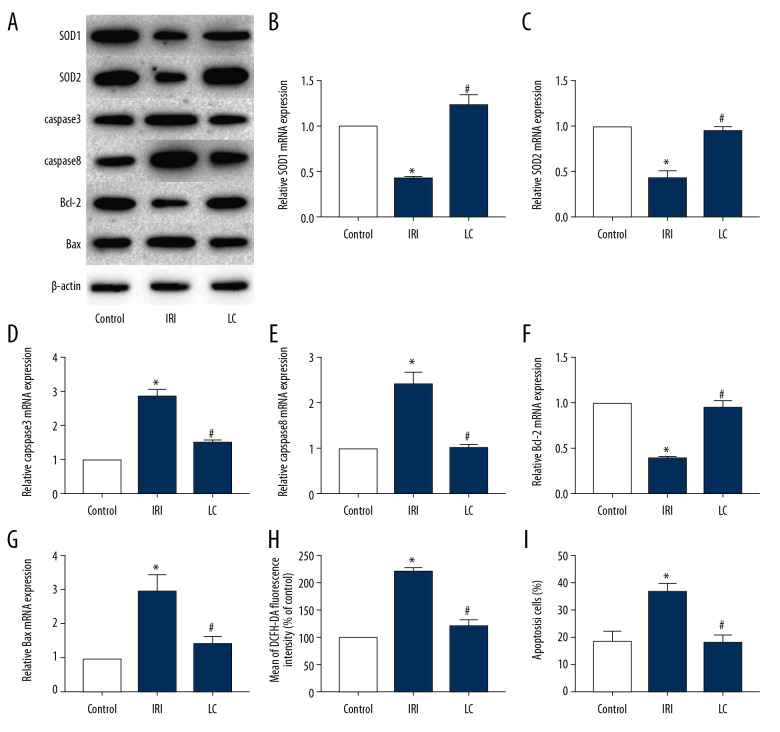
LC reduced oxidative stress and apoptosis of rat cardiomyocytes in vivo
The rat myocardium had high levels of oxidative stress and apoptosis during the injury process. Our results showed that the protein expression of SOD1, SOD2, and Bcl-2 in myocardial tissue of rats in the IRI group was significantly decreased, while the protein expression of caspase3, caspase8, and Bax was significantly increased. LC can alleviate this effect (Figure 2A). The results of qRT-PCR (Figure 2B–2G) were similar to those of Western blot. In addition, the results of flow cytometry also found that LC can reduce ROS levels (Figure 2H) and apoptosis levels (Figure 2I) of cardiomyocytes.
Figure 2.
LC reduced oxidative stress and apoptosis of rat cardiomyocytes in vivo. Expression of SOD1, SOD2, caspase3, caspase8, Bcl-2, and Bax in the 3 groups was detected by Western blot (A) and qRT-PCR (B–G). (H, I) ROS level (H) and cell apoptosis level (I) were determined by flow cytometry. (“*” means statistical difference vs. control group and “#” means statistical difference vs. IRI group).
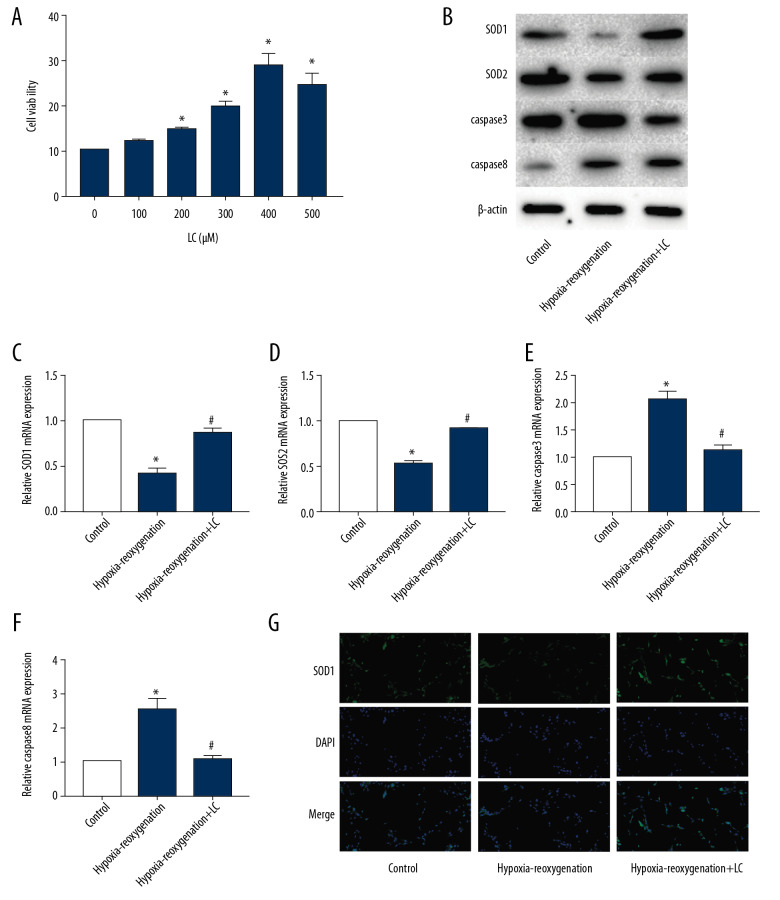
LC reduced oxidative stress and apoptosis of H9c2 cells in vitro
H9c2 cells were cultured to observe the effect of LC on cardiomyocytes. We determined the effect of LC (10, 50, 100, 200, and 500 μM) on the proliferative capacity of H9c2 cells by CCK8 assay (Figure 3A). We found that 200 μM is the optimum concentration. Western blot (Figure 3B) and qRT-PCR (Figure 3C–3F) showed that the SOD1 and SOD2 levels in H9c2 cells treated with hypoxia-reoxygenation decreased and the expression of caspase3 and caspase8 increased, while LC significantly attenuated this effect. In addition, IF results also found that LC significantly increased the expression of SOD1 (Figure 3G).
Figure 3.
LC reduced oxidative stress and apoptosis of H9c2 cells in vitro. (A) CCK8 assay detected the optimum concentration. (B–F) Expression of SOD1, SOD2, caspase3, and caspase8 in the 3 groups was determined by Western blot (B) and qRT-PCR (C–F). (G) IF detected the expression of SOD1 (G). (“*” means statistical difference vs. control group and “#” means statistical difference vs. hypoxia-reoxygenation group).
LC increased the activity of Nrf2/HO-1 signaling pathway in rat myocardial tissue and H9c2 cells
To examine the effect of LC on the Nrf2/HO-1 signaling pathway, we assessed the expression of Nrf2/HO-1 signaling pathway-related molecules in rat myocardial tissue and H9c2 cells. The results of Western blot (Figure 4A) and qRT-PCR (Figure 4B, 4C) showed that the expression of Nrf2 and HO-1 in myocardial tissue of rats in IRI group was significantly lower than that in the IRI group. In vitro, LC significantly attenuated the inhibitory effect of hypoxia-reoxygenation on the Nrf2/HO-1 signaling pathway in H9c2 cells (Figure 4D–4F).
Figure 4.
LC increased the activity of Nrf2/HO-1 signaling pathway in rat myocardial tissue and H9c2 cells. (A–C) Expression of Nrf2 and HO-1 in rat myocardial tissue was determined by Western blot (A) and qRT-PCR (B, C). (D–F) Expression of Nrf2 and HO-1 in H9c2 cells was determined by Western blot (D) and qRT-PCR (E, F). (“*” means statistical difference vs. control group and “#” means statistical difference vs. IRI group or hypoxia-reoxygenation group).
ML385 attenuated the anti-oxidative stress and anti-apoptotic effect of LC on cardiomyocytes
ML385 was used to inhibit the Nrf2/HO-1 signaling pathway. The expression of SOD1 and SOD2 proteins in H9c2 cells treated with ML385 was significantly lower than that in the LC group, while the expression of caspase3 and caspase8 increased (Figure 5A). The results of qRT-PCR (Figure 5B–5E) were similar to those of Western blot. This suggested that the protective effect of LC was reduced after ML385 inhibited the Nrf2/HO-1 signaling pathway, indicating that the protective effect of LC on cardiomyocytes was caused by activating the Nrf2/HO-1 signaling pathway.
Figure 5.
ML385 attenuates the anti-oxidative stress and anti-apoptotic effect of LC on cardiomyocytes. (A–E) Expression of SOD1, SOD2, caspase3, and caspase8 in the 4 groups was determined by Western blot (A) and qRT-PCR (B–E). (“*” means statistical difference vs. control group, “#” means statistical difference vs. hypoxia-reoxygenation group, and “##” means statistical difference vs. hypoxia-reoxygenation+LC group).
Discussion
Myocardial IRI is an important factor affecting the prognosis of AMI patients, but currently there is no effective treatment for IRI [13]. LC, which is an important amino acid with lipid metabolism regulation function, was found to have potential therapeutic effect on myocardial IRI in this study. We found that LC can reduce the levels of oxidative stress and apoptosis in cardiomyocytes and activate the antioxidant Nrf2/ho-1 signaling pathway.
The injury mechanism in myocardial IRI includes free radical damage. Free radical damage includes lipid free radicals and oxygen free radicals. MDA is one of the lipid metabolites, which can reflect the body’s lipid metabolism and reflect the degree of damage of cardiomyocytes [14]. In this study, the MDA content of the IRI group was significantly higher than that of the control group, indicating that the model was successfully established. The MDA content in the myocardial tissue of the rats in the LC group was significantly decreased, indicating that LC effectively reduced lipid metabolism of cardiomyocytes and reduced cardiomyocyte injury. SOD can reflect the body’s ability to scavenge oxygen free radicals. When the activity of SOD is lowered, a large amount of oxygen radicals is accumulated, and the membrane lipid is oxidized, so that the structure and function of cardiomyocytes are impaired [15]. A previous study also showed that 22-oxacalcitriol promotes the activity of antioxidant enzymes such as SOD, glutathione, and catalase in serum by inhibiting the NF-κB/TNF-α signaling pathway, thereby alleviating myocardial IRI [16]. Our study showed that the myocardium of the IRI group expressed lower SOD levels, indicating that myocardial IRI reduced the antioxidant capacity of cardiomyocytes. Increased activity of SOD in the myocardium of rats in the LC group indicated that LC can protect myocardial cell from the injury induced by ischemia-reperfusion. Serum CK and LDH markers of myocardial damage can reflect the degree of myocardial cell damage [17]. In this study, the levels of CK and LDH in rats injected with LC were significantly lower than those in the IRI group, showing that LC can reduce the production of myocardial injury markers, and further verifies the protective effect of LC on myocardium.
When myocardial tissue is subjected to IRI, tissue perfusion is insufficient, anaerobic glycolysis is increased, and ATP production is reduced. The main function of Na+-K+-ATPase is to pump out sodium ions in the cells and pump the extracellular potassium ions [18]. After myocardial IRI, Na+-K+-ATPase activity was significantly reduced, and sodium ions accumulated in the cardiomyocytes and extracellular potassium ions increased. A study on myocardial IRI found that with the aggravation of myocardial injury, the activity of Na+-k+-ATPase also decreased, and they found that DRm217 attenuates oxidative stress-induced cardiomyocyte damage by activating Na+-k+-ATPase [19]. In our study, Na+-K+-ATPase activity was elevated in the myocardial tissue of rats administered LC, restoring normal sodium and potassium ion balance. The main role of Ca2+-Mg2+-ATPase is to transport calcium ions out of the cell to maintain calcium ion balance within the cell. Similarly, Ca2+-Mg2+-ATPase activity is significantly reduced after myocardial IRI, resulting in accumulation of calcium ions in cardiomyocytes. In this study, the Ca2+-Mg2+-ATPase activity of rats in the LC group was significantly increased, and the calcium ion balance was restored.
Nrf2 is a key transduction factor regulating oxidative stress, and it is also a regulator of maintaining the body’s redox homeostasis [20]. Nrf2 regulates the glutathione redox system in the body, maintains cell redox balance, reduces cellular oxidative damage, and plays an important role in clearing free radicals [21]. Nrf2 has been shown to play an important antioxidant role in myocardial protection [22]. Some studies have also demonstrated that activation of the Nrf2/HO-1 signaling pathway contributes to the relief of myocardial oxidative injury [23,24]. In this study, we found that SOD expression and Nrf2/HO-1 signaling pathway activity were significantly increased in myocardial tissue in rats injected with LC and in LC-treated H9c2 cells. This indicated that LC activated the Nrf2/HO-1 signaling pathway in cardiomyocytes and reduced oxidative stress in cardiomyocytes.
The protective effect of LC on myocardial IRI was also studied in rheumatic valvular heart disease. In patients with rheumatic valvular heart disease who underwent cardiopulmonary bypass (CPB) surgery, Li et al. [25] added LC to the crystalloid cardioplegia to study the therapeutic effects of LC. However, since the crystalloid cardioplegia itself has significant protective effects on the myocardium, this may interfere with the study of the efficacy of LC. In addition, myocardial IRI during CPB surgery is different from AMI. In AMI, partial coronary artery occlusion leads to ischemic necrosis of the heart and presents a wavefront evolution. In CPB surgery, the blood flow of the human body completely bypasses the heart, causing the coronary artery of the heart to stop the blood flow supply and causes the heart to stop beating temporarily. However, the crystalloid cardioplegia added by the surgeon in the coronary artery can partially replace the blood, so there is a clear difference from the ischemic effect of AMI. In addition, Li et al. [25] did not distinguish among patients based on the type of rheumatic valvular disease (mitral valve, tricuspid valve, pulmonary valve, and aortic valve), which may have increased the effects of confounding factors and affected the reliability of the results. Therefore, we selected littermates and randomly divided them into a control group, a model group, and a treatment group to minimize the influence of confounding factors on the research results. In addition, we accurately and directly demonstrated the protective effect of LC on cardiomyocytes through cell experiments. In conclusion, our study of rats and H9c2 cells, as well as the cardioprotective effects of LC on patients with CPB surgery, demonstrates the promising future of LC for the treatment of myocardial IRI.
Conclusions
This study revealed that LC alleviates myocardial IRI by reducing oxidative stress and apoptosis of cardiomyocytes. This role is related to activation of the Nrf2/HO-1 signaling pathway. Our data elucidate a previously unrecognized effect of LC on myocardial IRI and may provide a new clinical target for the treatment of myocardial IRI.
Footnotes
Conflict of interests
None.
Source of support: Basic Scientific Research Business Expenses of Heilongjiang Provincial Department of Education Basic Scientific Research Project (2018-KYYWF-0961)
References
- 1.Zheng HF, Sun J, Zou ZY, et al. MiRNA-488-3p suppresses acute myocardial infarction-induced cardiomyocyte apoptosis via targeting ZNF791. Eur Rev Med Pharmacol Sci. 2019;23:4932–39. doi: 10.26355/eurrev_201906_18083. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj A, Sethi A, Rathor P, et al. Acute complications of myocardial infarction in the current era: Diagnosis and management. J Investig Med. 2015;63:844–55. doi: 10.1097/JIM.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 3.Russo I, Penna C, Musso T, et al. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc Diabetol. 2017;16:71. doi: 10.1186/s12933-017-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Zhang B, Chen Y, et al. Image-guided hydrogen gas delivery for protection from myocardial ischemia-reperfusion injury via microbubbles. ACS Appl Mater Interfaces. 2017;9:21190–99. doi: 10.1021/acsami.7b05346. [DOI] [PubMed] [Google Scholar]
- 5.Magruder JT, Crawford TC, Lin YA, et al. Selective localization of a novel dendrimer nanoparticle in myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2017;104:891–98. doi: 10.1016/j.athoracsur.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Mock CM, Schwetschenau KH. Levocarnitine for valproic-acid-induced hyperammonemic encephalopathy. Am J Health Syst Pharm. 2012;69:35–39. doi: 10.2146/ajhp110049. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi T. Effects of levocarnitine on cardiac function and renal anemia in hemodialysis patients. Contrib Nephrol. 2018;196:96–100. doi: 10.1159/000485706. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira GC, McKenna MC. L-Carnitine and acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem Res. 2017;42:1661–75. doi: 10.1007/s11064-017-2288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A, Choi Y, Back JH, et al. High-resolution metabolomics study revealing l-homocysteine sulfinic acid, cysteic acid, and carnitine as novel biomarkers for high acute myocardial infarction risk. Metabolism. 2020;104:154051. doi: 10.1016/j.metabol.2019.154051. [DOI] [PubMed] [Google Scholar]
- 10.Surendran A, Aliani M, Ravandi A. Metabolomic characterization of myocardial ischemia-reperfusion injury in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. Sci Rep. 2019;9:11742. doi: 10.1038/s41598-019-48227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinert CH, Empl MT, Kruger R, et al. The influence of a chronic L-carnitine administration on the plasma metabolome of male Fischer 344 rats. Mol Nutr Food Res. 2017;61:1600651. doi: 10.1002/mnfr.201600651. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Li X, Zhang W, et al. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism. 2018;83:256–70. doi: 10.1016/j.metabol.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansari J, Kaur G, Gavins FNE. Therapeutic potential of Annexin A1 in ischemia reperfusion injury. Int J Mo Sci. 2018;19(4):1211. doi: 10.3390/ijms19041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Houmani H, Rodriguez-Ruiz M, Palma JM, et al. Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma. 2016;253:885–94. doi: 10.1007/s00709-015-0850-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhou CN, Yao W, Gong YN, et al. 22-oxacalcitriol protects myocardial ischemia-reperfusion injury by suppressing NF-κB/TNF-α pathway. Eur Rev Med Pharmacol Sci. 2019;23:5495–502. doi: 10.26355/eurrev_201906_18219. [DOI] [PubMed] [Google Scholar]
- 17.Dash PC, Patro D. Role of CSF CK, LDH, GGTP enzyme levels in diagnostic and prognostic evaluation of meningitis. J Clin Diagn Res. 2014;8:C19–22. doi: 10.7860/JCDR/2014/9675.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obradovic M, Stanimirovic J, Panic A, et al. Regulation of Na+/K+-ATPase by estradiol and IGF-1 in cardio-metabolic diseases. Curr Pharm Des. 2017;23:1551–61. doi: 10.2174/1381612823666170203113455. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, Xun M, Dou X, et al. Activation of Na-K-ATPase with DRm217 attenuates oxidative stress-induced myocardial cell injury via closing Na-K-ATPase/Src/Ros amplifier. Apoptosis. 2017;22:531–43. doi: 10.1007/s10495-016-1342-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonelli C, Chio I, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–45. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Chou G, Li Q. Cardioprotective role of azafrin in against myocardial injury in rats via activation of the Nrf2-ARE pathway. Phytomedicine. 2018;47:12–22. doi: 10.1016/j.phymed.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Shanmugam G, Challa AK, Litovsky SH, et al. Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol. 2019;27:101212. doi: 10.1016/j.redox.2019.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Xu S, Geng Y, et al. The protective effects of L-carnitine on myocardial ischaemia-reperfusion injury in patients with rheumatic valvular heart disease undergoing CPB surgery are associated with the suppression of NF-kappaB pathway and the activation of Nrf2 pathway. Clin Exp Pharmacol Physiol. 2019;46:1001–12. doi: 10.1111/1440-1681.13155. [DOI] [PubMed] [Google Scholar]