Abstract
Background
Insomnia and other types of sleep disturbance are highly prevalent during withdrawal across many different types of substance use disorders (SUDs). It is largely unknown how sleep impacts SUD treatment outcomes, including treatment completion.
Methods
A retrospective chart review was conducted to obtain information about sleep disturbance and treatment completion in individuals beginning an intensive outpatient (IOP) SUD treatment program. Demographic data were collected along with number of sessions completed, treatment completion, comorbid psychiatric diagnosis, pertinent lab results, and scores on three self-reported measures of sleep: the Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), and Epworth Sleepiness Scale (ESS).
Results
Pertinent information was available for 110 individuals. The majority had clinically significant scores on the ISI and PSQI but not the ESS. ISI, but not PSQI or ESS, was associated with treatment completion, such that those with more insomnia were less likely to complete treatment.
Conclusion
The high prevalence of insomnia symptoms and poor sleep quality coupled with the relationship between insomnia severity and treatment completion may indicate that more severe symptoms of insomnia are a risk factor for treatment completion and subsequent relapse across many substance types. Applying evidence-based insomnia interventions in SUD treatment programs may have meaningful implications for outcomes.
Keywords: Sleep, Insomnia, Alcohol, Drug, Substance, Treatment, Outcomes, Comorbidity
1. Introduction
Substance use disorders (SUDs) are highly prevalent and pose a major public health problem (McLellan, Carise, & Kleber, 2003). According to a recent national survey, approximately 21 million people (1 in 12 of the United States population) met criteria for a SUD and were in need of treatment over a one-year period (Bose et al., 2016). Depending on the type and severity of the SUD, best-practice treatments vary from individual therapy to inpatient or intensive outpatient therapy, along with medication management.
Upon entering treatment, individuals are often encouraged, if not required, to abstain from substance use, at which time they may experience various types of self-reported sleep disturbance commonly seen in withdrawal and early abstinence. Specifically, insomnia, defined as difficulty falling asleep or staying asleep, is one of the few withdrawal symptoms listed in the DSM-5 across five major classes of substances (i.e., alcohol, cannabis, opioids, sedative/hypnotic/ anxiolytics, stimulants; American Psychiatric Association, 2013). Additionally, disturbing dreams are classified as a symptom of cannabis withdrawal, and hypersomnia (characterized by excessive sleepiness) as a symptom of stimulant withdrawal. Indeed, both self-reported sleep disturbance as well as changes in objective measures of sleep parameters (e.g., sleep onset, sleep duration, etc.) during this time have been associated with withdrawal from alcohol (Brower, Aldrich, Robinson, Zucker, & Greden, 2001), cannabis (Bolla et al., 2010), opioids (Sharkey et al., 2011), and stimulants (Pace-Schott et al., 2005; see Angarita, Emadi, Hodges, & Morgan, 2016 and Conroy & Arnedt, 2014 for comprehensive reviews).
Little is known regarding the similarities and differences of sleep disturbance between different types of SUDs, as the vast majority of studies exploring the relationship between sleep and substance use have collected sleep data in individuals with a single type of SUD. This makes comparison across studies difficult due to methodological differences, such as type of sleep measure and procedures implemented to control for confounding variables. Additionally, the term “sleep disturbance” lacks a widely accepted operational definition and may refer to any number of aspects of nocturnal sleep or subsequent daytime impact. For example, shorter sleep duration, increased perceived difficulty falling asleep, and greater daytime sleepiness may all be referred to as sleep disturbance.
The few studies that have looked at the prevalence of sleep disturbance across multiple types of SUDs suggest the majority of individuals with SUD have poor sleep quality during active use (Mahfoud, Talih, Streem, & Budur, 2009), in early abstinence (Tang et al., 2015), and during and following substance use treatment (Colvonen, Ellison, Haller, & Norman, 2018), regardless of substance type. However, these studies are limited by small sample size (Mahfoud et al., 2009), use of a single measure to assess sleep (Colvonen et al., 2018; Tang et al., 2015), and lack of substance-use outcome data.
This gap in the literature is important for two reasons. First, sleep disturbance has been linked to worse treatment outcomes, including higher rates of relapse, in each major SUD type, though this has not been examined longitudinally within the same sample across multiple SUD types. As such, it is possible that sleep is a modifiable risk factor across all substances (Brower & Perron, 2010); however, methodological differences between studies limit the strength of such a conclusion. Second, individuals with SUDs are often treated together through group therapy in intensive outpatient, inpatient, and residential treatment facilities. As such, understanding any patterns in sleep complaints across substances and their relationship to treatment outcomes could serve as an invaluable intervention target to be addressed concurrently alongside other evidence-based interventions. If sleep disturbance is indeed a “universal risk factor,” (Brower & Perron, 2010, p. 928) of treatment outcome and/or relapse, then implementing an evidence-based sleep intervention into treatment programs may help promote treatment completion and prevent relapse regardless of the type of primary SUD.
The purpose of the current study was to determine the prevalence of sleep disturbance by substance type in early SUD treatment and the relationship between sleep disturbance and treatment completion. Adults presenting for SUD intensive outpatient treatment were asked to complete three well-validated self-report measures to capture various aspects of sleep disturbance. These measures were selected as they all pertain to at least one of the sleep-related DSM-5 diagnostic criteria described above. Specifically, insomnia symptoms were measured via the Insomnia Severity Index (ISI; Morin, 1993), overall sleep quality was measured via the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), and sleepiness was measured via the Epworth Sleepiness Scale (ESS; M.W. Johns, 1991; Murray W Johns, 2000).
It was hypothesized that the majority of patients would exceed the clinically significant cutoff on each of the three measures, regardless of type of substance use disorder. It was also hypothesized that severity of sleep disturbance on each measure at the beginning of treatment would be related to treatment completion, such that those with more severe scores would be less likely to complete treatment.
2. Materials and methods
2.1. Procedure
This was a retrospective chart review of patients who attended the Intensive Outpatient Program (IOP) in the Center for Drug and Alcohol Programs (CDAP) at the Medical University of South Carolina (MUSC) from November 2014 to November 2015 when sleep measures were administered as part of a clinical care quality improvement project. Before beginning IOP, treatment-seeking adults with a SUD completed an intake interview where they provided demographic information as well as a brief history of alcohol and drug use, medical and mental health history, and social history. In the event of multiple problematic substances, the individual and clinician worked together to determine the most problematic substance in his or her current use or most recent use. The IOP provides treatment for individuals who meet criteria for at least one SUD and have completed detoxification or do not require hospitalization.
The IOP is designed to be a 20-day program, though patients may “graduate” without attending all 20 days (e.g., sickness, competing medical appointments, and transportation difficulties are considered valid excuses for absence provided the patient is making progress and attending the majority of sessions). Conversely, patients may attend > 20 days of treatment if it is recommended by the care team and the patient agrees. Each day consists of 3-h of group therapy, with weekly individual therapy and family participation strongly encouraged. Regular urine and serum biomarker screen for use of drugs and alcohol, and psychiatric medication management is offered if needed. Prior to the first group therapy session patients were asked to complete the ISI, PSQI, and ESS but were not required to complete these measures before beginning treatment. Sleep data were collected and managed using REDCap electronic data capture tools hosted at MUSC (Harris et al., 2009).
For the current study a retrospective chart review was conducted to gather information regarding demographic characteristics, number of sessions completed, comorbid psychiatric diagnosis, and pertinent lab results (primarily presence of primary substance in urine drug screen at baseline). “Graduation” was not consistently documented in the chart; therefore, for the purposes of this study, treatment completion was coded as attending at least 15 sessions of group therapy, as this indicates the patient attended at least 75% of recommended sessions. Further, this represents when patients have completed the majority of the psychoeducation materials and are transitioning into after-care planning. All chart review procedures were approved by the Institutional Review Board at MUSC.
2.2. Participants
Participants were 110 adult patients beginning the IOP in CDAP at MUSC. Patients were referred to IOP through a walk-in evaluation clinical program utilizing the Patient Placement Criteria of the American Society of Addiction Medicine (Mee-Lee, 2001). Patients represented a diverse range of use disorders (i.e., alcohol, cannabis, opioids, sedatives/hypnotics/anxiolytics, and stimulants [i.e., cocaine, methamphetamine]).
2.3. Measures
2.3.1. Insomnia Severity Index (ISI; Morin, 1993)
The ISI is a 7-item self-report measure designed to assess the perceived severity of insomnia. Each item uses a Likert scale from 0 to 4 with increasing value corresponding to increased symptom severity. The items are summed, yielding a minimum score of 0 and a maximum score of 28. A score of 0–7 indicates no insomnia symptoms, 8–14 indicates subthreshold/mild insomnia, 15–21 indicates moderate insomnia, and a score > 21 indicates severe insomnia.
2.3.2. Pittsburg Sleep Quality Index (PSQI; Buysse et al., 1989)
The PSQI is a 19-item questionnaire that assesses subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances (i.e., time taken to fall asleep, time awake throughout the night, difficulty breathing, coughing, snoring, feeling too hot or cold, bad dreams, pain), use of sleep medications, and daytime dysfunction. The PSQI generates seven subscores that correspond to the seven domains previously mentioned. The domain subscores range from 0 (no difficulty) to 3 (severe difficulty) and when combined produce a global score (0−21) with a score > 5 suggesting disordered sleep.
2.3.3. Epworth Sleepiness Scale (ESS; M.W. Johns, 1991; Murray W Johns, 2000)
The ESS is an 8-item questionnaire that assesses perceived severity of daytime sleepiness and insomnia-related interference with daytime functioning. Each item inquires about the likelihood of falling asleep in a given scenario using a Likert scale from 0 (not at all likely) to 3 (very likely). The items are summed, yielding a minimum score of 0 and a maximum score of 24. A score of 10–15 indicates higher than normal sleepiness and a score > 15 is considered severe excessive daytime sleepiness.
2.4. Data analysis
Data were analyzed using IBM SPSS version 24. ISI, PSQI, and ESS measures were screened for normality and missing data. ESS was logarithmically transformed to reduce positive skewness. One patient discontinued the survey before completing any of the measures and was subsequently excluded from analyses. All other patients who began the measures completed every item of every measure. Baseline and demographic variables for patients who completed the surveys were compared to patients who entered treatment at the same time and completed a general intake packet but did not complete the surveys using independent samples t-tests and x2 tests. Sleep measures were compared via one-way ANOVA to test for differences based on primary substance use disorder type (i.e., alcohol, opioid, cannabis, sedative-hypnotic, or stimulant use disorder). The relationship between baseline subjective sleep disturbance and treatment completion was examined by step-wise logistic regression covarying for presence of primary problematic substance in urine drug screen at baseline, comorbid psychiatric diagnosis, and SUD type, as these factors are also highly related to treatment completion (Adamson, Sellman, & Frampton, 2009; Kampman et al., 2001). Finally, correlations were run on all sleep measures to assist in interpretation of findings.
3. Results
3.1. Sample characteristics
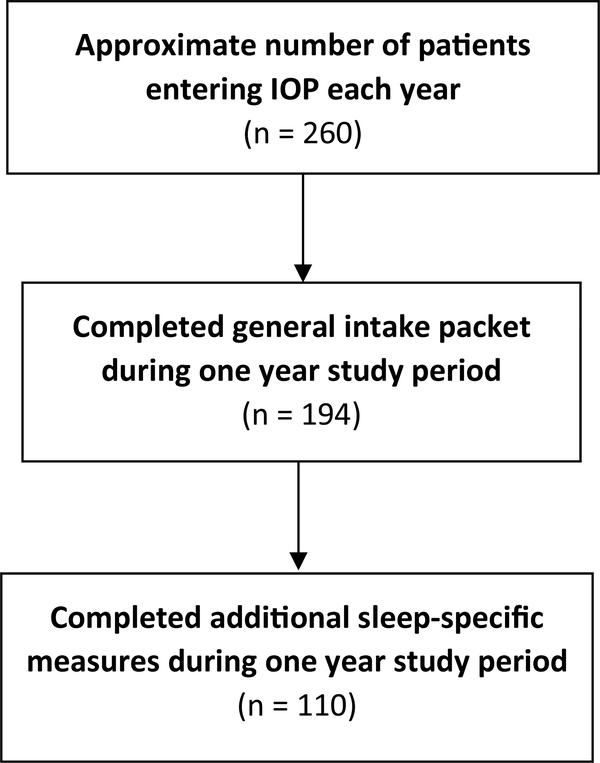
A detailed record of appointments of all patients attending IOP is accessible in the institution’s electronic medical record for only one year (i.e., the 365 days immediately preceding the date of any data query), and this chart review was conducted 18 months following the period of interest. Therefore, the total number of patients that began IOP during the one-year period when the measures were collected is unknown. However, a more recent four-month chart review indicates approximately five new patients join IOP each week, resulting in roughly 260 patients per year. For the time of interest (November 2014–November 2015) demographic information was available via clinic specific introduction packets for 194 patients (74.6% of estimated total number of patients). Of those patients, 110 (41.7% of estimated total number of patients) completed the sleep measures. See Fig. 1 for a representation of patient participation in completing sleep measures. Among the 194 patients whose demographic information was known, there were no significant differences between those who completed sleep surveys (n = 110) and those who did not (n = 84; see Table 1 for demographic information). The sample who completed sleep measures (n = 110) was 40% female (n = 44), with an age range of 18 to 71. The majority of patients presented with alcohol use disorder, with opioid use disorder being the second most common. See Table 2 for demographic information by SUD type.
Fig. 1.
Participant disposition flow chart during one year period when sleep measures were requested of new patients.
Table 1.
Demographic characteristics of survey completers and non-completers.
| Completed measures | ||||
|---|---|---|---|---|
| Characteristic | Yes | No | ||
| Total N | 110 | 84 | ||
| t | p | |||
| Age M (SD) | 36.3 (12.9) | 40.1 (15.6) | 1.82 | 0.07 |
| x2 | p | |||
| Gender, N (%) | 0.536 | 0.464 | ||
| Male | 66 (60) | 46 (55) | ||
| Female | 44 (40) | 38 (45) | ||
| Marital status, N (%) | 5.47 | 0.242 | ||
| Single | 53 (48) | 35 (42) | ||
| Engaged | 5 (5) | 2 (2) | ||
| Living with partner | 2 (2) | 7 (8) | ||
| Married | 31 (28) | 25 (30) | ||
| Separated/divorced | 19 (17) | 15 (18) | ||
| Education, N (%) | 5.81 | 0.325 | ||
| Less than 12th grade | 10 (9) | 11 (13) | ||
| High school/GED | 22 (20) | 12 (14) | ||
| Some college | 34 (31) | 19 (23) | ||
| Completed college | 18 (16) | 21 (25) | ||
| Graduate degree | 4 (4) | 6 (7) | ||
| Unanswered | 22 (20) | 15 (18) | ||
| Employment, N (%) | 3.8 | 0.433 | ||
| Employed | 55 (50) | 43 (51) | ||
| Unemployed | 37 (34) | 23 (28) | ||
| Retired | 5 (4) | 6 (7) | ||
| Disability | 13 (12) | 10 (12) | ||
| Unanswered | 0 (0) | 2 (2) | ||
| Primary substance, N (%) | 1.07 | 0.898 | ||
| Alcohol | 49 (45) | 37 (44) | ||
| Opiates | 39 (35) | 32 (38) | ||
| Cannabis | 6 (6) | 6 (7) | ||
| Sedative hypnotics | 5 (4) | 2 (3) | ||
| Stimulants | 11 (10) | 7 (8) | ||
Table 2.
Demographic characteristics based on substance use type.
| Characteristic | |||||
|---|---|---|---|---|---|
| Substance | Alcohol | Opiates | Cannabis | Sedative Hypnotics | Stimulants |
| Total, N (%) | 49 (45) | 39 (35) | 6 (5) | 5 (5) | 11 (10) |
| Age M(SD) | 40.9 (13.5) | 31.3 (8.4) | 28.8 (7.1) | 38.8 (23.6) | 36.4 (13.8) |
| Gender, N (%) | |||||
| Male | 35 (71) | 16 (41) | 3 (50) | 3 (60) | 9 (82) |
| Female | 14 (29) | 23 (59) | 3 (50) | 2 (40) | 2 (18) |
| Marital status, N (%) | |||||
| Single | 18 (37) | 26 (67) | 3 (50) | 2 (40) | 4 (36) |
| Engaged | 1 (2) | 4 (10) | 0 (0) | 0 (0) | 0 (0) |
| Living with partner | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Married | 19 (39) | 3 (8) | 2 (33) | 3 (60) | 4 (36) |
| Separated/divorced | 9 (18) | 6 (15) | 1 (17) | 0 (0) | 3 (28) |
| Education, N (%) | |||||
| Less than 12th grade | 2 (4) | 5 (13) | 3 (50) | 0 (0) | 0 (0) |
| High school/GED | 5 (10) | 11 (28) | 1 (17) | 2 (40) | 3 (27) |
| Some college | 14 (29) | 13 (33) | 2 (33) | 2 (40) | 3 (27) |
| Completed college | 14 (29) | 3 (8) | 0 (0) | 0 (0) | 1 (9) |
| Graduate degree | 4 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unanswered | 10 (20) | 7 (18) | 0 (0) | 1 (20) | 4 (37) |
| Employment, N (%) | |||||
| Employed | 29 (59) | 15 (43) | 1 (17) | 2 (40) | 8 (73) |
| Unemployed | 9 (19) | 20 (57) | 5 (83) | 2 (40) | 1 (9) |
| Retired | 3 (6) | 0 (0) | 0 (0) | 1 (20) | 1 (9) |
| Disability | 8 (16) | 4 (100) | 0 (0) | 0 (0) | 1 (9) |
| Positive UA, N (%) | 28 (57) | 17 (44) | 5(83) | 4 (80) | 2 (18) |
| Comorbidity, N (%) | 34 (69) | 25 (64) | 3 (50) | 3 (60) | 5 (46) |
Note: Comorbidity = documented diagnosis of PTSD, mood, or anxiety disorder by clinician at initial intake.
3.2. Prevalence of self-reported sleep disturbance
3.2.1. ISI
The mean ISI score of all subjects was 13.01 (SD = 8.31). Eighty participants (73%) had a total ISI score indicative of at least mild insomnia (≥ 8). Forty-six (42%) had scores indicative of moderate to severe insomnia (≥ 15). Mean scores within each substance disorder type revealed all substance types exceeded the cutoff for at least mild insomnia, with the exception of cannabis use disorder, as shown in Fig. 2. Given the low number of subjects with cannabis (n = 6), sedative-hypnotic (n = 5), and stimulant (n = 11) use disorder, further analyses were not run on these groups as meaningfulness of results would be limited due to small sample size. An independent samples ttest was run comparing those with alcohol (n = 49) and opioid (n = 39) use disorders to examine differences between these SUD types. Participants with opioid use disorder (M = 15.77, SD = 7.99) reported significantly worse insomnia symptoms than those with alcohol use disorder (M = 11.18, SD = 8.15; t = −2.64, p = 0.01).
Fig. 2.
Insomnia Severity Index score by SUD type.
3.2.2. PSQI
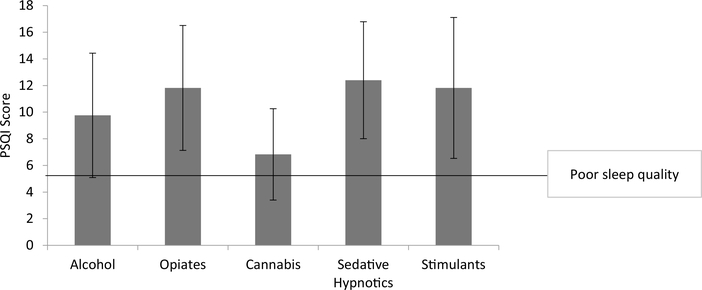
The mean PSQI score for all subjects was 10.65 (SD = 4.80). Ninetyfour patients (86%) had a total PSQI score suggestive of clinically significant sleep disturbance (≥ 6). Mean scores within each substance disorder type revealed all substance types exceeded the cutoff for clinically significant sleep disturbance, as shown in Fig. 3. Further analysis comparing alcohol and opioid groups revealed subjects with opioid use disorder again reported significantly worse sleep quality (M = 11.82, SD = 4.69) than subjects with alcohol use disorder (M = 9.76, SD = 4.67; t = −2.057, p = 0.04).
Fig. 3.
Pittsburgh Sleep Quality Index score by SUD type.
3.2.3. ESS
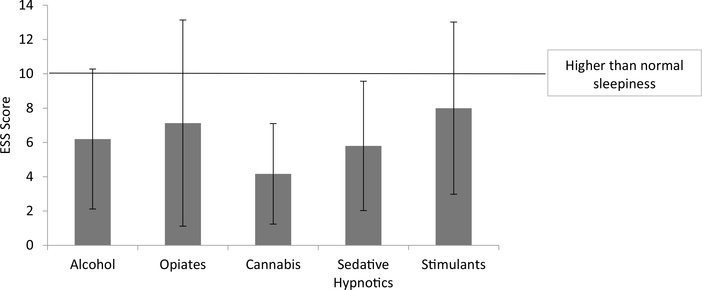
The mean ESS score for all patients was 6.58 (SD = 4.89). Twentyfive patients (23%) had total ESS scores indicative of higher than normal sleepiness (≥10). Only 7 patients (6%) had scores indicative of excessive daytime sleepiness (≥16). Mean scores within each SUD type revealed no type reached this cutoff, as shown in Fig. 4. Further analysis comparing alcohol and opioid groups revealed no significant differences between groups (t = 0.18, p = 0.85)
Fig. 4.
Epworth Sleepiness Scale score by SUD type.
3.3. Self-reported sleep disturbance and treatment completion
Seventy-nine patients (72% of the 110 who completed sleep measures) completed treatment, defined as attending at least 15 of 20 prescribed sessions. The median number of sessions completed was 26.5 (IQR = 22.25), indicating most patients continued past the minimum expectation before moving to an after-care plan. Half of patients (51%) had urine drug screens at baseline that were positive for the substance related to their primary SUD, and the majority of patients (64%) were given a comorbid psychiatric diagnosis (mood, anxiety, or PTSD) upon intake. Positive drug screen, comorbidity, and SUD type were not significantly related to treatment completion (X2 [1, N = 101] = 1.15, p = 0.28 and X2 [1, N = 110] = 0.58, p = 0.45, and X2 [4, N = 110] = 4.36, p = 0.36, respectively) and were therefore not included in the logistic regression model examining the relationship between each sleep measure and treatment completion. ISI severity score was significantly associated with treatment completion, such that for every increase in 5 points on the ISI an individual is 1.4 times less likely to complete treatment (X2 [1, N = 110] = 9.01, p = 0.003; OR: 1.08; 95% CI 0.1.03–1.15), accounting for 11.3% of the model’s variability. Treatment completion was not associated with PSQI (X2 [1, N = 110] = 1.62, p = 0.20; OR: 1.06; 95% CI 0.97–1.16) or ESS (X2 [1, N = 110] = 0.003, p = 0.96; OR: 1.03; 95% CI 0.30–3.60).
3.4. Relationship between sleep measures
There were significant, positive correlations between all three sleep measures (Table 3). Specifically, the ISI and the PSQI were significantly correlated with a large effect size, r (110) = 0.84, p < 0.001. The ISI and ESS were significantly related with a medium effect size, r (110) = 0.35, p < 0.001, and the PSQI and the ESS were significantly related with a small effect size, r (110) = 0.28 p = 0.003.
Table 3.
Pearson correlations among sleep measures.
Note: ISI = Insomnia Severity Index; PSQI = Pittsburg Sleep Quality Index; ESS = Epworth Sleepiness Scale.
p < 0.01.
4. Discussion
As hypothesized, the majority of patients with alcohol, opioid, sedative hypnotic, and stimulant use disorder in this cohort who were in early abstinence exceeded the clinical cutoff on the ISI and PSQI, indicating significant insomnia symptoms as well as poor overall sleep quality. Generally speaking, these findings in a sample of mixed substance use disorders, are concordant with other studies looking at sleep in individual SUDs (Angarita et al., 2016; Conroy & Arnedt, 2014), as well as the small body of literature that has examined sleep in multiple types of SUDs within the same sample (Mahfoud et al., 2009; Tang et al., 2015). Those with cannabis use disorder exceeded the clinically significant cutoff on the PSQI but not the ISI. The small sample of patients with cannabis use disorder (n = 6) makes these findings difficult to interpret in the current study, calling for further exploration.
Contrary to the hypothesis, patients in all SUD types denied problematic daytime sleepiness via the ESS. These results may seem counterintuitive, as one might expect lack of sleep and/or poor sleep quality to be followed by daytime sleepiness. Indeed, it has been found that approximately half of individuals with insomnia experience excessive daytime sleepiness (Hein, Lanquart, Loas, Hubain, & Linkowski, 2017). However, lack of sleepiness is consistent with the hyperarousal model of insomnia, which proposes those with insomnia have increased arousal levels both at night and during the day, making sleepiness less likely than might be expected given the degree of insomnia (Riemann et al., 2010). It is also possible that an underlying disorder that increases the likelihood of co-occurring insomnia symptoms and excessive sleepiness, such as sleep apnea (Chung, 2005), was not present in the majority of this sample, though this information was not explicitly collected and therefore this cannot be definitively determined.
Regarding sleep and treatment completion, as hypothesized, higher ISI score when beginning treatment was significantly associated with IOP treatment completion. Specifically, those with higher ISI scores, indicating more severe insomnia, were less likely to complete treatment. Notably, ISI accounted for only 11% of the variance in this model, indicating other factors may be involved. PSQI and ESS scores were not significantly related to treatment completion. Examining the items within each measure may assist in the interpretation of these results. The ISI focuses specifically on symptoms of insomnia (i.e., perceived difficulty falling asleep/maintaining sleep and subsequent daytime problems related to difficulty sleeping). The PSQI asks questions related to insomnia symptoms but also collects information on other aspects of sleep disruption and subsequent daytime dysfunction, including quantitative sleep parameters (e.g., time to fall asleep, sleep duration, time in bed), sources of sleep disturbance (e.g., snoring, feeling too hot or cold, bad dreams, pain), use of sleep medication, and sleepiness. The ESS is focused solely on perceived sleepiness during situations when wakefulness is typically expected. Considering the targeted constructs of each measure, it was not surprising there was a significant correlation between the ISI and PSQI and a significant, though smaller, relationship between each of these measures with the ESS. However, given the strong correlation between the ISI and PSQI, it is interesting ISI was associated with treatment completion while PSQI was not. These findings may indicate that insomnia-specific symptoms (difficulty falling and staying asleep) are more related to treatment outcomes than some of the other aspects of sleep disturbance. These findings add to a growing body of literature linking insomnia to comorbid psychiatric diagnoses (e.g., mood and anxiety disorders; Buysse et al., 2008; Taylor, Lichstein, & Durrence, 2003) and medical problems (e.g., heart disease, hypertension, gastrointestinal complaints, migraines; Taylor et al., 2007; Wang et al., 2017), as well as increased work absenteeism and use of the healthcare system (Bramoweth & Taylor, 2012; Daley et al., 2009). These results highlight the need for further research in this area, as treatment retention is a highly important aspect in overall recovery. Future studies would benefit from a larger sample size with the power to explore more specific aspects of sleep, such as examining each subscale of the PSQI.
Although important, these findings are potentially limited by several factors. First, questionnaire administration was part of a timelimited clinical care improvement effort, with inconsistent administration of questionnaires due to patient preference and competing demands of staff. Specifically, it is estimated 52% of new patients either opted out or were not asked to complete the questionnaires during the 12-month period of data collection; however, comparing demographic information with a subset of those who began treatment at the same time but did not complete the sleep measures revealed no significant differences between groups. Another potential limitation of this chart review is that treatment completion, as defined in this study, may not necessarily indicate a patient truly completed treatment (i.e., the patient attended 15 sessions but may have been advised to complete more based on severity of substance use). Further, presence of other modifiable risk factors that have previously been linked to treatment completion were not consistently collected at baseline and therefore could not be controlled for in the analysis. Specifically, social support (Dobkin, Civita, Paraherakis, & Gill, 2002), duration of abstinence (Marcovitz, McHugh, Volpe, Votaw, & Connery, 2016), and severity of use (Mckellar, Harris, & Moos, 2006) have all been linked to substance use treatment completion and were not included in the analyses. Including these variables into future models could increase the variance accounted for to above the 11% found in the present study and provide insight into how the intersection of these factors with insomnia symptoms impact progression through SUD treatment.
Despite these limitations, the results of this study indicate the majority of patients who enter IOP treatment for SUDs have significant sleep disturbances, regardless of the type of SUD, and that insomnia in particular might predict lower likelihood of IOP completion. Thus, IOP completion may be improved by assessing and providing interventions for sleep disturbances, including insomnia symptoms, during the treatment for SUDs. To the authors’ knowledge this is the first study to assess the relationship between sleep in early treatment and treatment completion using well-validated sleep measures in an IOP with patients diagnosed with one of the five most prevalent SUDs. It will be important for future studies to incorporate objective measures of sleep, such as actigraphy or polysomnography, as differences in self-reported sleep and objective sleep disturbances may have different treatment implications. Additionally, assessing sleep during and after SUD treatment and assessing other treatment outcomes (e.g., relapse following treatment completion, symptoms of depression and anxiety, and overall quality of life) will help determine if insomnia symptoms subside after initial withdrawal and will aid in the development and implementation of sleep-based interventions during and after treatment to improve SUD treatment outcomes.
5. Conclusion
Taken together, high prevalence of insomnia symptoms and poor sleep quality and the relationship between insomnia severity and treatment completion in this diverse sample of SUD types support the notion that sleep disturbance is highly prevalent and may be a “universal risk factor” for relapse regardless of problematic substance type (Brower & Perron, 2010). This relationship has meaningful implications for optimizing the treatment of SUDs. Specifically, introducing components of evidence based, non-pharmacological interventions for sleep, such as cognitive behavioral therapy of insomnia into already existing treatment settings might positively impact treatment completion and other treatment outcomes through improving the insomnia symptoms that are likely present in the majority of patients.
Acknowledgments
Funding
This work was supported by the National Institute on Drug Abuse (K12 DA031794 and K23DA043628).
References
- Adamson SJ, Sellman JD, & Frampton CM (2009). Patient predictors of alcohol treatment outcome: A systematic review. Journal of Substance Abuse Treatment, 36(1), 75–86. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). (Washington, DC: ). [Google Scholar]
- Angarita GA, Emadi N, Hodges S, & Morgan PT (2016). Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: A comprehensive review. Addiction Science & Clinical Practice, 11(1), 9 10.1186/s13722-0160056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang NY, Funderburk FR, & Cadet JL (2010). Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Medicine, 11(9), 882–889. 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Hedden SL, Lipari RN, Park-Lee E, Porter JD, & Pemberton MR (2016). Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration website; https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf (Published September). [PubMed] [Google Scholar]
- Bramoweth AD, & Taylor DJ (2012). Chronic insomnia and health care utilization in young adults. Behavioral Sleep Medicine, 10(2), 106–121. 10.1080/15402002.2011.587067. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, & Greden JF (2001). Insomnia, self-medication, and relapse to alcoholism. The American Journal of Psychiatry, 158(3), 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, & Perron BE (2010). Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Medical Hypotheses, 74(5), 928–933. 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, & Rossler W (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep, 31(4), 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Chung KF (2005). Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration, 72(5), 460–465. [DOI] [PubMed] [Google Scholar]
- Colvonen PJ, Ellison J, Haller M, & Norman SB (2018). Examining insomnia and PTSD over time in veterans in residential treatment for substance use disorders and PTSD. Behavioral Sleep Medicine, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DA, & Arnedt JT (2014). Sleep and substance use disorders: An update. Current Psychiatry Reports, 16(10), 487 10.1007/s11920-0140487-3. [DOI] [PubMed] [Google Scholar]
- Daley M, Morin C, LeBlanc M, Gregoire J, Savard J, & Baillargeon L (2009). Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Medicine, 10(4), 427–438. [DOI] [PubMed] [Google Scholar]
- Dobkin PL, Civita MD, Paraherakis A, & Gill K (2002). The role of functional social support in treatment retention and outcomes among outpatient adult substance abusers. Addiction, 97(3), 347–356. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein M, Lanquart J-P, Loas G, Hubain P, & Linkowski P (2017). Prevalence and risk factors of excessive daytime sleepiness in insomnia sufferers: A study with 1311 individuals. Journal of Psychosomatic Research, 103, 63–69. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep, 14(6), 540–545. [DOI] [PubMed] [Google Scholar]
- Johns MW (2000). A sleep physiologist’s view of the drowsy driver. Transportation Research Part F: Traffic Psychology and Behaviour, 3(4), 241–249. [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, & Mulvaney FD (2001). Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychology of Addictive Behaviors, 15(1), 52. [DOI] [PubMed] [Google Scholar]
- Mahfoud Y, Talih F, Streem D, & Budur K (2009). Sleep disorders in substance abusers: How common are they? Psychiatry, 6(9), 38–42. [PMC free article] [PubMed] [Google Scholar]
- Marcovitz DE, McHugh RK, Volpe J, Votaw V, & Connery HS (2016). Predictors of early dropout in outpatient buprenorphine/naloxone treatment. The American Journal on Addictions, 25(6), 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckellar JD, Harris AH, & Moos RH (2006). Predictors of outcome for patients with substance-use disorders five years after treatment dropout. Journal of Studies on Alcohol, 67(5), 685–693. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, & Kleber HD (2003). Can the national addiction treatment infrastructure support the public’s demand for quality care? Journal of Substance Abuse Treatment, 25(2), 117–121. 10.1016/s0740-5472(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Mee-Lee D (2001). ASAM patient placement criteria for the treatment of substance-related disorders. American Society of Addiction Medicine. [Google Scholar]
- Morin CM (1993). Insomnia: Psychological assessment and management. New York, NY: The Guilford Press. [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, & Hobson JA (2005). Sleep quality deteriorates over a binge–abstinence cycle in chronic smoked cocaine users. Psychopharmacology, 179(4), 873–883. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, & Nissen C (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, & Stein MD (2011). Assessing sleep in opioid dependence: A comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug & Alcohol Dependence, 113(2), 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liao Y, He H, Deng Q, Zhang G, Qi C, & Liu T (2015). Sleeping problems in Chinese illicit drug dependent subjects. BMC Psychiatry, 15, 28 10.1186/s12888-015-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, & Durrence HH (2003). Insomnia as a health risk factor. Behavioral Sleep Medicine, 1(4), 227–247. 10.1207/S15402010BSM0104_5. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, & Bush AJ (2007). Comorbidity of chronic insomnia with medical problems. Sleep, 30(2), 213–218. [DOI] [PubMed] [Google Scholar]
- Wang Y-M, Song M, Wang R, Shi L, He J, Fan T-T, & Gao Y-Y (2017). Insomnia and multimorbidity in the community elderly in China. Journal of Clinical Sleep Medicine, 13(04), 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]