Abstract
Increase in the number of patients with chronic kidney disease (CKD) calls for improved management of these patients. In stage 5 CKD, when the initiation of renal replacement therapy (RRT) becomes necessary, there is an increase in the infection risk of the patients and immunological tests for hepatitis C virus (HCV) detection turn positive at an alarmingly higher rate compared to general population. With the introduction into clinical practice of diagnostic tests, the increased prevalence of HCV among CKD patients has been known since the 1990s. Also, the negative impacts of HCV infection on CKD evolution as well as the unfavorable evolution of grafts received by HCV infected patients are known. Chronic hemodialysis patients are a category of patients whose risk of HCV infection is substantial. Currently, in the hemodialysis centers, at the base of the transmission of HCV infection there are a multitude of factors. Infection with HCV has a different impact on patient with end-stage renal disease (ESRD). Comorbidities in this case have significant sources of mortality and morbidity. It was proven that the post transplantations problems were prevented and mortality was reduced for patients who were diagnosed with HCV and in whom the infection was treated before the kidney transplant (KT). Consequently, early detection of the infection and the application of specific treatment has a considerable impact on the outcome of the patients. Another important component of the management of HCV infection in the chronic hemodialysis patients is the prevention of the infection transmission by applying specific methods.
Keywords: chronic kidney disease, hemodialysis, HCV infection, screening, prevention
1. Introduction
Chronic kidney disease (CKD) is a worldwide public health problem, due to the continuous increase in the number of patients. The rising prevalence of CKD is due especially to the increasing number of patients with diabetes mellitus (DM) and high blood pressure (HBP), which are the major risk factors of the disease. Besides inducing the risk of CKD, HBP may also be the consequence of CKD, occurring in most patients sooner or later in the evolution of the disease (1-3).
Irrespective of the etiology of CKD, the progressive loss of kidney functions has a strong impact on the health status of the patient and renal replacement therapy (RRT) by dialysis (hemodialysis or peritoneal dialysis) or kidney transplant, which is the necessary treatment for advanced stage CKD (4,5).
The infection risk in hemodialysis patients is considerable, explained by impaired immunity and by the need for frequent hospitalizations and surgical interventions. Moreover, hemodialysis itself involves frequent and/or prolonged exposure to blood by means of vascular access and the extracorporeal circuit, and by the proximity of other patients during dialysis, contact with the medical staff, and change of the dialysis machine. The infection with hepatitis C virus (HCV), a particular type of blood-borne viral infection, is relatively common in hemodialysis patients (6).
The association of chronic hepatitis with HCV with an increased morbidity-mortality of the transplanted and the hemodialysis patients is described in numerous studies in the specialized literature. Moreover, statistical studies show a prevalence of infection among CKD patients between 5 and 60% (in developed countries) with certain predominance in patients with chronic hemodialysis patients (7-9). In addition, the data available until 2006 confirm the increased incidence of HCV worldwide, 1.47 per 100 patient-years with a clear difference between developed and poorly developed countries (10,11).
The predominance among hemodialysis patients is supported by numerous studies in the specific literature (12,13). Despite development of prevention guidelines, the risk of transmission of the virus has not been substantially reduced. This justifies the need to increase the education of the medical staff and the patients in order to learn the rules of prevention of HCV transmission in the hemodialysis center (14-18). At the same time, in order to prevent the transmission of the infection, it is necessary to know the immunological status of the patients. Thus, the periodic check-up and screening of patients is especially important because most patients do not present clinical symptomatology suggestive for HCV infection (19).
2. Infection with HCV in patients on chronic hemodialysis
Demographic factors have an inconsistent role in the predominance of HCV, lowest percentage is found in Northern Europe, while the highest incidence is in Central and East Asia and North America. US has 1.6% while in the rural areas of Romania HCV prevalence is up to even 3% (20). HCV is responsible for ~10% of acute hepatitis cases, 75-85% of chronic hepatitis cases, and ~10-20% will develop liver cirrhosis over 20-30 years (20-22). Among patients with cirrhosis, there is: 1-5% annual risk of hepatocellular carcinoma and 3-6% annual risk of hepatic decompensation, for which the risk of death in the following year is 15-20% (20-22). It is estimated that worldwide 71 million individuals are infected with HCV, a great number of patients dying each year of HCV-related complications (23). HCV infection becomes chronic in a considerable proportion of patients (~75% of cases) (23). Among these, 27% are diagnosed with liver cirrhosis and 25% with liver carcinoma, 20 years after acquiring the infection (23,24).
The prevalence of HCV virus in US hemodialysis (HD) centers (2002 statistical data) has been estimated at ~8% and is higher compared to the general population (25,26). The increased prevalence of chronic hepatitis with HCV in the HD centers was also maintained in the analysis carried out in Europe (27,28). In this context, we emphasize that these observations are valid also in Romania. Comparing with the general population, studies in the literature also describe for Romania a higher prevalence of HCV virus among chronically hemodialysis patients. Despite efforts to improve the methods of preventing the transmission of HCV infection, in Romania both the incidence and the prevalence of the infection are still at an increased rate in chronic hemodialysis patients (29).
HCV was discovered in 1989 and is an RNA virus, member of the Hepacivirus genus, belonging to Flaviviridae family, which is divided into three species: Flavivirus, pestivirus and hepacivirus (30). It is ~30-80 nm in size and has a predominantly lipid content (31). HCV is currently classified into six major genotypes, which differ from each other by >30% at the nucleotide level, as well as numerous subtypes (655) (32).
HCV is transmitted predominantly by contact with blood or infected blood products: Transfusions, use of unsterilized needles and syringes often passed from one person to another, tattoos or vaccinations with unsterilized equipment, sharing of toothbrushes, shaving devices, pincers or manicure toolkits. HCV is also found in bodily fluids (saliva, mother's milk, vaginal secretions, seminal fluid, etc.), but in lower concentrations than hepatitis B virus (HBV). HCV can also be transmitted from mother to fetus (33,34). Vertical transmission rate in anti-HCV positive pregnant women has been estimated as 2.7-4.4%, and as high as 5.4-8.6% for pregnant women with HCV-human immunodeficiency virus (HIV) coinfection (35).
Besides liver involvement, extra hepatic manifestations of chronic HCV infection are common, including kidney involvement. The histopathological spectrum of CKD induced by HCV infection includes membrane-proliferative glomerulonephritis, IgA nephropathy, focal segmental glomerulosclerosis, fibrillary glomerulonephritis, tubulointerstitial nephropathy and thrombotic microangiopathy. The most frequent transmission route of HCV is exposure to infected blood - consequently, hemodialysis patients are at a high risk for infection (23,36), resulting in a higher prevalence of HCV infection than in the general population (37). It has been shown that infection risk increases with the duration of hemodialysis (23,36).
In patients on chronic hemodialysis, HCV positivity is associated with poorer survival (38), the risk of liver disease-related death being compounded by the higher risk of cardiovascular death attributed to chronic malnutrition and inflammation (37,39,40).
The need for early diagnosis and treatment of HCV infection in chronic hemodialysis patients calls for improved screening (17). In US, recent studies have revealed a 5% prevalence of HCV infection in patients on chronic hemodialysis compared to 1% in the general population (41). Romania is among the countries with a high prevalence of HCV infection (~6%) in the general population (42). Furthermore, the prevalence of HCV positivity in chronic hemodialysis patients is high, reaching 50% in some centers (29,42). These facts impose early detection of HCV infected patients and the application of methods for preventing infection transmission within the dialysis center (11,37).
3. Screening and diagnosis of the HCV infection in patients on chronic hemodialysis
According to the American Association for the Study of the Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) there are categories of individuals more exposed to the risk of HCV infection and in whom testing for the infection is recommended (41). Among them should be counted patients on chronic hemodialysis, and intravenous or intranasal drug users, patients who received a transplant before July 1992, previous prison inmates, and individuals with documented HIV infection. Testing for HCV infection is also recommended for at-risk individuals, namely in persons who suffered puncture injuries by needles or other sharp objects possibly contaminated, as well as in people born between 1945-1965(41). The higher prevalence of HCV infection in CKD patients compared to the general population and the rapid evolution to more advanced stages in the presence of HCV infection justify the need for screening in these patients (43).
For patients on chronic hemodialysis, testing for HCV infection is performed when RRT is initiated or when the patient is transferred from another dialysis center, or when the RRT method is changed (from peritoneal dialysis to hemodialysis), as well as periodically, every six months (43).
The diagnosis of the HCV infection calls for two categories of laboratory tests: Serological tests for anti-HCV antibodies detection and molecular tests for viral particles detection (44-46).
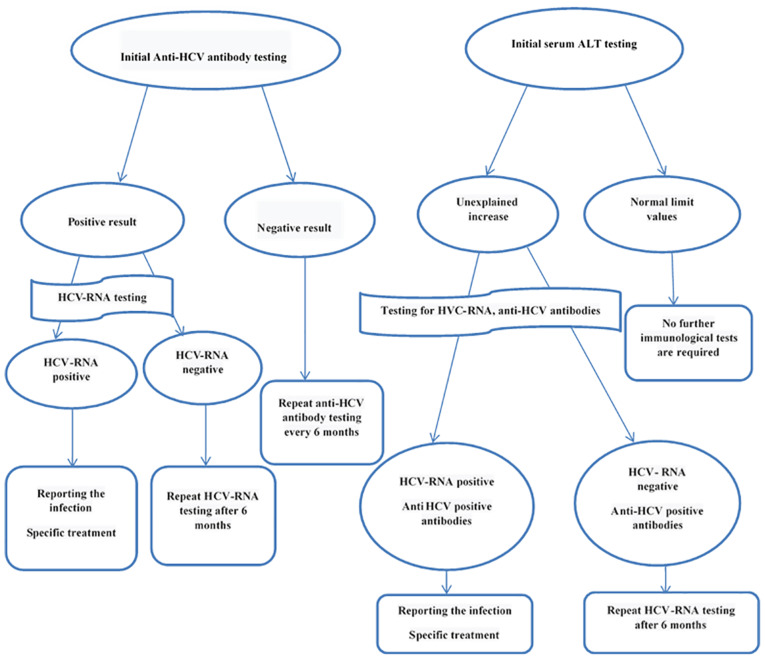
This approach is also appropriate for the diagnosis of HCV infection in chronic hemodialysis patients (Fig. 1). There is a time lapse between the time of HCV infection and the antibody detection in blood. This time span, called ‘serological window’ varies from patient to patient. The currently available serological tests allow for anti-HCV antibodies detection on average 7-8 weeks after the moment of the infection. After eradication of the infection, anti-HCV antibodies may have a lifelong persistence or their titer may progressively decrease until disappearance in several years. In patients with chronic infection, anti-HCV antibodies may persist indefinitely. If currently available laboratory tests yield positive results, confirmation by a supplementary test is necessary, namely recombinant immunoblot assay (RIBA) which indicates the reactivity against two viral antigens. As the screening tests and molecular techniques improve, confirmation by RIBA will become increasingly less necessary (except blood banks). Testing for HCV-RNA [by polymerase chain reaction (PCR)] confirms the diagnosis and quantifies the number of viral copies in the blood (viremia). Almost all patients with chronic infection have HCV-RNA detectable in blood (34,47).
Figure 1.
Diagnosis algorithm of HCV infection in hemodialysis patients (48).
In each patient who started on hemodialysis, testing for anti-HCV antibodies and assessing of liver transaminases (especially alanine transaminase) is recommended. Detection of anti-HCV antibodies does not make the distinction between current and past infection. HCV-RNA detection is necessary to confirm the diagnosis of active HCV infection (47). In contrast to anti-HCV antibodies, which may be detected in the blood 7-8 weeks after exposure, testing for HCV-RNA may detect HCV infection 1 week after exposure.
As there are several HCV genotypes and the specific treatment depends on the genotype, genotype identification is important. However, direct antiviral medication, which inhibits viral replication, covers several HCV genotypes, so that genotype specification does not have to be included in the diagnosis and treatment protocol of the infected patient (48).
The patients on chronic hemodialysis are usually tested for anti-HCV antibodies and for the serum level of alanine-transaminase (ALT). In the dialysis centers with a high prevalence of HCV infection, initial testing for HCV-RNA is recommended (43).
The patients in whom anti-HCV antibodies are detected should also be tested for HCV-RNA. In the patients with undetectable HCV-RNA levels, cured spontaneously or after antiviral treatment, testing should be performed every six months. In the positive patients for anti-HCV antibodies and with detectable HCV-RNA, declaring the infection and applying the specific treatment are mandatory (49).
For patients in whom HCV screening detects no infection, monitoring ALT every month and anti-HCV antibodies every six months are recommended (49). If ALT level is increasing, immunological testing for detecting HCV infection is recommended, otherwise it is unnecessary (49).
When a new case of HCV infection, seroconversion, is detected among the elderly patients of a dialysis center, an epidemiological inquiry should be performed, which should evaluate the possibility that the viral infection might have been acquired within the hemodialysis center or outside it, in other circumstances. An internal audit of the clinical practice should be performed regarding the correct application of the rules for preventing infection transmission and for proper hand hygiene, as well as the adequate employment of protective gloves. Furthermore, the viral status of the other patients of the dialysis center exposed to the infection risk should be assessed. For a good management, great importance should be attached to spotting and correcting any error in the control of infection prevention (48).
4. Preventing the dissemination of HCV infection in the hemodialysis center
Although HCV infection remains present in hemodialysis centers, a decrease in transmission between patients has been observed in recent years (9). Properly performing blood donor screening as well as reducing the need for transfusions in CKD patients (secondary to ESA treatment) are the most important elements that contributed to the slight decrease in the transmission rate of HCV virus in hemodialysis centers (50). Currently, an important cause of transmission of HCV infection within the hemodialysis center remains the failure to comply with the transmission prevention measures described in the specialized guides (11,17,51-54). In this sense, currently, within the hemodialysis centers the most frequently incriminated methods for transmitting HCV infection are represented by the inappropriate use of the medical equipment, the non-observance of the regular hygiene or of the administration of the parenteral treatment (55,56).
The above is supported by the data provided by the CDC in the period 2008-2015. According to reports, during this period, the incorrect application of the protocols to prevent the transmission of infection within the hemodialysis center was the main cause of HCV outbreaks (57).
Thus, in order to prevent the transmission of HCV infection, it is necessary to intensify the training actions of the medical personnel (58).
The importance of this decision is emphasized by the following: The persistence period of HCV on various surfaces (at room temperature) is over 16 h (11,17,29,38-42) and the incorrect manipulation of products that get into contact with the contaminated blood of patients can easily result in the dissemination of blood-borne viral infections (11).
Organizing treatment area in a hemodialysis center
In order to prevent HCV infection in the hemodialysis center, optimal compartmentalization of the space is necessary, such that an individual area for each patient should be provided, in which the hemodialysis session should be performed, called ‘patient's area’ (11). This area includes the treatment bed or armchair, the dialysis machine, and the appropriate surrounding space and should be considered contaminated. For this reason, a set of rules to prevent infection transmission should be observed, namely: Clean-up and disinfection should be performed after the patient has left the area, the sterile materials for dialyzing the next patient should be fetched into the patient's area after disinfection, and taking out sterile materials from one patient's area to be used in another patient's area is forbidden.
Isolation of infected patients and the employment of dialysis machines
In order to prevent the transmission of HCV infection in hemodialysis patients, the application of the same practice as in the case of chronically infected HBV patients as discussed, and there is a need to isolate patients. Thus, in the case of HBV infected patients, it is prudent to isolate patients by referring to the use of dedicated rooms and devices for the prevention of the transmission of viral infection. The medical staff is also required to follow a strict hygiene regulation and this includes the highest precautions regarding the use of the work equipment. Compliance with all these protection rules limits the transmission of HBV infection within the hemodialysis center (59). This is not the case, however, in the case of HCV-infected patients, which is confirmed by the literature studies (60).
Several studies have supported the utility of isolating chronic hemodialysis HCV positive patients and performing the treatment on dedicated machines, with the purpose of preventing the transmission of the viral infection (61). There are even studies recommending the isolation of HCV positive patients in a separate treatment room (62).
Recent research has proved that isolating HCV infected patients conveys no benefit for preventing infection transmission, the most indicated method to achieve it is to rigorously comply to the general rules of hygiene (11,12,63-66).
In infected hemodialysis patients, the approach depends on the type of hepatitis virus (B or C). Bearing in mind that the risk of HCV crossing the dialysis membrane is very low, in the case of HCV positive patients the use of dedicated dialysis machines it is not firmly recommended and the used machines may be placed in the treatment room for seronegative patients (11,67). According to the 2018 conclusions of Hepatitis C Work Group of Kidney Disease: Improving Global Outcomes (KDIGO), when HCV positive patients are dialyzed in the close vicinity of seronegative patients, with no or only cursory observance of the general hygiene rules, the risk of HCV transmission increases (11,67).
Hepatitis B virus (HBV) infected patients need to be isolated in separate rooms and hemodialysis treatment should be performed on dedicated machines, given the high infectious risk imparted by the large number of detected viral copies coupled with the long-term (up to seven days) persistence of HBV on surfaces (67).
In Romania, HCV positivity is highly prevalent among hemodialysis patients (29,42). According to the current legislation, a dialysis nurse cares for at least four patients (68). Ideally, connecting and blood restitution of the seropositive patients should be performed by a dedicated dialysis nurse. Nonetheless, it is allowed that the same nurse might treat both seropositive and seronegative patients during the same round. Under such circumstances, standard hygiene rules for preventing infection transmission should be strictly observed, as well as the following practice rules: the nurse should perform both the connection to the hemodialysis machine (at the beginning of the hemodialysis session) and the disconnection (at the end of the hemodialysis session) first for the seronegative patients and only thereafter for the seropositive ones (69).
Hand hygiene
In the hemodialysis center, rigorous observation of the hygiene rules is decisive in preventing HCV infection transmission. Hand hygiene should be performed by the medical staff before every contact with the patient or after every maneuver and after every contact with a potentially infected biological fluid or with the hemodialysis machine (11,70). Hand washing on the arrival and on the departure from the dialysis center and in the treatment room is recommended both for the medical staff and for the patients in RRT (11). Hemodialysis patients should also thoroughly wash their skin with soap and water at the level of the arteriovenous fistula, be it native or prosthetic (Fig. 2).
Figure 2.
Summary of the general rules for preventing HCV infection in a hemodialysis center (11,70).
Disinfection of the surfaces, of the dialysis machine, and of the reusable materials
Disinfection of dialysis machine, both internal and external, should be performed after each treatment, in compliance with good practice guidelines. Disinfection of the internal circuit should be done according to the producer's recommendations, using the specified substances during the recommended time span. If chemical substances are employed for internal disinfection, residue testing before starting dialysis is mandatory. At the end of dialysis session, disinfection of the external surfaces of the dialysis machine with low-level disinfectants is recommended if there is no sign of contamination with biologic fluids (such as blood), and with hypochlorite as disinfectant if such a contamination is noticeable. These maneuvers should always be performed after the patient has left the treatment area (11).
Besides disinfecting the dialysis machines and the reusable materials employed for treatment, proper disinfection of the surfaces is important, given the risk of being contaminated with biological fluids. Depending on the necessary level of disinfection (low, intermediary or high) use of the specific disinfecting substance, in the appropriate dilution is recommended (70).
Reusing the dialyzers
Reusing the dialyzers is a method commonly incriminated for increasing the risk of HCV transmission in dialysis centers (11).
One of the factors incriminated in favoring the transmission of HCV infection among patients is the switch of the blood port caps between patients or ineffective sterilization of blood port caps. Also, if there is spillage of contaminated blood or mixing of the reused dialyzers during transport it may lead to the infection of other HCV patients. Compliance with hygiene rules is salutary in preventing the infection of other patients. In the specialized literature there are two large studies that did not identify this method as an important tool in preventing the transmission of HCV infection within the hemodialysis center (25,63). Instead, in specialty literature we have identified a study where this association is described (71).
There are clear procedures of disinfecting and employing again the reusable dialyzers, so that the risk of infection transmission is eliminated. Currently, in most hemodialysis centers single-use materials are employed (11).
Parenteral treatment administration
The transmission of HCV infection is attributed to the incorrect administration of parenteral treatments. A current practice in dialysis centers incriminated for HCV infection transmission is the employment of multi-dose vials and of the blood glucose monitoring devices (11).
In dialysis centers, the multi-dose vials conditioned medication (such as heparin) should be prepared in a clean area exclusively dedicated to the preparation of injectable medication. Using the same multi-dose vial for bedside preparation of the injectable medication is forbidden. Individual syringes should be employed for administering the treatment from multi-dose vials (49).
Besides, preparing the medication in a clean area, which should be situated far from the dialysis machine, and using sterile syringes, the rules for preventing HCV infection transmission include hand hygiene both before and after medication preparation and administration, as well as changing the sterile gloves before each of these maneuvers. Antiseptics such as chlorhexidine, alcohol 70%, povidone-iodine or iodine tincture should be used (48). In hemodialysis centers with a high prevalence of HCV, replacement of multi-dose vials of heparin with individual vials is recommended (72) (Fig. 2).
Clinical audit
Periodic controls (clinical audit) have a critical role in the evaluation of the correct implementation of the clinical procedures designed for preventing HCV infection transmission in dialysis. Clinical audit should be performed by an impartial observer, based on clear criteria, and implies observing the activity in the dialysis center during a day or over several days. If flaws in the application of work procedures are noticed, including in those specific for HCV infection prevention transmission, medical staff will be instructed so that the flaws should be eliminated and the quality of provided health services should be improved. A time span of six months between two consecutive evaluations is recommended (11).
Aiming to a better assessment of the activity in the dialysis center, a change in the examination manner must be considered, together with the possibility of video monitoring the hygiene practices. This is the result of acknowledging the increased tendency of the staff to rigorously apply procedures when aware of being evaluated by the auditor, although this is not current practice (11,73).
Monitoring viral status
Knowing the immunological status of hemodialysis patients is important both for treatment administration and for preventing HCV transmission (69). Periodic evaluation of patients is therefore justified with the purpose of detecting as early as possible blood-borne viral infections and to prevent seroconversions in the patients of the hemodialysis center (17).
Antiviral treatment
Treatment of patients detected with HCV infection is another way of preventing the transmission, but it does not replace the other methods of preventing HCV infection transmission within the dialysis center (11).
Monitoring viral status
Knowing the immunological status of hemodialysis patients is important both for treatment administration and for preventing HCV transmission (43). Periodic evaluation of the patients is therefore justified with the purpose of detecting as early as possible blood borne viral infections and for preventing seroconversions in the patients of the hemodialysis center (16).
Antiviral treatment
Treatment of patients detected with HCV infection is another way of preventing the transmission, but it does not replace the other methods of preventing HCV infection transmission within the dialysis center (11).
5. Conclusions
The association of HCV infection with an increased mortality in the chronic hemodialysis patients emphasizes the great importance of prevention in the disease transmission within hemodialysis center. The application of the measures recommended by the international guidelines regarding the prevention of infection transmission within hemodialysis centers is of great importance. However, further studies are necessary for evaluating the way these universal rules should be applied to the geographical location of each hemodialysis center, with the aim of elaborating individualized good practice protocols.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
DT, DD, AEBS, DGB, OS and DI designed the study and wrote the manuscript. DT, DD, AEBS, DGB, OS, DI, MDT, AA and AT performed a literature search and selected the included studies. DT, DD, AEBS, DGB, OS and DI critically revised the manuscript. All authors read and approved the final manuscript. The contributions of all the authors on this review are greatly valued and appreciated.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mandita A, Timofte D, Balcangiu-Stroescu AE, Balan D, Raducu L, Tanasescu MD, Diaconescu A, Dragos D, Cosconel CI, Stoicescu SM, et al. Treatment of high blood pressure in patients with chronic renal disease. Rev Chim Buchar. 2019;70:993–995. [Google Scholar]
- 2.Balan DG, Tanasescu MD, Diaconescu A, Raducu L, Mihai A, Tanase M, Stanescu II, Ionescu D, Balcangiu-Stroescu AE. Nutritional intervention in patients with diabetic renal disease a brief presentation. Rev Chim Buchar. 2018;69:4078–4082. [Google Scholar]
- 3.Tanasescu MD, Diaconescu A, Raducu L, Balan DG, Mihai A, Tanase M, Stanescu II, Ionescu D, Balcangiu-Stroescu AE. Diabetic nephropathy: Aconcise assessment of the causes, risk factors and implications in diabetic patients. Rev Chim Buchar. 2018;69:4018–4021. [Google Scholar]
- 4.Zilisteanu DS. Chronic kidney disease, a major public health problem. Rom Med J. 2013;LX:18–26. [Google Scholar]
- 5.Piccoli GB, Alrukhaimi M, Liu ZH, Zakharova E, Levin A. What we do and do not know about women and kidney diseases; questions unanswered and answers unquestioned: Reflection on World Kidney Day and International Woman's Day. BMC Nephrol. 2018;19(66) doi: 10.1111/nep.13193. World Kidney Day Steering Committee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization: Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed December 15, 2019. [Google Scholar]
- 7.Covic A, Iancu L, Apetrei C, Scripcaru D, Volovat C, Mititiuc I, Covic M. Hepatitis virus infection in haemodialysis patients from Moldavia. Nephrol Dial Transplant. 1999;14:40–45. doi: 10.1093/ndt/14.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Vere CC, Streba CT, Streba L, Rogoveanu I. Statins in the treatment of hepatitis C. Hepat Mon. 2012;12:369–371. doi: 10.5812/hepatmon.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selcuk H, Kanbay M, Korkmaz M, Gur G, Akcay A, Arslan H, Ozdemir N, Yilmaz U, Boyacioglu S. Distribution of HCV genotypes in patients with end-stage renal disease according to type of dialysis treatment. Dig Dis Sci. 2006;51:1420–1425. doi: 10.1007/s10620-005-9025-9. [DOI] [PubMed] [Google Scholar]
- 10.Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: A systematic review and meta-analysis. Hemodial Int. 2013;17:532–541. doi: 10.1111/j.1542-4758.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 11.KDIGO 2018 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in Chronic Kidney Disease. Kidney Int Suppl. 2018;8:91–165. doi: 10.1016/j.kisu.2018.06.001. Kidney Disease; Improving Global Outcomes (KDIGO) Hepatitis C Work Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 13.Sauné K, Kamar N, Miédougé M, Weclawiak H, Dubois M, Izopet J, Rostaing L. Decreased prevalence and incidence of HCV markers in haemodialysis units: A multicentric French survey. Nephrol Dial Transplant. 2011;26:2309–2316. doi: 10.1093/ndt/gfq696. [DOI] [PubMed] [Google Scholar]
- 14.Hmaied F, Ben Mamou M, Saune-Sandres K, Rostaing L, Slim A, Arrouji Z, Ben Redjeb S, Izopet J. Hepatitis C virus infection among dialysis patients in Tunisia: Incidence and molecular evidence for nosocomial transmission. J Med Virol. 2006;78:185–191. doi: 10.1002/jmv.20526. [DOI] [PubMed] [Google Scholar]
- 15.Izopet J, Sandres-Sauné K, Kamar N, Salama G, Dubois M, Pasquier C, Rostaing L. Incidence of HCV infection in French hemodialysis units: A prospective study. J Med Virol. 2005;77:70–76. doi: 10.1002/jmv.20415. [DOI] [PubMed] [Google Scholar]
- 16.Mbaeyi C, Thompson ND. Hepatitis C virus screening and management of seroconversions in hemodialysis facilities. Semin Dial. 2013;26:439–446. doi: 10.1111/sdi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen DB, Gutowski J, Ghiselli M, Cheng T, Bel Hamdounia S, Suryaprasad A, Xu F, Moulton-Meissner H, Hayden T, Forbi JC, et al. A large outbreak of hepatitis C virus infections in a hemodialysis clinic. Infect Control Hosp Epidemiol. 2016;37:125–133. doi: 10.1017/ice.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, Desenclos JC. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol. 2005;26:752–760. doi: 10.1086/502613. [DOI] [PubMed] [Google Scholar]
- 19.Alghamdi AS, Sanai FM, Ismail M, Alghamdi H, Alswat K, Alqutub A, Altraif I, Shah H, Alfaleh FZ. SASLT practice guidelines: Management of hepatitis C virus infection. Saudi J Gastroenterol. 2012;18 (Suppl 1):S1–S32. doi: 10.4103/1319-3767.101155. Saudi Association for the Study of Liver Diseases and Transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61 (Suppl 1):S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Han R, Zhou J, François C, Toumi M. Prevalence of hepatitis C infection among the general population and high-risk groups in the EU/EEA: A systematic review update. BMC Infect Dis. 2019;19(655) doi: 10.1186/s12879-019-4284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SM, Song IH. Hepatitis C virus infection in chronic kidney disease: Paradigm shift in management. Korean J Intern Med. 2018;33:670–678. doi: 10.3904/kjim.2018.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 26.Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371–378. doi: 10.1053/j.ajkd.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Barril G, Traver JA. Decrease in the hepatitis C virus (HCV) prevalence in hemodialysis patients in Spa in: Effect of time, initiating HCV prevalence studies and adoption of isolation measures. Antiviral Res. 2003;60:129–134. doi: 10.1016/j.antiviral.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Taskapan H, Oymak O, Dogukan A, Utas C. Patient to patient transmission of hepatitis C virus in hemodialysis units. Clin Nephrol. 2001;55:477–481. [PubMed] [Google Scholar]
- 29.Caragea DC, Mihailovici AR, Streba CT, Schenker M, Ungureanu B, Caragea IN, Popa R, Obleaga C, Vere CC. Hepatitis C infection in hemodialysis patients. Curr Health Sci J. 2018;44:107–112. doi: 10.12865/CHSJ.44.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranjith-Kumar CT, Kao CC. Biochemical activities of the HCV NS5B RNA-dependent RNA polymerase. In: Hepatitis C Viruses: Genomes and Molecular Biology. Tan SL (ed). Horizon Bioscience, Norfolk, 2006. [PubMed] [Google Scholar]
- 31.Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The Viruses and their Replication. In: Fields Virology. 5th edition. Knipe DM and Howley PM (eds). Lippincott-Raven Publishers, Philadelphia, pp1101-1152, 2007. [Google Scholar]
- 32.Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 33.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 34.Wallach J. Hepatobiliary and pancreatic disorders. In: Interpreting Diagnostic Tests. 7th edition. Wallach J (ed). Medical Sciences Publishing House, Romania, pp316-323, 2001. [Google Scholar]
- 35.Gronowski AM. Screening and diagnosis of congenital infections. In: Handbook of Clinical Laboratory Testing During Pregnancy. Gronowski AM (ed). Totowa, NJ, p257, 2004. [Google Scholar]
- 36.Martin P, Jadoul M, Pol S. HCV in the hemodialysis population: Treat now or later? J Viral Hepat. 2020;27:233–234. doi: 10.1111/jvh.13224. [DOI] [PubMed] [Google Scholar]
- 37.Fabrizi F, Messa P, Martin P. Recent advances on hepatitis C virus in dialysis population. Kidney Blood Press Res. 2014;39:260–271. doi: 10.1159/000355803. [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, McAllister CJ, Miller LG. Clinical characteristics and mortality in hepatitis C-positive haemodialysis patients: A population based study. Nephrol Dial Transplant. 2005;20:1662–1669. doi: 10.1093/ndt/gfh895. [DOI] [PubMed] [Google Scholar]
- 39.Balcangiu-Stroescu AE, Tanasescu MD, Diaconescu A, Raducu L, Constantin AM, Balan DG, Tarmure V, Ionescu D. Cardiovascular comorbidities, inflammation and serum albumin levels in a group of hemodialysis patients. Rev Chim Buchar. 2018;69:926–929. [Google Scholar]
- 40.Diaconu C. Treatment of diabetes in patients with heart failure. In: Proceedings of the 3rd International Conference on Interdisciplinary Management of Diabetes Mellitus and its Complications -Diabetes mellitus as cardiovascular disease. INTERDIAB, Bucharest, pp170-177, 2017. [Google Scholar]
- 41.Gordon CE, Berenguer MC, Doss W, Fabrizi F, Izopet J, Jha V, Kamar N, Kasiske BL, Lai CL, Morales JM, et al. Prevention, diagnosis, evaluation, and treatment of Hepatitis C virus infection in chronic kidney disease: Synopsis of the kidney disease: Improving Global Outcomes 2018 Clinical Practice Guideline. Ann Intern Med. 2019;171:496–504. doi: 10.7326/M19-1539. [DOI] [PubMed] [Google Scholar]
- 42.Curescu M, Golea O, Brinzan F. Mihăilescu M and Cotospan E: Prevalence of anti-HCV antibodies in patients undergoing hemodialysis and in medical staff in hemodialysis centers. Rom J Infect Dis. 2007;X:145–148. [Google Scholar]
- 43.Jadoul M, Berenguer MC, Doss W, Fabrizi F, Izopet J, Jha V, Kamar N, Kasiske BL, Lai CL, Morales JM, et al. Executive summary of the 2018 KDIGO Hepatitis C in CKD Guideline: Welcoming advances in evaluation and management. Kidney Int. 2018;94:663–673. doi: 10.1016/j.kint.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Gheorghe G, Stoian AP, Găman MA, Socea B, Neagu TP, Stănescu AM, Bratu OG, Mischianu DL, Suceveanu AI, Diaconu CC. The benefits and risks of antioxidant treatment in liver diseases. Rev Chim Buchar. 2019;70:651–655. [Google Scholar]
- 45.Suceveanu AI, Pantea Stoian A, Mazilu L, Voinea F, Hainăroșie R, Diaconu CC, Pițuru S, Nițipir C, Badiu DC, Ceaușu I, et al. Interferon-free therapy is not a trigger for hepatocellular carcinoma in patients with chronic infection with hepatitis C virus. Farmacia. 2018;66:904–908. [Google Scholar]
- 46.Fierbinteanu-Braticevici C, Papacocea R, Tribus L, Cristian B. Role of 13C methacetin breath test for non invasive staging of liver fibrosis in patients with chronic hepatitis C. Indian J Med Res. 2014;140:123–129. [PMC free article] [PubMed] [Google Scholar]
- 47.Lothar T (ed) Hepatitis C. In: Clinical Laboratory Diagnostics - Use and Assessment of Clinical Laboratory Results. 1st edition. TH-Books, Germany, pp1273-1276, 1998. [Google Scholar]
- 48.Nguyen DB, Bixler D, Patel PR. Transmission of hepatitis C virus in the dialysis setting and strategies for its prevention. Semin Dial. 2019;32:127–134. doi: 10.1111/sdi.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. https://www.asn-online.org/ntds/resources/20190509-NCHHSTP_NTDS-HCV_Subcommittee_Algorithm.pdf. Accessed December 17, 2019. [Google Scholar]
- 50.Marwaha N, Sachdev S. Current testing strategies for hepatitis C virus infection in blood donors and the way forward. World J Gastroenterol. 2014;20:2948–2954. doi: 10.3748/wjg.v20.i11.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson ND, Novak RT, White-Comstock MB, Xia GL, Ganova-Raeva L, Ramach S, Khudyakov YE, Bialek SR, Williams IT. Patient-to-patient hepatitis C virus transmissions associated with infection control breaches in a hemodialysis unit. J Nephrol Therapeutics. 2012;10(2) [Google Scholar]
- 52.Aho-Glélé LS, Giraudon H, Astruc K, Soltani Z, Lefebvre A, Pothier P, Bour JB, Manoha C. Investigation of a case of genotype 5a hepatitis C virus transmission in a French hemodialysis unit using epidemiologic data and deep sequencing. Infect Control Hosp Epidemiol. 2016;37:134–139. doi: 10.1017/ice.2015.263. [DOI] [PubMed] [Google Scholar]
- 53.Thompson ND, Novak RT, Datta D, Cotter S, Arduino MJ, Patel PR, Williams IT, Bialek SR. Hepatitis C virus transmission in hemodialysis units: Importance of infection control practices and aseptic technique. Infect Control Hosp Epidemiol. 2009;30:900–903. doi: 10.1086/605472. [DOI] [PubMed] [Google Scholar]
- 54.Fabrizi F, Messa P. Transmission of hepatitis C virus in dialysis units: A systematic review of reports on outbreaks. Int J Artif Organs. 2015;38:471–480. doi: 10.5301/ijao.5000437. [DOI] [PubMed] [Google Scholar]
- 55.Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43. No authors listed. [PubMed] [Google Scholar]
- 56.Bianco A, Bova F, Nobile CG, Pileggi C, Pavia M. Healthcare workers and prevention of hepatitis C virus transmission: Exploring knowledge, attitudes and evidence-based practices in hemodialysis units in Italy. BMC Infect Dis. 2013;13(76) doi: 10.1186/1471-2334-13-76. Collaborative Working Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Centers for Disease Control and Prevention: Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention 2008-2015. https://www.cdc.gov/hepatitis/outbreaks/healthcarehepoutbreaktable.htm. Accessed July 27, 2018. [Google Scholar]
- 58.CDC urging dialysis providers and facilities to assess and improve infection control practices to stop hepatitis C virus transmission in patients undergoing hemodialysis. Am J Transplant. 2016;16:1633–1634. The Centers for Disease Control and Prevention. [Google Scholar]
- 59.Labriola L, Jadoul M. The decades-long fight against HBV transmission to dialysis patients: Slow but definite progress. Nephrol Dial Transplant. 2010;25:2047–2049. doi: 10.1093/ndt/gfq238. [DOI] [PubMed] [Google Scholar]
- 60.Jadoul M. Should hemodialysis patients with hepatitis C virus antibodies be isolated? Semin Dial. 1995;8:1–3. [Google Scholar]
- 61.Soliman AR, Momtaz Abd Elaziz M, El Lawindi MI. doi: 10.5402/2013/395467. Evaluation of an isolation program of hepatitis C virus infected hemodialysis patients in some hemodialysis centers in Egypt. ISRN Nephrol: Oct 31, 2013 (Epub ahead or print). doi: 10.5402/2013/395467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal SK. Hemodialysis of patients with HCV infection: Isolation has a definite role. Nephron Clin Pract. 2011;117:c328–c332. doi: 10.1159/000319984. [DOI] [PubMed] [Google Scholar]
- 63.Jadoul M, Cornu C, van Ypersele de Strihou C. Universal precautions prevent hepatitis C virus transmission: A 54 month follow-up of the Belgian Multicenter Study. Kidney Int. 1998;53:1022–1025. doi: 10.1111/j.1523-1755.1998.00823.x. The Universitaires Cliniques St-Luc (UCL) Collaborative Group. [DOI] [PubMed] [Google Scholar]
- 64.Petrosillo N, Gilli P, Serraino D, Dentico P, Mele A, Ragni P, Puro V, Casalino C, Ippolito G. Prevalence of infected patients and understaffing have a role in hepatitis C virus transmission in dialysis. Am J Kidney Dis. 2001;37:1004–1010. doi: 10.1016/s0272-6386(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 65.Mactier R, Davies S, Dudley C, Harden P, Jones C, Kanagasundaram S, Lewington A, Richardson D, Taal M, Andrews P, et al. Summary of the 5th edition of the Renal Association Clinical Practice Guidelines (2009-2012) Nephron Clin Pract. 2011;118 (Suppl 1):c27–c70. doi: 10.1159/000328060. [DOI] [PubMed] [Google Scholar]
- 66.Section VI. Haemodialysis-associated infection. Nephrol Dial Transplant. 2002;17 (Suppl 7):72–87. European Best Practice Guidelines Expert Group on Hemodialysis European Renal Association. [PubMed] [Google Scholar]
- 67.Garthwaite E, Reddy V, Douthwaite S, Lines S, Tyerman K, Eccles J. Clinical practice guideline management of blood borne viruses within the haemodialysis unit. BMC Nephrol. 2019;20(388) doi: 10.1186/s12882-019-1529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Regulament din 23 Decembrie 2004. http://legislatie.just.ro/Public/DetaliiDocumentAfis/61116. Accessed December 18, 2019. [Google Scholar]
- 69. Recommendations for Preventing Transmission of Infections Among Chronic Hemodialysis Patients. MMWR Recomm Rep 50: 1-43, 2001. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5005a1.htm. Accessed December 18, 2019. [Google Scholar]
- 70.Constancio NS, Gomes Ferraz ML, Tzanno C, Martins B, Campos Kraychete A, Bitencourt PL, Mazza do Nascimento M. doi: 10.1590/2175-8239-JBN-2018-0177. Hepatitis C in hemodialysis units: Diagnosis and therapeutic approach. J Bras Nefrol 41: 539-549 (In English, Portuguese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.dos Santos JP, Loureiro A, Cendoroglo Neto M, Pereira BJ. Impact of dialysis room and reuse strategies on the incidence of hepatitis C virus infection in haemodialysis units. Nephrol Dial Transplant. 1996;11:2017–2022. doi: 10.1093/oxfordjournals.ndt.a027090. [DOI] [PubMed] [Google Scholar]
- 72.Alashek WA, McIntyre CW, Taal MW. Hepatitis B and C infection in haemodialysis patients in Libya: Prevalence, incidence and risk factors. BMC Infect Dis. 2012;12(265) doi: 10.1186/1471-2334-12-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. National Kidney Foundation: Hepatitis C management and hemodialysis. https://www.kidney.org/professionals/KDOQI/12-10-1601. Accessed December 18, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.