Abstract
Angiotensin-converting enzyme inhibitors (ACEIs) represent an important group of pharmacological compounds, largely prescribed for more than 30 years. They have been extensively evaluated in clinical trials, demonstrating significant reduction of morbidity and mortality of patients with cardiovascular diseases, mainly high blood pressure, myocardial infarction, heart failure and stroke. Besides their beneficial effects and a general good safety profile, it was proven that ACEIs might also induce adverse effects in some patients, most notably angioedema (AE) and chronic cough. The occurrence rate of adverse events induced by ACEIs is low, but the number of suffering patients is relatively high, since ACEIs is one of the most frequently prescribed medication worldwide. The aim of our study was to evaluate clinical pattern, risk factors and general management of ACEI-induced angioedema in a cohort of patients addressed for allergist evaluation in one university hospital in Romania, during a period of 32 months. It was found that ACEI-induced angioedema (ACEI-AE) represented more than half of the total number of patients addressed for angioedema without urticaria, with variable clinical and time-patterns. Most of the patients were referred by general practitioners (GPs) with diagnosis of urticaria or other skin allergy and continued to take ACEIs for months and years after onset of angioedema. We concluded that the awareness of acquired, non-allergic angioedema induced by ACEI therapy in medical practice is still low and there is a need for improved knowledge and interdisciplinary collaboration in this field.
Keywords: acquired angioedema, angiotensin-converting enzyme inhibitors, allergy center, safety profile
Introduction
Angiotensin-converting enzyme inhibitors (ACEIs) are known as a potent antihypertensive medication since early 1980s, representing an important and largely prescribed group of pharmacological compounds (1). They have been extensively evaluated in clinical trials, demonstrating significant reduction of morbidity and mortality of patients with cardiovascular diseases, mainly high blood pressure, myocardial infarction, heart failure and stroke. It was found that ACEIs represented the most commonly used antihypertensive medication in the United States since 2002(2). Besides their beneficial effects and a general good safety profile, it was proven that ACEIs might also induce adverse effects in some patients, most notably chronic cough and angioedema (AE) (3). The occurrence rate of adverse events to ACEIs is low, with estimated incidence of angioedema between 0.1-2.2%, but the number of suffering patients is relatively high, since ACEIs is one of the most frequently prescribed classes of medication worldwide (4,5). Besides angioedema and cough, other less frequent adverse reactions were mentioned, such as hyperkalemia, hypotension, liver dysfunction and bone marrow depression.
Adverse reactions induced by ACEIs are secondary to interference with the renin-angiotensin-aldosterone system (RAAS), which is responsible for the development and progression of cardiovascular and renal disorders. ACEIs block angiotensin-converting enzyme (ACE) activity, decrease conversion of angiotensin I to angiotensin II and also impact the degradation metabolism of bradykinin, leading to its increasing accumulation. The increased level of bradykinin is responsible for cough and angioedema in some patients, who become intolerant to this class of medication. Bradykinin is a potent local mediator of vasodilatation and vascular permeability, with very short half-life, being rapidly metabolized by peptidases in tissues and serum (6). The mechanism of ACEIs intolerance is not fully understood and genetic and/or environmental risk factors are considered to play an important role (7). It was proven that black race and female sex are genetic risk factors, while other possible mixed genetic and environmental risk factors are age over 65, history of drug rash, concomitant seasonal or persistent allergic diseases, concomitant medication with nonsteroidal anti-inflammatory drugs (NSAIDs) or immunosuppressive agents and smoking, while diabetes seems to be a protective factor (8,9).
Angioedema is a potentially severe, life-threatening adverse event associated with ACEI medication and the reported number of affected patients is accepted to be continuously increasing (5). Retrospective studies from the beginning of ACEI therapy mentioned that ACEI-induced angioedema (ACEI-AE) represented 30-40% of the patients with angioedema presenting to emergency departments (ED) (10). Despite long experience with this class of medication, ACEIs-AE is still underestimated and mislabeled as allergic AE or urticaria by many physicians and its potential for severe outcome is ignored. Recent publications on angioedema update diagnosis criteria and management recommendations for both hereditary and acquired angioedema, including mechanisms and characteristics of ACEI-induced angioedema (11). There are two main forms of acquired angiodema in clinical practice: Mast cell-mediated and bradykinin-mediated angioedema (12,13).
The aim of our study was to evaluate clinical pattern, risk factors, outcome and general management of ACEI-induced angioedema in a cohort of patients addressed for allergist evaluation in one university hospital from Romania, during a period of 32 months.
Materials and methods
A retrospective study of hospital data was performed of the patients addressed for allergy consultation to the hospital-based Allergy center during a 32 months period, between January 2017 to August 2019. The following data were recorded from the patients files: Age at hospital presentation, sex, indication for ACEIs therapy, types of ACE inhibitors used, treatment duration before occurrence of the first AE episode, AE localization, frequency and recurrence rate, concomitant diseases and medication, allergist recommendations and management. The study group consisted of 50 patients who presented for angioedema during the study period, being treated with ACEIs for variable time periods previously. The study group represented 56.81% from the total number of 88 patients addressed to the department for angioedema without urticaria, during the same period of time. The patients were aged between 45-83 years, with a mean age of 63 years and the sex distribution showed a 64% female predominance. From the total study group of 50 patients, 45 (90%) were treated with ACEIs for hypertension, 4 patients were treated for chronic heart failure and one patient had ischemic heart disease associated with chronic kidney disease. Patients characteristics in terms of age, sex, hypertension stage and concomitant diseases are showed in Table I. The patients signed an informed consent for publication of their health data
Table I.
Patients characteristics.
| Mean | 63 years |
|---|---|
| Age | |
| <65 years | 27 pts (54%) |
| ≥65 years | 23 pts (46%) |
| Sex | |
| Female | 32 pts (64%) |
| Male | 18 pts (36%) |
| Hypertension stage | |
| Stage II | 14 pts (28%) |
| Stage III | 36 pts (72%) |
| Concomitant diseases | |
| Diabetes mellitus type II | 11 pts (22%) |
| Chronic intermittent urticaria | 16 pts (30%) |
| COPD | 2 pts (4%) |
| Autoimmune thyroiditis | 6 pts (12%) |
| Degenerative osteoarticular disease | 12 pts (24%) |
Results
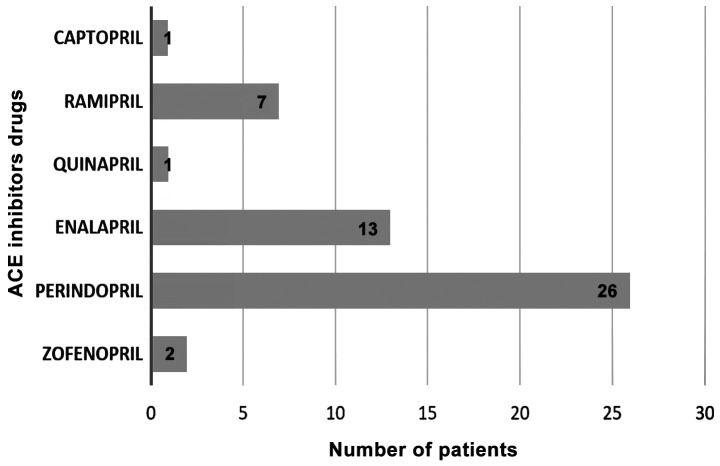
In total, 34 patients out of 50 (68%) received ACEIs in monotherapy and 16 patients used fixed dose combinations with ACE inhibitors. The most frequently used pharmacological substances were perindopril in 52% of patients and enalapril in 26% (Fig. 1).
Figure 1.
Pharmacological substances from ACEIs class used by the study group (number of patients for each substance). ACEIs, angiotensin-converting enzyme inhibitors.
Double or triple combinations with ACEIs were: Perindopril plus indapamide/perindopril plus amlodipine/perindopril plus indapamide plus amlodipine.
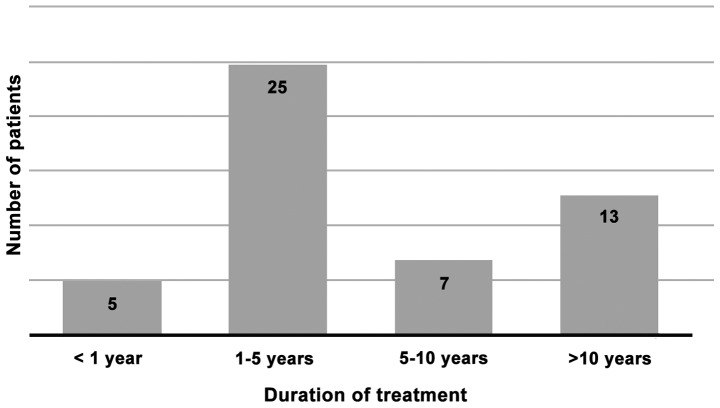
Regarding the duration of ACEI therapy before onset of angioedema, this was less than one year in only 5 patients (10%). In one case angioedema occurred during the same day of the first ACEI intake in one fixed dose combination, in two cases AE occurred during the first month and in further 2 patients AE occurred after 7 and 12 months, respectively. In half of the studied group, the AE episodes occurred within a time period between 1-5 years after ACEI therapy initiation, in 7 patients (14%) AE occurred after variable time period between 5-10 years, while in 13 patients (26%) AE occurred after more than 10 years (Fig. 2).
Figure 2.
Duration of treatment with ACEIs (number of patients for each time period). ACEIs, angiotensin-converting enzyme inhibitors.
The patients continued to take ACEIs after AE onset, except three cases who discontinued ACEIs and replaced it with angiotensin-receptor blocker (ARBs) before hospital presentation.
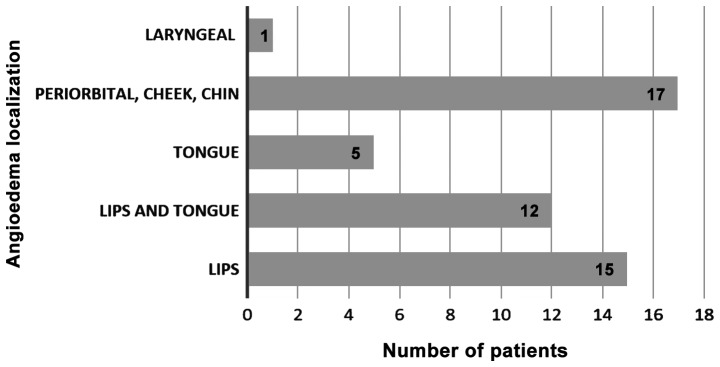
Regarding the site of angioedema, all patients presented with facial angioedema, 15 patients had angioedema of the lips, 12 patients had angioedema of both lips and tongue and 5 patients developed tongue angioedema alone. Laryngeal edema was found in one case only (Fig. 3).
Figure 3.
Angioedema localization (number of patients for each site).
Regarding the frequency of AE episodes, about half of the study group (24 patients) presented for allergist evaluation after 1 or 2 episodes of angioedema and 26 patients presented after 3 or more AE episodes. The AE recurrence rate was lower, ~1-3 AE episodes per year in cases of patients with longer AE history before hospital presentation. Only 18 patients had AE at the time of hospital presentation, including the patient with laryngeal AE confirmed by ENT examination.
It was found that 16 patients (32%) declared history of chronic mild urticaria, but not concomitant with AE episodes and 12 patients (24%) declared intermittent intake of non-steroidal anti-inflammatory drugs (NSAIDs) for chronic osteo-articular symptoms, which was coincident with AE episodes in only two cases.
Written documentation regarding medical attitude at ED could be found in less than half of the patients, with confirmation of adrenaline administration in only six cases.
Allergist recommendations on hospital presentation consisted of monitoring until complete resolution of AE, immediate discontinuation of ACEIs and replacement with other antihypertensive therapy, mostly diuretics and/or calcium blockers and ARBs in five cases, evaluation of severity and possible risk factors for AE, treatment with systemic corticosteroids and antihistamines. The clinical outcome was good in all cases, with almost complete remission of AE in less than 24 h. In 6 patients (12%) recurrence of AE episodes was recorded during the first month after discontinuation of ACEIs, but with reduced intensity and duration.
Discussion
The study showed that ACEI-induced angioedema represented more than half of the patients with acquired angioedema addressed to one hospital-based Allergy center during a period of 32 months. Almost all of the patients from the study group were referred by their general practitioners (GPs) with diagnosis of urticaria or other skin allergies, being mislabeled as allergy in most of the cases. It was found that a significant number of patients had a long history of angioedema, until diagnosis confirmation, more than 10 years after initiation of ACEI treatment. This is a longer period comparing with published data from the literature, reporting that ACEI-AE can appear from few hours up to 8 years after ACEI therapy initiation (14). Previous publications showed that AE most often occurred within the first month of ACEI treatment and ~27% of cases occurred after more than 6 months after treatment initiation (10). Regarding the risk factors for ACEI induced angioedema mentioned in the literature, the results of this study showed a 64% female predominance, with not relevant contribution of age >65 years, co-morbidities or concomitant medication, possible due to rather small study group.
Data from the literature showed that ACEI-related angioedema has an increasing prevalence and severity during the last decades, being underestimated in clinical practice (15). It was proven that failure to recognize the relation between angioedema and ACEI treatment may lead to unnecessary morbidity and mortality, caused by the lack of medical provider knowledge and low frequency of this type of reaction. A published survey of physician's knowledge, including cardiologists, GPs and allergists, demonstrated a rather poor awareness of the ACEI adverse reactions, with correct responses in about half of the group (16). Majority of the patients with angioedema are considered having food or drug allergy and treated with antihistamines, corticosteroids and epinephrine, despite unclear response. Difficulties in recognizing allergies and discriminating between allergic and intolerant patients are due to limited university or postgraduate training of medical staff in the field of allergic diseases, despite increasing prevalence and severity of this pathology (17). A recent local study investigating knowledge and attitude of GPs in the field of drug allergies revealed a significant need for training and updated information in allergic diseases, as well as for interdisciplinary collaboration (18).
Angioedema without urticaria may raise significant problems in clinical practice, mainly due to unclear etiopathology, variable clinical presentation and potential for severe outcome (19). The classic clinical presentation of ACEI-AE is recurrent episodes of lips and tongue edema, without urticaria and pruritus (20). History of atopy or concomitant allergies are usually missing and clinical outcome is not significantly influenced by antihistamines and corticosteroids, due to non-allergic underlining mechanism (21). The late onset as well as variable clinical pattern of ACEI-AE can be confusing for both patients and medical professionals, who should take into consideration the potential role of risk factors and triggers (22). Depending on associated risk factors or concomitant diseases and medications, patients may have an increased risk for severe forms, even fatal angioedema due to laryngeal edema (23). This life-threatening reaction is possible even after the first dose of an ACEI, with higher risk in female patients and in African-American population (24). It has been proven that African-American patients have an increased sensitivity to bradykinin compared to Caucasian patients and that patients with heart failure have higher rates of angioedema due to ACEI medication (25,26). In rare cases of acquired angioedema in adult patients, this may be first attributed to ACEI therapy, but later revealing another mechanism and pathology, such is the acquired C1-inhibitor deficiency due to hematological neoplasia or autoimmune or unknown diseases (27). Regarding therapeutic approach of ACEI-induced angioedema, several new drugs used for the treatment of hereditary angioedema, such as icatibant (a bradykinin receptor antagonist), have been evaluated, due to similar pathophysiological mechanism (9).
The main limitation of our study is the small study group and experience of one allergy center only, during a rather short period of time.
It was concluded that acquired, non-allergic angioedema induced by ACEI therapy is a frequent and possible severe condition in medical practice and the awareness of both patients and medical staff is still low. It is important that all clinicians have adequate information and training with regard to safety profile of ACEIs and the most frequent possible adverse events, such as recurrent angioedema and chronic cough. A standardized medical attitude, including detailed written documentation of medication at emergency departments and improved interdisciplinary collaboration in this field are needed.
Acknowledgements
Not applicable.
Funding
Not funding was received.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
PML designed the current study, analyzed and interpreted datasets and wrote the manuscript. VFA composed and analyzed the datasets. CB, AM and DB contributed to the study design, drafting and revision of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The patients signed an informed consent for publication of their health data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests in relation with this study and manuscript.
References
- 1.Smith RE, Ashiya M. Antihypertensive therapies. Nat Rev Drug Discov. 2007;6:597–598. doi: 10.1038/nrd2354. [DOI] [PubMed] [Google Scholar]
- 2.Gu Q, Paulose-Ram R, Dillon C, Burt V. Antihypertensive medication use among US adults with hypertension. Circulation. 2006;113:213–221. doi: 10.1161/CIRCULATIONAHA.105.542290. [DOI] [PubMed] [Google Scholar]
- 3.Dunlap ME, Peterson RC. ACE inhibitors vs ARBs: Is one class better for heart failure. Cleve Clin J Med. 2002;69:433–438. doi: 10.3949/ccjm.69.5.433. [DOI] [PubMed] [Google Scholar]
- 4.Cicardi M, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: Outcome after switching to a different treatment. Arch Intern Med. 2004;164:910–913. doi: 10.1001/archinte.164.8.910. [DOI] [PubMed] [Google Scholar]
- 5.Banerji A, Clark S, Blanda M, LoVecchio F, Snyder B, Camargo CA Jr. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008;100:327–332. doi: 10.1016/S1081-1206(10)60594-7. [DOI] [PubMed] [Google Scholar]
- 6.Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621–622. doi: 10.1056/NEJM200208223470820. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, Fukui T, Bates DW. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoover T, Lippmann M, Grouzmann E, Marceau F, Herscu P. Angiotensin converting enzyme inhibitor induced angio-oedema: A review of the pathophysiology and risk factors. Clin Exp Allergy. 2010;40:50–61. doi: 10.1111/j.1365-2222.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- 9.Terreehorst I, Reitsma S, Cohn DM. Current treatment of Angioedema induced by ACE inhibitors. Curr Treat Options Allergy. 2019;6:18–26. [Google Scholar]
- 10.Gabb GM, Ryan P, Wing LM, Hutchinson KA. Epidemiological study of angioedema and ACE inhibitors. Aust N Z J Med. 1996;26:777–782. doi: 10.1111/j.1445-5994.1996.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 11.Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K, Caballero T, Farkas H, Grumach A, Kaplan AP, et al. HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology): Classification, diagnosis, and approach to treatment for angioedema: Consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602–616. doi: 10.1111/all.12380. [DOI] [PubMed] [Google Scholar]
- 12.Craig TJ, Bernstein JA, Farkas H, Bouillet L, Boccon-Gibod I. Diagnosis and treatment of bradykinin-mediated angioedema: Outcomes from an angioedema expert consensus meeting. Int Arch Allergy Immunol. 2014;165:119–127. doi: 10.1159/000368404. [DOI] [PubMed] [Google Scholar]
- 13.Nedelea I, Deleanu D. Isolated angioedema: An overview of clinical features and etiology (Review) Exp Ther Med. 2019;17:1068–1072. doi: 10.3892/etm.2018.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21–25. doi: 10.1016/s0162-3109(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 15.Zingale LC, Beltrami L, Zanichelli A, Maggioni L, Pappalardo E, Cicardi B, Cicardi M. Angioedema without urticaria: A large clinical survey. CMAJ. 2006;175:1065–1070. doi: 10.1503/cmaj.060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardi C, Crivellaro M, Dama A, Senna G, Gargioni S, Passalacqua G. Are physicians aware of the side effects of angiotensin-converting enzyme inhibitors?: A questionnaire survey in different medical categories. Chest. 2005;128:976–979. doi: 10.1378/chest.128.2.976. [DOI] [PubMed] [Google Scholar]
- 17.Shakib S, Caughey GE, Fok JS, Smith WB. Adverse drug reaction classification by health professionals: Appropriate discrimination between allergy and intolerance? Clin Transl Allergy. 2019;9(18) doi: 10.1186/s13601-019-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leru PM. Drug allergies in primary care practice in Romania: A questionnaire-based survey. Allergy Asthma Clin Immunol. 2014;10(16) doi: 10.1186/1710-1492-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aberer W. Angioedema is not just ‘deep urticaria’ but an entity of its own. Allergy. 2014;69:549–552. doi: 10.1111/all.12362. [DOI] [PubMed] [Google Scholar]
- 20.Agostoni A, Cicardi M. Drug-induced angioedema without urticaria. Drug Saf. 2001;24:599–606. doi: 10.2165/00002018-200124080-00004. [DOI] [PubMed] [Google Scholar]
- 21.Cicardi M, Zanichelli A. Acquired angioedema. Allergy Asthma Clin Immunol. 2010;6(14) doi: 10.1186/1710-1492-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller PI, Messmer SL, Haefeli WE, Schlienger RG, Bircher AJ. Angiotensin-converting enzyme inhibitor-induced angioedema: Late onset, irregular course, and potential role of triggers. Allergy. 1997;52:432–435. doi: 10.1111/j.1398-9995.1997.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 23.Krogh Nielsen T, Bygum A, Rye Rasmussen E. Life-threatening angio-oedema after the first dose of an ACE inhibitor-not an anaphylactic reaction. BMJ Case Rep. 2016;2016(pii: bcr2016214364) doi: 10.1136/bcr-2016-214364. doi: 10.1136/bcr-2016-214364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean DE, Schultz DL, Powers RH. Asphyxia due to angiotensin converting enzyme (ACE) inhibitor mediated angioedema of the tongue during the treatment of hypertensive heart disease. J Forensic Sci. 2001;46:1239–1243. [PubMed] [Google Scholar]
- 25.Makani H, Messerli FH, Romero J, Wever-Pinzon O, Korniyenko A, Berrios RS, Bangalore S. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383–391. doi: 10.1016/j.amjcard.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 26.McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332:1177–1181. doi: 10.1136/bmj.38803.528113.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leru PM, Anton VF, Bumbea H. Nine year follow-up of a rare case of angioedema due to acquired C1-inhibitor deficiency with late onset and good response to attenuated androgen. Allergy Asthma Clin Immunol. 2018;14(69) doi: 10.1186/s13223-018-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.