Abstract
The monitoring and care of patients with chronic kidney disease (CKD) before the dialysis initiation contribute to a better survival rate and an improvement in quality of life. The patients who do not benefit from a good predialysis management have a worse short and long-term prognosis. A retrospective, unicentric study was performed to evaluate the status of patients with stage 5 CKD at the time of initiation of renal replacement treatment. A total of 109 patients were included in the study. The evaluation of the patients included the clinical manifestations leading to hemodialysis initiation, the clinical and laboratory data of the patients when the hemodialysis was started. Based on the obtained data, a statistical analysis was performed using the Chi-square test, Fisher's exact test, ANOVA, and Kruskal-Wallis H test. The mean age of the patients was 64.61±13.59 years. Of the patients 51.38% were women. Vascular nephropathies and diabetes mellitus dominated the etiology of CKD. The comorbidities were high blood pressure, ischemic heart disease, history of myocardial infarction, heart failure, history of stroke, peripheral artery disease or atrial fibrillation. Only 43 (39.45%) of our patients were monitored before the hemodialysis initiation. Hemodialysis was initiated on central venous catheter (in most cases non-tunneled) in 78.90% of the patients. Most of the patients had an altered general status, fatigue/tiredness with poor exercise capacity when hemodialysis was initiated. Most of the patients (98.17%) had anemia, the average level of hemoglobin being 8.69±1.85 g/dl. In conclusion, careful monitoring of patients in the early stages of CKD would result in lower morbidity and mortality. These objectives can be achieved by implementing screening programs and early interventions.
Keywords: chronic kidney disease, predialysis management, dialysis initiation, renal replacement treatment, arterio-venous fistula, central venous catheters
Introduction
Chronic kidney disease (CKD) has a considerable impact on health, by progressive loss of kidney functions up to end-stage renal disease (ESRD) requiring renal replacement treatment (RRT), either by dialysis or by kidney transplant. Moreover, health and survival are endangered by the complications associated with CKD, such as anemia, disturbances of bone and mineral metabolism, high blood pressure (HBP), cardiovascular disease, anxiety or depression and altered quality of life (1,2).
Therefore, an early diagnosis is important, followed by adequate assessment, monitoring, and treatment of patients with CKD, because proper management may slow down the progression and even prevent the occurrence of ESRD (1).
Referring these patients to the nephrologist is one of the mainstays of the correct management of CKD. An early assessment by the nephrologist may unravel the etiology of kidney disease and may lead to prompt initiation of strategies able to delay the progressive decline of kidney function, prevent and treat the complications, and appropriately prepare for and timely initiate RRT (3).
Materials and methods
This is a retrospective, unicentric study regarding the status of patients with stage 5 CKD at the time of RRT initiation. The evaluation included the clinical manifestations leading to hemodialysis initiation, as well as the clinical and laboratory features of the patients when hemodialysis was started, including etiology of CKD, associated comorbidities, biological status, previous nephrological monitoring, preparation of patients by vascular access creation prior to the initiation of RRT. The subjects of this study were patients with stage 5 CKD admitted to the Nephrology Clinic of the Emergency University Hospital of Bucharest (Romania), in whom hemodialysis was initiated between January 1st, 2017 and March 31st, 2018. The inclusion criteria were age over 18 years and hemodialysis initiation. The exclusion criteria was acute kidney injury unresponsive to conservatory treatment, but with recovery of the kidney function after several hemodialysis sessions.
A total of 109 patients were included in the study. Information was obtained regarding age, gender, socioeconomic background, etiology and duration of CKD, risk factors, history, including HBP, diabetes mellitus (DM), myocardial infarction, left ventricular hypertrophy, ischemic heart disease (IHD), heart failure (HF), stroke, peripheral arterial disease (PAD), and cardiac arrhythmias. The assessment included also routine laboratory tests. Glomerular filtration rate was calculated by means of 2009 CKD-EPI Creatinine Equation. The statistical analysis was performed by means of Chi-square test, Fisher's exact test, ANOVA, and Kruskal-Wallis H test.
This study was approved by Ethics Committee of the Emergency University Hospital of Bucharest. Written informed consent was obtained from all patients prior to publication.
Results
Demographics
In our sample, there were 56 (51.38%) women and 53 men (48.62%). The mean age of the patients was 64.61±13.59 years, with a minimum age of 25 and a maximum of 91 years. In women, the average age was 66.46±14.09 years and in men 62.66±12.88 years. Of the patients 69.72% lived in an urban setting.
Etiology
In our sample, the etiology of CKD was: i) vascular nephropathies (28%); ii) DM (24%); iii) glomerular nephropathies (10%); iv) obstructive nephropathy (9%); v) tubulointerstitial nephropathy (5%); vi) other etiology including kidney polycystic disease (6%); and vii) unknown etiology in 12% of the patients. Gender predominance varied according to CKD etiology, with a female preponderance in diabetic nephropathy, obstructive nephropathy, tubulointerstitial nephropathy, and in kidney disease of unknown cause. By contrast, there was a male preponderance for vascular and glomerular nephropathies, and for polycystic kidney disease.
Comorbidities
Most of our patients (93 i.e., 85.32%) had HBP. There was a mild female preponderance (48 out of 93) among our hypertensive patients. There were 46 patients with left ventricular hypertrophy (LVH), most of these patients being males (31 males vs. 15 females). The higher proportion of males was statistically significant: Chi-squared with Yates correction = 3.87 with a P=0.049, while the P-value yielded by Fisher's exact test was 0.036. HF was present in 41 (37.61%) patients. A history of stroke was identified in 15 patients (7 females and 8 males). PAD was present in 27 patients (13 females and 14 males). Atrial fibrillation (AF) was present in 19.27% of the patients.
Duration of CKD
The duration of CKD prior to RRT was not known in 42 of our patients. In the other 67 patients, the mean duration of the known evolution of CKD (from the moment of diagnosis until RRT initiation) was 41.28±35.71 months.
Pre-dialysis monitoring
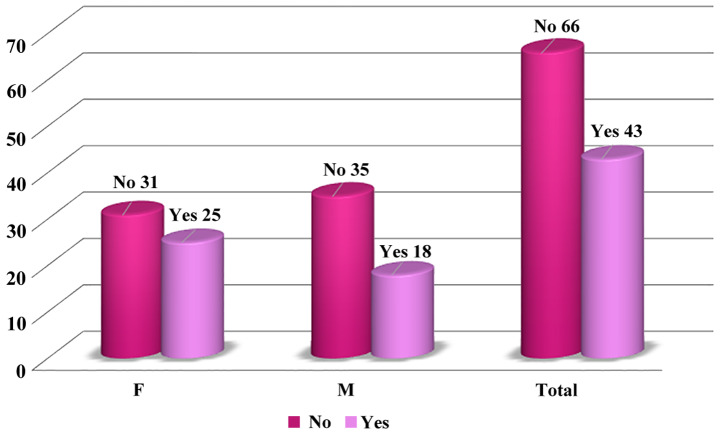
Only 43 (39.45%) of our patients were monitored prior to hemodialysis initiation. Among these patients followed by a nephrologist, there was a higher proportion of females (Table I; Fig. 1).
Table I.
The characteristics of the study group.
| General characteristics | Values |
|---|---|
| Total number of patients | 109 |
| Sex | F: 51.38%; M: 48.62% |
| Socioeconomic background | Urban: 69.72%; Rural: 30.28% |
| Mean age | 64.61±13.59 years |
| Duration of CKD | 41.28±35.71 months |
| Body mass index | 25.97±6.27 kg/m2 |
| CKD etiology (%) | |
| Vascular nephropathies | 28.44 |
| Diabetic nephropathy | 23.85 |
| Glomerular nephropathy | 10.09 |
| Tubulointerstitial nephropathy | 4.59 |
| Polycystic kidney disease | 6.42 |
| Obstructive nephropathy | 9.17 |
| Unknown etiology | 11.93 |
| Comorbidities (%) | |
| High blood pressure | 85.32 |
| Ischemic heart disease | 68.81 |
| Left ventricular hypertrophy | 42.20 |
| Cardiac failure | 37.61 |
| Stroke | 13.76 |
| Peripheral arterial disease | 24.77 |
| Atrial fibrillation | 19.27 |
F, female; M, male; CKD, chronic kidney disease.
Figure 1.
Distribution of patients as a function of predialysis monitoring. F, female; M, male.
Vascular access and preparation for hemodialysis
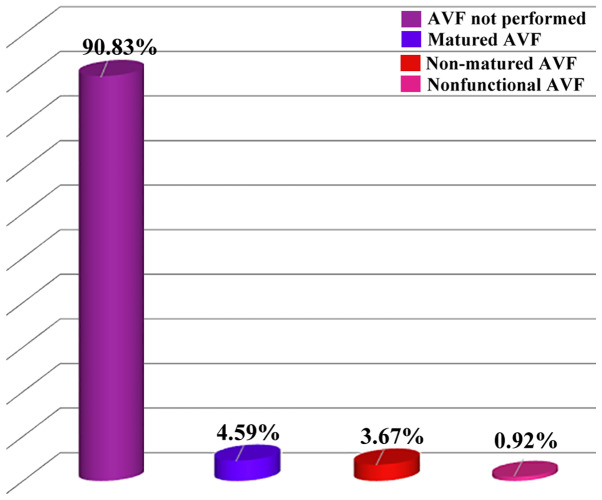
In our study, a low availability of arteriovenous fistula (AVF) was identified for hemodialysis initiation: only 10 of or patients (9.17%) presented AVF, and only 5 of them had AVF mature enough to be used for hemodialysis. Consequently, hemodialysis was initiated on central venous catheter (CVC) (in most cases non-tunneled) in 78.90% of the patients (Fig. 2).
Figure 2.
Distribution of the patients as a function of FAV existence at the initiation hemodialysis. AVF, arteriovenous fistula.
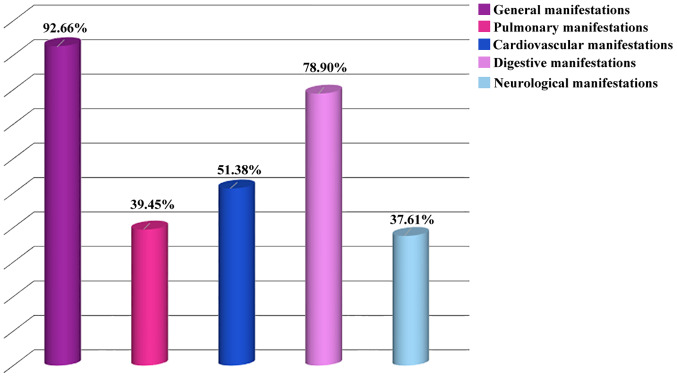
For the patients included in our study, hemodialysis was initiated at a GFR of 4 to 5 ml/min/1.73 m2. Hemodialysis was initiated at a higher GFR in males (5.32±2.14 ml/min/1.73 m²) than in females (4.25±1.96 ml/min/1.73 m²), a statistically significant difference (P=0.007 on ANOVA, P=0.0013, corresponding to a 10.34 statistic, on Kruskal-Wallis H test). Most of the patients included in our study had an altered general status and fatigue/tiredness with poor exercise capacity when started on hemodialysis (Fig. 3).
Figure 3.
Distribution of the patients as a function of clinical manifestations at hemodialysis initiation.
Pulmonary manifestations
Pulmonary manifestations were present in 39.45% of the patients and cardiovascular manifestations in more than half of the cases. Acute pulmonary edema was present in 17.43% of the patients, but 33.94% had clinical and/or laboratory signs of hyperhydration. Digestive manifestations, such as loss of appetite, nausea and vomiting were encountered in most of the patients (78.90%) enrolled in our study. Only a low proportion of patients (7.34%) had hemorrhagic events when hemodialysis was initiated, and 5.5% had an upper gastrointestinal bleeding.
Neurological manifestations
A proportion of 37.61% of the patients had neurological manifestations, but only 1.83% had uremic encephalopathy (Table II).
Table II.
Biological parameters of the study group.
| Parameters | Values |
|---|---|
| Hemoglobin | 8.6±1.8 g/dl |
| Cholesterol | 173.14±47.14 mg/dl |
| Triglycerides | 164.04±91.32 mg/dl |
| Calcium | 7.7±1.11 mg/dl |
| Phosphorus | 6.55±2.43 mg/dl |
| Calcium x Phosphorus | 33.2±27.2 mg/dl |
| Potassium | 5.06±1.32 mmol/dl |
| Uric acid | 7.4±2.7 mg/dl |
| Albumin | 3.35±0.70 g/dl |
Anemia
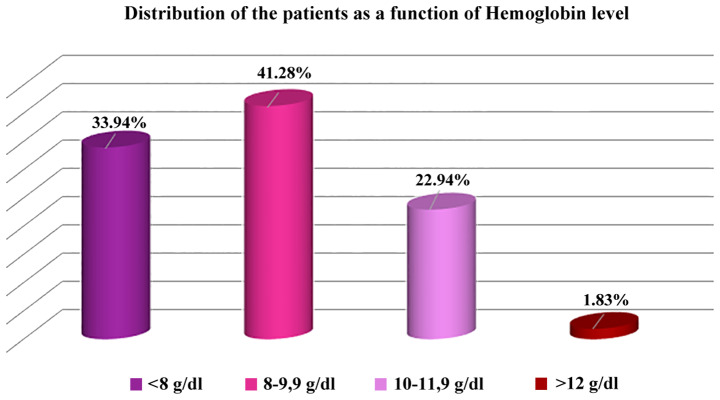
Most of the patients (98.17%) had anemia, the mean level of hemoglobin being 8.69±1.85 g/dl. Dividing the patients in three categories based on the level of hemoglobin (10-12 g/dl, 8-9.9 g/dl, and less than 8 g/dl), the middle category was the most numerous (41.28%).
In our study, the hemoglobin level did not differ significantly between the patients monitored by a nephrologist (8.71±1.65 g/dl) and the unmonitored ones (8.67±1.98 g/dl) (Fig. 4).
Figure 4.
Distribution of the patients as a function of hemoglobin level.
Nutritional status
The mean serum albumin level was 3.35±0.70 g/dl and mean serum uric acid 7.44±2.7 mg/dl, while the mean body mass index was 25.97±6.27 kg/m2.
Bone and mineral metabolism
The mean serum calcium level was 7.70±1.11 mg/dl and mean serum phosphorus 6.55±2.43 mg/dl. Most of the patients had hypocalcemia.
Discussion
In our study group there were slightly more females, although most of the currently available studies agree on a higher prevalence of CKD in males than in females, and also on a slower progression of CKD in females than in males (3). The fact that most of the patients included in the study lived in an urban setting may be explained by healthcare facilities being more readily available to the urban population. The poor access to medical care in the countryside is a consequence of the low socioeconomic level (4,5). Vascular nephropathies and DM were the dominant etiology of CKD, in agreement with available data pointing out HBP and DM as the main causes of CKD (6,7). However, it should be noted that HBP may also be the result of CKD, not only the cause (8).
Regarding gender predominance for diabetic nephropathy, a recent study concluded that female gender should be counted among the unmodifiable risk factors for diabetic nephropathy, while male gender is one of the factors incriminated for its progression (7). It is common knowledge that HBP is one of the major risk factors for glomerulosclerosis (9). Both systolic and diastolic arterial pressure were higher in males (10). These observations may explain the higher percentage of male patients in our study, with HBP as etiology for CKD. In our study, the patients with CKD secondary to a glomerular disease were more frequently males. As reported in the literature, the gender predominance differs according to the type of glomerular disease: females in lupus nephritis and males in acute post infectious nephritis, focal segmental glomerulosclerosis and IgA nephropathy (11). In our study, information was lacking about the exact type of glomerular disease responsible for ESRD, therefore we could not find an explanation for the higher percentage of males in the subset defined by glomerular disease etiology. The female predominance among the patients with obstructive nephropathy as the cause of ESRD in our sample is at odds with the data in the literature, where male gender dominates this particular etiology of ESRD (12). For autosomal dominant polycystic kidney disease, the data in the literature indicate that RRT is initiated in males earlier than in females, which may explain the results of our study (13).
Most of our patients had HBP, which is in agreement with the data from the literature indicating a 90% prevalence of HBP among ESRD patients at the time when hemodialysis is initiated (14). Of our patients 68.81% had IHD at the time when hemodialysis was started. IHD is an important comorbidity in patients started on hemodialysis, as it is associated with a higher mortality (15). Other identifiable comorbidities were left ventricular hypertrophy (LVH), HF, stroke, PAD and AF. The available studies confirm the presence of LVH in patients with ESRD, and associate it with an increased risk of sudden death, highlighting the need that the practitioners should pay particular attention to therapeutic strategies aimed at reducing LVH (16). Like IHD, HF is an important risk factor for death in ESRD patients started on hemodialysis, both on short and long-term (17). Stroke seems to occur with increased frequency in the 3 months preceding the onset of chronic hemodialysis, the highest risk being in the month preceding the initiation of RRT. However, after starting hemodialysis, the stroke risk declines (18). A history of PAD in patients started on chronic hemodialysis is associated with a higher need of either revascularization or amputation (19). Patients with AF are known to have a bad prognosis because of a higher risk of cardiovascular events (20-23).
According to literature, the evolution of CKD to more advanced stages is slower in elderly patients (24).
The patient with CKD needs specialized care. The guidelines state that specialized nephrological care is preferable for patients with a GFR <60 ml/min/1.73 m2 and mandatory for those with a GFR <30 ml/min/1.73 m2 (25). The occurrence of complications typical for CKD (HBP, cardiovascular disease, anemia, bone and mineral metabolism disturbances, anxiety or depression) has an important impact on the health status and on the survival of these patients (1). Early monitoring and implementation of therapeutic strategies aimed at slowing down the progressive decline of kidney function, as well as adequate preparation for and timely initiation of RRT, are important for ameliorating the prognosis of these patients (2). Delaying the referral to a nephrologist is one of the major issues leading to suboptimal care of a CKD patient. A patient not included soon enough in a monitoring program lead by a nephrologist is more likely to be started on RRT on an emergency basis, which may be deleterious for the outcome of the patient, resulting in lower quality of life and increased morbidity, mortality, and healthcare costs (26).
When RRT is required, the clinician together with the patient have to choose between hemodialysis, peritoneal dialysis and kidney transplant. The first option for treatment of choice (in Romania and in most other countries) is hemodialysis (27). Performing hemodialysis requires vascular (venous) access. The conclusion of most of the studies and the current recommendation in most of the guidelines is that the preferred vascular access for hemodialysis initiation is an arteriovenous fistula (AVF). However, in most ESRD patients hemodialysis is initiated on a CVC, either untunneled or tunneled (28). The data collected in our study are consistent with the literature. Suboptimal preparation of the patients is the result of poor referral to the specialist. Regarding GFR, there is no optimal level for hemodialysis initiation defined by the guidelines. The symptoms and the general lack of well-being of the patient, rather than a certain GFR are major criteria for starting hemodialysis (27). However, the occurrence of certain manifestations recommend earlier initiation of hemodialysis, even at a GFR of 15 ml/min/1.73 m2, including hyperkalemia, hyper hydration, weight loss and malnutrition, cognitive decline (29,30). The identified symptomatology of our patients is in line with specialty literature: pulmonary, cardiovascular and digestive manifestations, acute pulmonary edema.
CKD patients are known to have a higher risk of bleeding, gastrointestinal tract being frequently involved (31).
However, our study identified an unacceptably high number of patients with a hemoglobin level below 8 g/dl, which is an important issue because a level of hemoglobin below 8 g/dl is associated with higher mortality. This increased death risk persists even if the hemoglobin level is restored to above 10 g/dl after 4 months of dialysis (32). The most probable explanation is that among the 43 patients monitored by a nephrologist only a few (23.26%) received an erythropoiesis stimulating agent, and none of them reached the targeted level of hemoglobin, a sobering fact that underlines the necessity of improving anemia management in predialysis patients (32).
Both in predialysis and in chronically hemodialysis patients there is a close relation between the nutritional status and mortality (33). The nutritional status was assessed by means of the body mass index and the serum albumin level, lipid fractions, and uric acid. In most of the subjects the serum cholesterol level was below 150 mg/dl, while the serum triglycerides level was normal, which differs from the results of other studies in which the serum triglycerides level was increased (34,35). In our study, we evaluated markers of nutritional status (albumin, uric acid, body mass index), as well as bone and mineral metabolism (calcium, phosphorus) markers.
Unfortunately, data regarding the serum PTH level were not available. Assessing for bone and mineral metabolism disorders is of paramount importance for the survival of the patients on chronic hemodialysis, because these complications have a great impact on both morbidity and mortality (36,37), due to their close association with cardiovascular complications (38).
In conclusion, increasing the number of monitored patients in early stages of CKD and improving their management result in lower morbidity and mortality. These objectives may be achieved by implementing screening programs and early interventions. In the advanced stages of the disease, when RRT is needed, choosing the optimal moment and the type of treatment are decisions that should be taken by the physician together with the patient and his/her family members and individually adapted to the needs of each patient.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Due to confidentiality reasons data generated or analyzed during this study are not included in this published article.
Authors' contributions
DT, DD, AEBS, DGB, OS and DI designed the study, analysed and interpreted datasets and wrote the manuscript. MDT and LM collected the data and analysed the datasets. DT, DD, AEBS, DGB, OS, DI, LR and CRJ performed a literature search and selected the studies to be included. DT, DD, AEBS, DGB, OS and DI critically revised the manuscript. All authors read and approved the final manuscript. The contributions of all the authors on this article are greatly valued and appreciated.
Ethics approval and consent to participate
This study was approved by Ethics Committee of the Emergency University Hospital of Bucharest (Romania). Written informed consent was obtained from all patients prior to publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zilisteanu DS. Chronic kidney disease, a major public health problem. Rom Med J. 2013;70:18–26. [Google Scholar]
- 2.Piccoli GB, Alrukhaimi M, Liu ZH, Zakharova E, Levin A. What we do and do not know about women and kidney diseases; questions unanswered and answers unquestioned: Reflection on World Kidney Day and International Woman's Day. BMC Nephrol. 2018;19(66) doi: 10.1111/nep.13193. World Kidney Day Steering Committee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wavamunno MD, Harris DC. The need for early nephrology referral. Kidney Int Suppl. 2005;67:S128–S132. doi: 10.1111/j.1523-1755.2005.09429.x. [DOI] [PubMed] [Google Scholar]
- 4.Balcangiu-Stroescu AE, Tanasescu MD, Diaconescu A, Raducu L, Constantin AM, Balan DG, Tarmure V, Ionescu D. Cardiovascular comorbidities, inflammation and serum albumin levels in a group of hemodialysis patients. Rev Chim Buchar. 2018;69:926–929. [Google Scholar]
- 5.Francu V. Inequities in addressability and accessibility of vulnerable groups to health care services. Transilv Acta Med. 2013;2:174–176. [Google Scholar]
- 6.Balan DG, Balcangiu Stroescu AE, Tanasescu MD, Diaconescu A, Raducu L, Mihai A, Tanase M, Stanescu II, Ionescu D. nutritional intervention in patients with diabetic renal disease - A brief presentation. Rev Chim Buchar. 2018;69:4078–4082. [Google Scholar]
- 7.Balcangiu Stroescu AE, Tanasescu MD, Diaconescu A, Raducu L, Balan DG, Mihai A, Tanase M, Stanescu II, Ionescu D. Diabetic nephropathy: A concise assessment of the causes, risk factors and implications in diabetic patients. Rev Chim Buchar. 2018;69:4018–4021. [Google Scholar]
- 8.Mandita A, Timofte D, Balcangiu-Stroescu AE, Balan D, Raducu L, Tanasescu MD, Diaconescu A, Dragos D, Cosconel CI, Stoicescu SM, et al. Treatment of high blood pressure in patients with chronic renal disease. Rev Chim Buchar. 2019;70:993–995. [Google Scholar]
- 9.Fervenza FC. Nephrosclerosis. Medscape, 2018. https://emedicine.medscape.com/article/244342-overview. Accessed September 19, 2019. [Google Scholar]
- 10.August P. Hypertension in men. J Clin Endocrinol Metab. 1999;84:3451–3454. doi: 10.1210/jcem.84.10.6124. [DOI] [PubMed] [Google Scholar]
- 11.O'Shaughnessy MM, Hogan SL, Thompson BD, Coppo R, Fogo AB, Jennette JC. Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey. Nephrol Dial Transplant. 2018;33:661–669. doi: 10.1093/ndt/gfx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klahr S. Obstructive nephropathy. Intern Med. 2000;39:355–361. doi: 10.2169/internalmedicine.39.355. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa I, Maeda K, Nakai S, Kawaguchi Y. Gender difference in the mean age at the induction of hemodialysis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:1072–1075. doi: 10.1016/s0272-6386(00)70042-4. [DOI] [PubMed] [Google Scholar]
- 14.Malliara M. The management of hypertension in hemodialysis and CAPD patients. Hippokratia. 2007;11:171–174. [PMC free article] [PubMed] [Google Scholar]
- 15.Inaguma D, Koide S, Takahashi K, Hayashi H, Hasegawa M, Yuzawa Y. Relationship between history of coronary heart disease at dialysis initiation and onset of events associated with heart disease: A propensity-matched analysis of a prospective cohort study. BMC Nephrol. 2017;18(79) doi: 10.1186/s12882-017-0495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charytan D. Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr Opin Nephrol Hypertens. 2014;23:578–585. doi: 10.1097/MNH.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: A systematic integrative review. BioMed Res Int. 2014;2014(937398) doi: 10.1155/2014/937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol. 2013;24:1166–1173. doi: 10.1681/ASN.2012080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plantinga LC, Fink NE, Coresh J, Sozio SM, Parekh RS, Melamed ML, Powe NR, Jaar BG. Peripheral vascular disease-related procedures in dialysis patients: Predictors and prognosis. Clin J Am Soc Nephrol. 2009;4:1637–1645. doi: 10.2215/CJN.02220409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka A, Inaguma D, Shinjo H, Murata M, Takeda A. Asami Takeda the Aichi cohort study of prognosis in patients newly initiated into dialysis (AICOPP) study group, presence of atrial fibrillation at the time of dialysis initiation is associated with mortality and cardiovascular events. Nephron. 2016;132:86–92. doi: 10.1159/000443314. Aichi Cohort Study of Prognosis in Patients Newly Initiated into Dialysis (AICOPP) Study Group. [DOI] [PubMed] [Google Scholar]
- 21.Laslo C, Pantea Stoian A, Socea B, Paduraru D, Bodean O, Socea L, Neagu TP, Stanescu AMA, Marcu D, Diaconu C. New oral anticoagulants and their reversal agents. J Mind Med Sci. 2018;5:195–201. [Google Scholar]
- 22.Tica OA, Tica O, Antal L, Hatos A, Popescu MI, Pantea Stoian A, Bratu OG, Gaman MA, Pituru SM, Diaconu CC. Modern oral anticoagulant treatment in patients with atrial fibrillation and heart failure: Insights from the clinical practice. Farmacia. 2018;66:972–976. [Google Scholar]
- 23.Gaman MA, Dobrica EC, Pascu EG, Cozma MA, Epingeac ME, Gaman AM, Pantea Stoian A, Bratu OG, Diaconu CC. Cardiometabolic risk factors for atrial fibrillation in type 2 diabetes mellitus: Focus on hypertension, metabolic syndrome and obesity. J Mind Med Sci. 2019;6:157–161. [Google Scholar]
- 24.Giannelli SV, Graf CE, Herrmann FR, Michel JP, Patel KV, Pizzarelli F, Ferrucci L, Guralnik J. Natural history of older adults with impaired kidney function: The InCHIANTI study. Rejuvenation Res. 2011;14:513–523. doi: 10.1089/rej.2011.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(Part 1). Section I. Measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transplant. 2002;17:7–15. doi: 10.1093/ndt/17.suppl_7.7. European Best Practice Guidelines for Haemodialysis. [DOI] [PubMed] [Google Scholar]
- 26.Jones C, Roderick P, Harris S, Rogerson M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant. 2006;21:2133–2143. doi: 10.1093/ndt/gfl198. [DOI] [PubMed] [Google Scholar]
- 27.Chan CT, Blankestijn PJ, Dember LM, Gallieni M, Harris DCH, Lok CE, Mehrotra R, Stevens PE, Wang AY, Cheung M, et al. Conference Participants: Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96:37–47. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Xue H, Ix JH, Wang W, Brunelli SM, Lazarus M, Hakim R, Lacson E Jr. Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am J Kidney Dis. 2013;61:123–130. doi: 10.1053/j.ajkd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurella Tamura M, O'Hare AM, McCulloch CE, Johansen KL. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis. 2010;56:1117–1126. doi: 10.1053/j.ajkd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abra G, Kurella Tamura M. Timing of initiation of dialysis: Time for a new direction? Curr Opin Nephrol Hypertens. 2012;21:329–333. doi: 10.1097/MNH.0b013e328351c244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang CC, Chou CY, Chang CT, Wang IK, Huang CC. Upper gastrointestinal bleeding as a risk factor for dialysis and all-cause mortality: A cohort study of chronic kidney disease patients in Taiwan. BMJ Open. 2016;6(e010439) doi: 10.1136/bmjopen-2015-010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaboyas A, Morgenstern H, Waechter S, Fleischer NL, Vanholder R, Jacobson SH, Sood MM, Schaubel DE, Inaba M, Pisoni RL, et al. Low hemoglobin at hemodialysis initiation: An international study of anemia management and mortality in the early dialysis period. Clin Kidney J. 2019;sfz065 doi: 10.1093/ckj/sfz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon YE, Kee YK, Yoon CY, Han IM, Han SG, Park KS, Lee MJ, Park JT, Han SH, Yoo TH, et al. Change of nutritional status assessed using subjective global assessment is associated with all-cause mortality in incident dialysis patients. Medicine (Baltimore) 2016;95(e2714) doi: 10.1097/MD.0000000000002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandya V, Rao A, Chaudhary K. Lipid abnormalities in kidney disease and management strategies. World J Nephrol. 2015;4:83–91. doi: 10.5527/wjn.v4.i1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang WL, Zhu XY, Zhu N, Su CY, Han QF, Wang T, Zhang AH. What's the optimal lipids level for dialysis patients? A cohort study from a Chinese dialysis center in a university hospital. PLoS One. 2016;11(e0167258) doi: 10.1371/journal.pone.0167258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafeez E, Raza H, Khan RU, Anwar MA, Hussain T, Beg MA. CKD-MBD spectrum at the time of initiation of hemodialysis in Pakistani chronic kidney disease patients. Saudi J Kidney Dis Transpl. 2015;26:823–826. doi: 10.4103/1319-2442.160227. [DOI] [PubMed] [Google Scholar]
- 37.Soohoo M, Feng M, Obi Y, Streja E, Rhee CM, Lau WL, Wang J, Ravel VA, Brunelli S, Kovesdy CP, et al. Changes in markers of mineral and bone disorders and mortality in incident hemodialysis patients. Am J Nephrol. 2016;43:85–96. doi: 10.1159/000444890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komaba H, Igaki N, Takashima M, Goto S, Yokota K, Komada H, Takemoto T, Kohno M, Kadoguchi H, Hirosue Y, et al. Calcium, phosphorus, cardiovascular events and all-cause mortality in hemodialysis patients: A single-center retrospective cohort study to reassess the validity of the Japanese Society for Dialysis Therapy guidelines. Ther Apher Dial. 2008;12:42–48. doi: 10.1111/j.1744-9987.2007.00539.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to confidentiality reasons data generated or analyzed during this study are not included in this published article.