Abstract
Background
Ultrasound (US) in psoriasis (PsO) and psoriatic arthritis (PsA) is an important tool in several situations to detect joint ecostructural damage as well as other tissue alterations, such as those that occur in the larger vessels. The objective of this study was to detect and correlate the changes that indicate the inflammatory and atherosclerotic process in two groups of patients, using nail US and carotid artery intima–media thickness radiofrequency (RF) software.
Methods
A total of 30 patients diagnosed with (PsO) and (PsA) were selected. About 15 patients were present in each group, assigned by the Dermatology and Rheumatology Service of the Universidade Pontifícia Católica de Campinas, São Paulo, Brazil, and were assessed using carotid artery US (radiofrequency quality intima–media thickness [RF-QIMT]), joint US, clinical evaluation, and laboratory tests.
Results
Spearman and Pearson correlations between US variables per group were Psoriasis Area and Severity Index (PASI) and loss of the nail pattern trilaminar: r=0.658, p=0.015; Framingham Score (FS) and Internal Resistance Index (IR): 0.351 to 0.526, p=0.034 to 0.002; the significant correlations by the Bayesian factor (BF) were those with a BF greater than 2.5, between QIMT expected with FS: r=0.677, BF=10.06, with total cholesterol: r=0.5232, BF=2.60, and QIMT-RF with low density lipoproteins: r=0.633, BF=3.70.
Conclusion
The use of US in the evaluation of these patients showed significant correlations between clinical and laboratory measures, characterized by QIMT and FS as well as changes in nail insertion. Future studies may demonstrate an even better interaction.
Keywords: atherosclerosis, carotid artery, psoriasis, psoriatic arthritis, spectral Doppler, ungueal disease
Introduction
Psoriatic arthritis (PsA) and psoriasis (PsO) are chronic inflammatory diseases, immunomediated with a broad clinical spectrum, involving inflammation of the tendon, enthesis, and nails.1,2 These diseases are closely associated with cardiovascular diseases and metabolic syndrome, which are the main causes of mortality of PsA and PsO patients.3,4 This is due to a persistent systemic inflammation, which induces endothelial dysfunction and an inflammatory process in the vessel walls, promoting, as a consequence, atherosclerosis and thickening of the carotid intima–media layer.5,6
The use of ultrasound (US) in PsA and PsO is an important tool in several conditions to detect joint ecostructural damage as well as other tissue changes, such as those that occur in the vessels.
Both gray scale and Doppler are US tools that detect early and established injuries, such as joint and vascular injuries of small, medium, and large vessels. With power Doppler, we can evaluate subclinical joint injuries in PsA due to the presence of blood flow, on account of the progressive neo-angiogenesis caused by joint tissue inflammation damage. Using spectral Doppler, which evaluates hemodynamically the inflammatory process through the Internal Resistance Index (IR), we are able to quantify all the joint tissue damage due to inflammation.
Investigations have attempted to track the correlation between IR and the presence of inflammation in different joint sites, including the nail, especially if there is a flow change in the nail insertion, as well as gray scale changes, due to the thickening of the nail bed and alteration of the trilaminar pattern of the nail plate.1,7
On the one hand, using US with the quality intima–media thickness (QIMT) method, we are able to assess the inflammatory activity of these diseases and also estimate the cardiovascular risk by assessment of the carotid artery. On the other hand, in the metabolic context, through US analysis of the carotid intima–media layer thickness, we are able to estimate the cardiovascular risk: a difference of 0.1 mm increases the risk of acute myocardial infarction from 10 to 15% and stroke from 13 to 18%.8
Therefore, considering the greater predisposition of these patients to cardiovascular impact and systemic inflammatory changes, we evaluated the carotid, other metabolic variables, and sonographic findings of the nail bed and nail lamina in PsO and PsA patients.
This study aimed to detect and correlate the changes in an inflammatory and atherosclerotic process in two groups of patients, using spectral Doppler, nail gray scale’s parameters, and the arteries’ QIMT radiofrequency (RF) software.
Methods
Patients and study design
This non-randomized cross-sectional study was conducted at the Pontifícia Universidade Católica de Campinas and was approved by the ethics committee (number 43488115.4.0000.5481/1.082.100) on May 27, 2015, with patient recruitment performed during the years 2016–2017. A total of 30 patients diagnosed with PsO and PsA were selected, 15 patients for each disease. The clinical diagnosis in the PsO group was performed by the dermatology team of the Dermatology and Rheumatology Service, and in the PsA group, patients were classified using the Classification Criteria for Psoriatic Arthritis (CASPAR) by the rheumatology team. A total of 300 nail structures: 286 nail plates, 284 nail bed thicknesses, and 265 IR were assessed. The plates, beds, and IR of the nails not evaluated by US were for a history of previous trauma, flow, or other measures not detected and recorded by the device. In addition, 60 carotid arteries were assessed.
Inclusion criteria included patients followed up at the service, with or without nail clinical alteration, and with or without metabolic syndrome changes and asymptomatic from a cardiovascular and articular perspective. Exclusion criteria included failure to meet some of the criteria proposed in the study design.
To assess disease activity and the patients’ metabolic profile, the following variables were evaluated: low density lipoproteins (LDL), serum glucose level, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and Framingham Score (FS). In addition, data on the treatment of each group were also obtained.
Ultrasound assessments
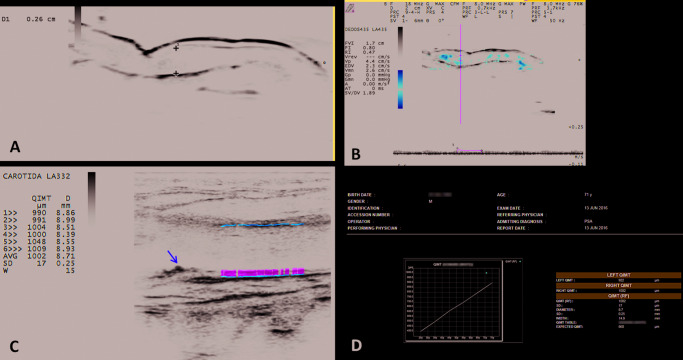
Sonographic evaluation was performed in all patients by a single rheumatologist with 12 years’ experience in examination. The measurement took place in a controlled (23°C) room in order to avoid measurement bias – especially in the nail bed evaluation where blood flow is influenced by temperature, thus affecting the Doppler measurement. The device used in all patients was an Esaote MyLab 50 with an 18 MHz linear probe for nail bed evaluation, with power Doppler frequency of 6.6–8 MHz, pulse repetition frequency that varied from 0.5 Hz to 1.0 MHz, and low filter. For the carotid assessments, a linear probe with 3.5–10 MHz frequency, Doppler of 3.3–5.0 MHz, and an average filter were used. Prior to the examination, the patients were kept resting for approximately 10 minutes, and the evaluation was performed in the supine position. The Doppler velocimetric parameters of the nail insertion were assessed. The RF software was used for the assessment of the QIMT for the carotid artery.
This software provides, in real time, a measurement of the average of six mean values obtained during six consecutive cardiac cycles. IMT was measured in the wall of the most distal 15-mm length of the common carotid artery, 10 mm from the distal extremity, just next proximal to the bifurcation. According to age-categorized values (based on the standard deviation for a sample population aged ≥25 years), mean RF-QIMT values of between 0.4 and 0.65 mm are considered normal (expected QIMT), between 0.65 and 0.75 mm are abnormal, and between 0.75 and 1.5 mm are very abnormal.9
Statistical methods
A descriptive evaluation of the study sample and analysis of parametric and non-parametric variables were performed, with data presented as mean (standard deviation [SD]) or percentages. Spearman’s and Pearson’s correlation analyses were performed to identify significant relationships between US, clinical, and laboratory parameters. Statistical significance was indicated by p<0.05. Statistical analysis was performed using MATLAB2014B software.
Another method was also used to evaluate the statistical significance of the correlation coefficients by the Bayesian factors (BFs), in which the statistically significant correlations are those with a BF greater than 2.5, which could be moderate, strong, or extreme evidence. Those correlations that do not show a BF greater than 2.5 are not considered sufficient for experimental evidence.
The groups were further divided according to the Psoriasis Area and Severity Index (PASI) and Nail Psoriasis Severity Index (NAPSI) variables.10,11
Result
The PsO patient group included 7 men (46.6%) and 8 women (53.4%); 66.6% were Caucasian and 33.4% of African descent, with mean±SD equal to 15.04±9.80 disease years. The PsA group included 10 women (66.6%) and 5 men (33.4%); 53.3% were Caucasians and 46.67% of African descent, with mean±SD equal to 7.88±6.68 disease years. All immunobiologicals used in the two groups were anti-tumor necrosis factor (anti-TNF) agents.
We observed a greater prevalence of metabolic syndrome, and of antihypertensive, hypolipidemic, hypoglycemic, and topical therapies in the PsO group when compared to the PsA group (Table 1). In contrast, we observed a greater use of immunomodulators and corticosteroids in the PsA group.
Table 1.
Medication classes and comorbidities in PsO and PsA groups.
| Medication classes and comorbidities | PsO group percentage (%) | PsA group percentage (%) |
|---|---|---|
| Antihypertensive drugs | 66.6 | 24.6 |
| Hypolipidemic drugs | 40 | 6.7 |
| Hypoglycemic drugs | 33.3 | 33.3 |
| Methotrexate | 26.6 | 33.3 |
| Topical medication | 20 | 13.3 |
| Phototherapy | 13.3 | 0 |
| Antidepressant drugs | 13.3 | 20 |
| Corticosteroids | 6.6 | 26.6 |
| Immunobiologicals | 6.6 | 53.3 |
| Metabolic syndrome | 53.3 | 30 |
PsA, psoriatic arthritis; PsO, psoriasis.
The t-test was performed to compare the mean of the variables between the two groups concerned (PsA and PsO); significance for all groups was p<0.05. There was a statistical difference between the groups, only due to the NAPSI variable (Table 2). Considering the clinical metabolic variables studied, we observed that the PsA group presented higher serum glucose levels, larger abdominal waist measurements, and larger body mass index. In the PsO group, patients were older and exhibited higher cholesterol levels, higher cardiovascular risk measured by the FS, and a higher QIMT (p=0.006), that is, 56.25% compared with 40% in the PsA group (QIMT expected). There were no statistical differences in relation to IR and nail-bed thickening, but the PsO group showed an increase in these two variables, although some patients with PsA presented with important nail-bed thickening, above 2.5 mm (Figure 1).
Table 2.
Mean and standard deviation of ultrasound, clinical, and laboratory variables by clinical group.
| PsA | PsO | ||
|---|---|---|---|
|
| |||
| Metabolic variables | Value, mean (SD) | p-value | |
|
| |||
| Age (years) | 54.66 (0.6) | 57.13 (14.3) | 0.514 |
| AW (cm) | 103.04 (18.8) | 101.03 (13.49) | 0.739 |
| Weight (kg) | 78.17 (20.1) | 73.9 (14.4) | 0.509 |
| BMI (kg/m2) | 30.79 (8.6) | 28.6 (4.69) | 0.395 |
| Total cholesterol (mg/dL) | 194.28 (41.6) | 198.73 (41.7) | 0.772 |
| LDL (mg dL) | 111.07 (35.4) | 130.5 (37.3) | 0.154 |
| HDL (mg/dL) | 50.5 (15) | 50.9 (15.1) | 0.942 |
| Serum glucose (mg/dL) | 136.5 (87.7) | 102.53 (20.3) | 0.164 |
| Glycated hemoglobin (%) | 6.37 (3.0) | 5.60 (1.83) | 0.403 |
| Uric acid (mg/dL) | 5.72 (1.5) | 5.78 (1.2) | 0.904 |
| Homocysteine (umol/L) | 10.93 (3.4) | 11.33 (4.1) | 0.773 |
| FS (%) | 14.9 (0.12) | 15.5 (12) | 0.849 |
|
| |||
| Ultrasound variables | |||
|
| |||
| IR | 0.58 (0.09) | 0.59 (0.10) | 0.775 |
| NBT | 1.60 (0.78) | 1.68 (0.50) | 0.740 |
| QIMT-RF (mm) | 0.667 (0.131) | 0.794 (0.212) | 0.058 |
| QIMT expected (mm) | 0.686 (0.105) | 0.695 (0.134) | 0.839 |
|
| |||
| Other clinical and laboratory variables | |||
|
| |||
| CRP (mg/dl) | 0.75 (1.1) | 0.54 (0.7) | 0.537 |
| Erythrocyte sedimentation rate (mm/h) | 27.5 (24.9) | 20.4 (14.9) | 0.351 |
| Rheumatoid factor (IU/mL) | 1.86 (6.73) | 11.86 (34.3) | 0.285 |
| NAPSI | 45.73 (36.08) | 19.06 (18.31) | 0.018 |
| PASI | 5.18 (5.77) | 5.84 (8.4) | 0.803 |
AW, abdominal waist circumference; BMI, body mass index; CRP, C-reactive protein; FS, Framingham Score; HDL, high-density lipoprotein; IR, internal resistance index; LDL, low density lipoprotein; NAPSI: Nail Psoriasis Severity Index; NBT, nail bed thickening; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; SD, standard deviation.
Figure 1.
The significant correlations by BF were those with a BF greater than 2.5, between the QIMT expected with FS: r=0.677, BF=10.06, with total cholesterol: r=0.5232, BF=2.60, and QIMT-RF with LDL: r=0.633, BF=3.70.
In the evaluation of Spearman and Pearson correlations, there was statistical significance between the clinical, laboratory, and ultrasonographic variables (Tables 3 and 4).
Table 3.
Spearman statistically significant correlations between clinical and laboratory variables by group PsO and PsA.
| Spearman correlations | r |
|---|---|
| VLDL × CRP | 0.574, p=0.025 |
| Uric acid × TGL | 0.532, p=0.041 |
| Uric acid × homocysteine | 0.666, p=0.009 |
| Uric acid × HbAc | 0.625, p=0.013 |
| TGL × CRP | 0.581, p=0.023 |
| HDL × CRP | −0.730, p=0.002 |
| Fasting glucose × PASI | 0.658, p=0.015 |
| ESR × CRP | 0.534, p=0.040 |
| CRP × weight | 0.516, p=0.049 |
| CRP × AW | 0.615, p=0.015 |
| CRP × BMI | 0.573, p=0.025 |
AW, abdominal waist; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation; HbAc, glycated hemoglobin; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; TGL, triglycerides; VDRL, very low density lipoprotein.
Table 4.
Spearman and Pearson clinically significant correlations between ultrasound and metabolic variables by group PsO and PsA.
| Correlations | r (p-value) |
|---|---|
| PASI × GS | 0.658, p=0.015 |
| FS × IR | 0.351, p=0.034 to 0.526, p=0.0002 |
| IR × TNB | 0.542, p=0.0001 to 0.694, p=0.001 |
| Weight × RI | 0.351, p=0.012 to 0.415, p=0.029 |
| WA × IR | 0.351, p=0.029 to 0.484, p=0.006 |
| FS × IR | 0.351, p=0.034 to 0.526, p=0.002 |
FS, Framingham Score; GS, loss of nail trilaminar pattern; IR, Internal Resistance Index; NBT, nail bed thickening; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; WA, abdominal waist.
Discussion
With the recognition of the inflammatory nature of atherosclerosis pathophysiology, some authors have suggested that PsO and PsA are multisystem conditions that affect even the coronary arteries and the heart.12–14
In addition to macrophages, atheromatous plaques also present lymphocytes, which act by releasing interferons, TNF-α, and Th1 and Th17 cytokines that promote atherosclerosis, enthesitis, and synovitis.9 The CRP is nested in this inflammatory and metabolic correlation. Titration of this protein is used both as a marker of inflammatory activity and as a prognostic marker, as high CRP levels are associated with greater cardiovascular risk as well as worse outcomes in PsA and PsO.9,12,13
Within the whole spectrum of PsA and PsO manifestations, besides skin and joints, another important site of involvement is the nail, with prevalence of 30–50% in patients diagnosed with PsO and reaching 80% in patients with PsA.15,16
The US, besides helping in the study of joints, can measure the thickness of the nail matrix as well as the trilaminar pattern of the nail plate, which tends to augment and get disarranged in PsA and PsO, respectively. In addition, subclinical injuries can be detected.2
For an adequate cardiovascular risk stratification, in addition to clinical evaluation, complementary non-invasive methods are used, such as carotid US, which is widely used. US allows evaluation of not only hemodynamic aspects but also blood vessel wall thickness and the presence of atherosclerosis.17–20
Considering all the common pathology of atherosclerotic, joint, and nail lesions that can be subclinical manifestations, important correlations are shown in Tables 3 and 4.
It was also found, with a statistically significant difference, that even with a lower NAPSI, patients in the PsO group had an increased nail thickness when compared to the PsA group. This may explain the close subclinical relationship that may exist in PsO patients. We believe that these findings of small nail-thickening changes, which characterize enthesopathy and minor nail plate changes, may be associated with increased use of medications in the treatment of the PsA group.
The prevalence of use of biological and synthetic immunomodulators in the PsA group is 53.3 and 33.3%, respectively, while in the PsO group, it is 6.6 and 26.6%, respectively. This can be explained by inflammatory joint involvement, characterizing an impact on the functional capacity of PsA patients, which requires a more aggressive treatment than for PsO patients.
This fact may also be associated with higher metabolic changes and a QIMT above average in the PsO group, reflecting an increase in the carotid intima–media thickening. This was observed in our study by laboratory and clinical measurements, in which higher LDL and FS measurements were detected as well as the use of hypolipidemic and antihypertensive drugs in the PsO group; however, this was not statistically significant. In the literature, the use of anti-TNF agents was associated with decreased carotid intima–media thickening. Perhaps, these changes were not observed in the PsA group due to the impact of the use of anti-TNF agents in these patients.18,20,21
There are discrepancies in the literature about US data regarding blood flow in the nail insertion evaluated by IR. Some articles report that this index increases in PsO and decreases in PsA patients, thus requiring further studies to better clarify this information. In our study, the mean RI did not appear to be different between the two groups; however, low IR may indicate inflammation, even when laboratory inflammatory marker values are not high. Similar findings were observed in the literature – a study that evaluated IR of nail insertion in PsA patients found a reduced IR even in patients with controlled disease.2
The limitations of this study may be related to non-randomization and heterogeneity of the groups. In addition, failure to calculate the sample size and the limited number of samples may have affected the statistical significance of the results.
The pathophysiological processes of PsO, PsA, and metabolic syndrome intermingle apparently constituting the same syndrome. In our study, we obtained data that corroborate this hypothesis. In addition, the comparison of variables between the PsO and PsA groups provided interesting data, such as a worse metabolic profile and increased nail-bed thickness in the PsO group even with better NAPSI. This suggests that nail-bed thickening could be a risk factor associated with a worse metabolic profile in these patients.
Based on current literature data, it is not feasible to determine the exact impact of anti-TNF treatment on metabolic syndrome. Further studies are needed to determine the role of this class of medication on metabolic syndrome and to find out if this type of immunobiologicals and other drugs can present effects in the framework of inflammatory diseases.
In any case, patients with PsA and PsO should be viewed as high cardiovascular risk patients.
Also, concomitant control of inflammatory and metabolic conditions is mandatory in the management of these patients. With regard to treatment, the available literature shows that early intervention improves patient outcome.22–24
In conclusion, the use of US in the evaluation of patients with PsA and PsO showed significant correlations between clinical and laboratory measures, represented by QIMT and FS, that help assess atherosclerosis and stratify cardiovascular risk, respectively. In addition, the inflammatory activity in nail entheses, evidenced by spectral Doppler, may be detected in psoriatic disease. These conditions can reflect in subclinical vascular and joint lesions.2,9,18 Therefore, US could be an important tool to obtain better treatment results in future.25
Acknowledgements
The authors thank the patients of the Dermatology and Rheumatology Service of the Pontifical Catholic University of Campinas, Brazil.
Footnotes
Contributions: All authors are part of the medical team of the Pontifical Catholic University of Campinas and contributed to the methodological and clinical aspects of the article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/05/dic.2020-1-2-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2020 Mendonça JA, Pansani LN, Mimoto MB, Ferreira ITG, Sanches FB, Pinto TFC, Leandro-Merhi VA, Aquino JLB. https://doi.org/10.7573/dic.2020-1-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 1 April 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Mendonça JA. Differences of spectral Doppler in psoriatic arthritis and onychomycosis. Rev Bras Reumatol. 2014;54(6):490–493. doi: 10.1016/j.rbre.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Mendonça JA, Aydin SZ, D’Agostino MA. The use of ultrasonography in the diagnosis of nail disease among patients with psoriasis and psoriatic arthritis: a systematic review. Adv Rheumatol. 2019;59(41):1–12. doi: 10.1186/s42358-019-0081-9. [DOI] [PubMed] [Google Scholar]
- 3.Contessa C, Ramonda R, Lo Nigro A, et al. Subclinical atherosclerosis in patients with psoriatic arthritis: a case-control study. Reumatismo. 2009;61(4):298–305. doi: 10.4081/reumatismo.2009.298. [DOI] [PubMed] [Google Scholar]
- 4.Milčić D, Janković S, Vesić S, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based cross-sectional study. An Bras Dermatol. 2017;92(1):46–51. doi: 10.1590/abd1806-4841.20175178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angel K, Provan SA, Hammer HB, Mowinckel P, Kvien TK, Atar D. Changes in arterial stiffness during continued infliximab treatment in patients with inflammatory arthropathies. Fundam Clin Pharmacol. 2011;25(4):511–517. doi: 10.1111/j.1472-8206.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 6.Di Minno MN, Ambrosino P, Lupoli R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med. 2015;47(4):346–353. doi: 10.3109/07853890.2015.1031822. [DOI] [PubMed] [Google Scholar]
- 7.Mendonça J, Pansani L, Mimoto M, et al. AB1194 Are there new parameters to be considered in spectral Doppler to evaluate the nail bed in psoriasis and psoriasis arthritis? Ann Rheum Dis. 2018;77:1697. doi: 10.1136/annrheumdis-2018-eular.3768. [DOI] [Google Scholar]
- 8.Di Minno MN, Iervolino S, Peluso R, Scarpa R, Di Minno G CaRRDs study group. Carotid intima-media thickness in psoriatic arthritis: differences between tumor necrosis factor-α blockers and traditional disease-modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol. 2011;31(3):705–712. doi: 10.1161/ATVBAHA.110.214585. [DOI] [PubMed] [Google Scholar]
- 9.Mendonça JA, Bisetto de Andrade B, Braga de Aquino JL, Leandro-Merhi VA, Damian GB. Spectral Doppler and automated software-guided ultrasound assessment of bilateral common carotid intima-media thickness in spondyloarthritis: is there a correlation with clinical findings? Drugs Context. 2018;3(7) doi: 10.7573/dic.212538. 212538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjalte F, Carlsson KS, Schmitt-Egenolf M. Sustained Psoriasis Area and Severity Index, Dermatology Life Quality Index and EuroQol-5D response of biological treatment in psoriasis: 10 years of real-world data in the Swedish National Psoriasis Register. Br J Dermatol. 2018;178(1):245–252. doi: 10.1111/bjd.15757. [DOI] [PubMed] [Google Scholar]
- 11.Klaassen KMG, van de Kerkhof PCM, Bastiaens MT, Plusjé LGJM, Baran RL, Pasch MC. Scoring nail psoriasis. J Am Acad Dermatol. 2014;70(6):1061–1066. doi: 10.1016/j.jaad.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Di Minno MN, Ambrosino P, Lupoli R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med. 2015;47(4):346–353. doi: 10.3109/07853890.2015.1031822. [DOI] [PubMed] [Google Scholar]
- 13.Eder L, Thavaneswaran A, Chandran V, et al. Increased burden of inflammation over time is associated with the extent of atherosclerotic plaques in patients with psoriatic arthritis. Ann Rheum Dis. 2015;74:1830–1835. doi: 10.1136/annrheumdis-2014-205267. [DOI] [PubMed] [Google Scholar]
- 14.Pirro M, Stingeni L, Vaudo G, et al. Systemic inflammation and imbalance between endothelial injury and repair in patients with psoriasis are associated with preclinical atherosclerosis. Eur J Prev Cardiol. 2014;22(8):1027–1035. doi: 10.1177/2047487314538858. [DOI] [PubMed] [Google Scholar]
- 15.Raposo I, Torres T. Nail psoriasis as a predictor of the development of psoriatic arthritis. Actas Dermosifiliogr. 2015;106(6):452–457. doi: 10.1016/j.ad.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68(4):915–923. doi: 10.1002/art.39494. [DOI] [PubMed] [Google Scholar]
- 17.Di Minno MN, Iervolino S, Peluso R, et al. Carotid intima-media thickness in psoriatic arthritis. Arterioscler Thromb Vasc Biol. 2011;31(3):705–712. doi: 10.1161/atvbaha.110.214585. [DOI] [PubMed] [Google Scholar]
- 18.Mendonça JA, Abrahão Machado ECF, Nucci LB, et al. FRI0644 analysis of cardiovascular risk and carotid intima-media thickness in patients with psoriasis. Ann Rheum Dis. 2019;78:1019–1020. doi: 10.1136/annrheumdis-2019-eular.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi SD, Mendonça JA, Palominos P, et al. FRI0423 nail ultrasonography findings in grayscale and power Doppler to differentiate psoriatic arthritis, psoriasis and control individuals. Ann Rheum Dis. 2019;78:900–901. doi: 10.1136/annrheumdis-2019-eular.2790. [DOI] [Google Scholar]
- 20.Azevedo Dias M, Maria Silva, de Siqueira L, Nascimento de Carvalho B, Pinheiro M, Mendonça JA, Luz K. The automated software-guided ultrasound assessment of bilateral common carotids intima-media thickness for investigation of cardiovascular risk in psoriasis: comparison between patients with or without arthritis [abstract] [Accessed May 19, 2020];Arthritis Rheumatol. 2015 67(suppl 10) https://acrabstracts.org/abstract/the-automated-software-guided-ultrasound-assessment-of-bilateral-common-carotids-intima-media-thickness-for-investigation-of-cardiovascular-risk-in-psoriasiscomparison-between-patients-with-or-withou/ [Google Scholar]
- 21.Ramonda R, Lo Nigro A, Modesti V, et al. Atherosclerosis in psoriatic arthritis. Autoimmun Rev. 2011;10(12):773–778. doi: 10.1016/j.autrev.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Sabry HH, Sabry JH, Daifalla AEH, Akl EM, Hamed AM, Torky AAA. Serum markers for asymptomatic atherosclerosis in Egyptian psoriatic patients: study controlled by Doppler estimation of carotid intima-media thickness. Vasc Health Risk Manag. 2018;9(14):145–152. doi: 10.2147/VHRM.S164274. . eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25(6):644–650. doi: 10.1038/ajh.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souto A, Salgado E, Maneiro JR, Mera A, Carmona L, Gómez-Reino JJ. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 2015;67(1):117–127. doi: 10.1002/art.38894. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease. Circulation. 2011;124(8):967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]