Abstract
Metabolic syndrome is associated with increased risk of cardiovascular disease. This study investigated the correlation between adipocyte and inflammation biomarkers, and metabolic syndrome and its components. The study included 80 patients with normal body-mass index and 80 obese patients. The groups were assessed for serum values of adiponectin, leptin and highly sensitive C reactive protein (hsCRP), the homeostatic model assessment of insulin resistance (HOMA-IR), as well as the influence of these biochemical markers on the prevalence of metabolic syndrome and its components. Leptin, HOMA-IR and hsCRP had statistically significant (P<0.01) higher values in the group of obese subjects, while adiponectin had statistically significant (P<0.01) lower values. The prevalence of metabolic syndrome was 35% in the obese group and 5% in the normal weight group. Adiponectin and HOMA-IR were the variables significantly associated with metabolic syndrome (P<0.01), adiponectin/HOMA-IR ratio and leptin/adiponectin ratio were also associated with metabolic syndrome (P<0.01). No relationship was found between metabolic syndrome and hsCRP. Adiponectin and adiponectin/HOMA-IR were associated with all the components of metabolic syndrome and they can be useful to identify patients with high risk of diabetes mellitus and cardiovascular disease.
Keywords: adipocyte biochemical markers, metabolic syndrome, inflammation, leptin, hsCRP
Introduction
Metabolic syndrome is an association of several risk factors for cardiovascular disease and other diseases (1,2). Many criteria have been proposed for the diagnosis of metabolic syndrome, all of them included the presence of high blood pressure, high fasting glycaemia, dyslipidaemia and obesity; however, the cut-off values for these variables differed depending on the organisations. A meeting of several major organisations including International Diabetes Federation and American Heart Association attempted to give unifying criteria for the definition of metabolic syndrome. For the diagnosis of metabolic syndrome 3 of the 5 following criteria must be present: increased waist circumference, in the case of Caucasian patients ≥94 cm for men and ≥80 cm for women; elevated triglycerides ≥150 mg/dl; elevated blood pressure, systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mg/dl; reduced high-density lipoprotein (HDL) cholesterol <40 mg/dl for men and ≤50 mg/dl for women (3).
The prevalence of metabolic syndrome began to increase worldwide because of obesity epidemics. The observation is supported by the findings of National health and nutrition examination survey (NHANES) where the prevalence of metabolic syndrome was 5% among individuals with normal body mass index (BMI) and 60% among individuals with obesity (4). The importance of metabolic syndrome consists in the high burden of complications it generates, epidemiological studies proving that subjects affected by metabolic syndrome have a 3-4 times greater risk of myocardial infarction and a 2-4 times greater risk of stroke, as well as therapeutic implications (5,6).
Lifestyle changes have triggered the obesity epidemic; obesity is the main cause for metabolic syndrome because obesity generates insulin resistance and insulin resistance is associated with all the components of metabolic syndrome (7). Adipocytes are metabolically active cells generating different metabolites called adipokines that act at different levels, some of them increasing the risk of diabetes and cardiovascular disease such as C-reactive protein (CRP), tumour necrosis factor α (TNFα) and interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), some of them decreasing, at least theoretically, the risk of diabetes and atherosclerosis such as adiponectin (8).
CRP is an inflammatory and endothelial dysfunction biomarker generally considered accurate in predicting cardiovascular disease. Correlations were established between highly sensitive C-reactive protein (hsCRP) levels, coronary events and different metabolic risk factors especially BMI and insulin resistance (9). It appears that CRP interferes with the insulin signalling pathway and therefore generates insulin resistance (10). Despite a variety of studies, the role of hsCRP in the pathogenesis of metabolic syndrome and cardiovascular disease remains controversial. Risk factors, such as smoking, age, BMI, blood pressure, triglycerides have been associated with elevated hsCRP levels (11).
In addition, because of the role of hsCRP levels in identifying prognosis and recurrent events in patients with stroke and peripheral arterial disease, it may be useful in assessing cardiovascular risks and in identifying high-risk populations as target for prevention (12). Racial and ethnic differences have been demonstrated in the hsCRP levels and sex difference (women having higher hsCRP levels than men). One of the possible explanations is relatively higher degree of adiposity in women so the body fat quantity and distribution appear to influence hsCRP levels to a greater extent in women than men (13).
Adiponectin or adipocyte complement-related protein is the most abundant peptide secreted by adipocytes that negatively correlates with obesity and type 2 diabetes because it acts by increasing insulin sensitivity; it is probably the only adipokine with higher levels correspond to lower BMI and decreased cardiovascular risk because of its anti-inflammatory, and antiatherogenic effects (14). These are reasons to be proposed as novel therapeutic target for diabetes and metabolic syndrome.
Human adiponectin is encoded by the Adipo Q gene, which spans 17 kb on chromosome locus 3q27. This chromosome has been identified as carrying a gene susceptible for type 2 diabetes and metabolic syndrome (15). Adiponectin is also involved in energy homeostasis by action in hypothalamus, therefore the name ‘starvation gene’ has been proposed.
AdipoR1 and AdipoR2, two structurally related seven transmembrane receptors, have been identified to function as adiponectin receptors, structurally and functionally distinct from classical G-protein coupled receptors. AdipoR1 is expressed ubiquitously, but most abundantly, in skeletal muscle while AdipoR2 is predominantly expressed in the liver (16). It was demonstrated that adiponectin improves the utilization of glucose at the level of the skeletal muscle, protects against atherosclerosis plaque formation, decreases liver glucose production and decreases visceral adiposity (17). Insulin and adiponectin interact with their receptors, fact that generates a cascade of metabolic actions such as increased protein synthesis, lipogenesis, reduces plasma glucose levels (increase glucose uptake and utilization), glycogen synthesis, lipolysis and gluconeogenesis (18).
Leptin is a polypeptide hormone produced by adipocytes in proportion to their triglyceride content. It is involved in the central control of energy balance. Its action consists in binding to and activating the long form of its receptor in the brain and the result is decreasing food intake while increasing energy expenditure. Plasma leptin concentration increases in proportion to body fat content, regulate food intake and energy expenditure to maintain body fat stores. Circulating leptin is secreted into the blood and after crossing the brain-blood and cerebrospinal fluid barrier, it acts in the hypothalamus, where leptin inhibits neuropeptide Y (NPY) neurons and causes anorexia. The arcuate nucleus has been proposed as an important site of leptin action. The central administration of leptin increases glucose turn-over and glucose uptake in peripheral tissues (heart, skeletal muscle, adipose tissue), stimulates hepatic gluconeogenesis and hepatic insulin sensitivity via the hepatic branch of the vagus nerve.
This study evaluated the correlation between the adipocyte biomarkers: adiponectin, leptin, HOMA-IR and inflammation biomarker, hs-CRP, and metabolic syndrome and its components.
Patients and methods
Patients
The study included 160 individuals, 80 with normal body weight defined as BMI ≥18.5 kg/m2 and <25 kg/m2 and 80 with obesity defined as BMI ≥30 kg/m2; all of them were selected from the list of patients of a primary care physician from Oradea, Romania. The inclusion criteria were as follows: subjects aged between 18 and 65 years, subjects that gave their written consent for the participation in the study. For the reference group, the additional criteria were BMI ≥18.5 kg/m2 and <25 kg/m2; for the control group the additional criteria was BMI ≥30 kg/m2. Since the study addresses to the general population, to the clinically healthy individuals (with the exception of obesity), the idea was to include individuals without significant chronic comorbidities therefore the exclusion criteria were: patients with diabetes mellitus, patients with stage II or III hypertension, patients with proved coronary artery disease or cerebrovascular disease (history of myocardial infarction or stroke), patients with other chronic diseases (cirrhosis, chronic obstructive pulmonary disease, chronic kidney disease, cancer, endocrine, haematological, psychiatric and neurological diseases), patients that were taking medications that can influence blood pressure or glycaemia. The exclusion criteria were similar for the reference and the control group. The research was performed according the WMA Declaration of Ethics, Helsinki - Medical Research Involving Human Principles for Subjects; the study was also approved by the Ethics Commission of the Council of Clinical County Emergency Hospital of Oradea (Romania). All subjects gave their written consent for the participation in the study and the medical practitioner gave written consent for the selection of the subjects from the patient lists.
Method
The method for selection of subjects was the following: in an interval of three months (1st June 2019-1st September 2019), the individuals that addressed the primary care physician for administrative reasons (requirement of medical certificate that attests the general health condition) were considered for inclusion in the study. When presented to the primary care physician, the subject was evaluated regarding the fulfilment of inclusion criteria and absence of exclusion criteria. A total of 80 obese subjects (reference group) and 80 normal weight subjects (control group) met the criteria and were further selected for clinical and biochemical evaluation. When selected, the subject was instructed to return the following day for physical examination that included measurement of height, weight, BMI calculation, waist circumference measurement and hip circumference measurement, determination of blood pressure, and collection of venous blood samples. Special determinations included serum insulin, adiponectin, leptin and hsCRP protein. hsCRP was determined using the immunoturbidimetric method, adiponectin and leptin were determined using ELISA method, insulin was determined by the chemiluminescence immunoassay. Quality control was conducted before testing samples. Normal values were: for adiponectin 12-25 ng/ml, for leptin (14.1-37.0) ng/ml in women and (3.3-14.3) ng/ml in men, for hsCRP <0.3 mg/dl and for insulin 2.5-25 µU/ml. Determinations were performed in the Laboratory Department of Clinical County Hospital of Oradea (Oradea, Romania).
Statistical analysis
Statistical analysis was performed using Biostat software. P<0.05 was considered statistically significant. The comparison of variables between the two groups was performed using t-test for variables with normal distribution and Mann-Whitney U test for variables with skewed distribution.
Results
Increased BMI is associated with statistically significant higher age, waist circumference, hip circumference, systolic blood pressure, diastolic blood pressure, fasting glycaemia, hemoglobin A1c (HbA1c), triglycerides and lower HDL-cholesterol as shown in Table I. The adipocyte biomarkers of the two groups are presented in Table II.
Table I.
Characteristics of the normal weight and obese subjects.
| Variable | Normal weight (n=80) | Standard deviation | Obese (n=80) | Standard deviation | P-value |
|---|---|---|---|---|---|
| Sex (women/men) % | 60/40 | - | 57.5/42.5 | - | 0.74 |
| Age (years) | 43.05 | 10.93 | 46.74 | 10.37 | 0.01 |
| Weight (kg) | 62.35 | 8.21 | 97.68 | 14.71 | <0.01 |
| Height (m) | 1.67 | 0.08 | 1.68 | 0.09 | 0.29 |
| BMI (kg/m2) | 22.32 | 1.81 | 34.68 | 3.82 | <0.01 |
| Waist circumference (cm) | 78.19 | 7.36 | 108.42 | 14.61 | <0.01 |
| Hip circumference (cm) | 94.29 | 6.43 | 117.08 | 10.98 | <0.01 |
| Waist/hip circumference | 0.83 | 0.08 | 0.93 | 0.08 | <0.01 |
| SBP (mmHg) | 116.8 | 11.41 | 127.51 | 14.27 | <0.01 |
| DBP (mmHg) | 72.24 | 7.49 | 80.28 | 9.61 | <0.01 |
| Glycaemia (mg/dl) | 88.11 | 8.6 | 92.9 | 9.76 | <0.01 |
| HbA1c (%) | 5.2 | 0.26 | 5.46 | 0.39 | <0.01 |
| Triglycerides (mg/dl) | 80.94 | (60, 91.5) | 114.08 | (73.25, 135.5) | <0.01 |
| Cholesterol (mg/dl) | 181.69 | 36.72 | 186.06 | 37.59 | 0.46 |
| LDL-cholesterol (mg/dl) | 106.65 | 31.8 | 114.94 | 34.3 | 0.11 |
| HDL-cholesterol (mg/dl) | 58.95 | 13.94 | 49.01 | 13.22 | <0.01 |
Table II.
Inflammation and adipocyte biomarkers in the two groups (P<0.01).
| Parameter | Normal weight (n=80) | Standard deviation | Obese (n=80) | Standard deviation |
|---|---|---|---|---|
| Insulin (µU/ml) | 6.17 | (3.8, 7.82) | 13.31 | (7.77, 15.75) |
| HOMA-IR | 1.36 | (0.79, 1.69) | 3.06 | (1.78, 3.55) |
| hsCRP (mg/dl) | 0.22 | (0.08, 0.21) | 0.58 | (0.17, 0.73) |
| Leptin (ng/ml) | 7.21 | (2.02, 10.75) | 25.7 | (12, 33.79) |
| Adiponectin (ng/ml) | 16.84 | (14.34, 17.69) | 7.52 | (6.16, 8.55) |
| Adiponectin/HOMA-IR | 17.11 | (9.62, 19.53) | 3.41 | (1.73, 4.51) |
| Leptin/adiponectin | 0.42 | (0.13, 0.61) | 3.47 | (1.77, 4.50) |
The prevalence of metabolic syndrome was statistically significantly higher (35%) in obese subjects than in normal weight subjects (5%) (P<0.01). The prevalence of metabolic syndrome components was statistically significantly higher (P<0.01) in obese subjects than in normal weight subjects (Fig. 1).
Figure 1.
Prevalence of metabolic syndrome components among normal weight and obese subjects.
Subjects with metabolic syndrome had statistically significantly higher weight, BMI, waist circumference, hip circumference, systolic blood pressure, diastolic blood pressure, glycaemia, HbA1c, triglycerides and lower HDL-cholesterol compared with subjects without metabolic syndrome (Table III).
Table III.
Characteristics of the subjects with and without metabolic syndrome.
| Parameter | Without metabolic syndrome (n=128) | Standard deviation | With metabolic syndrome (n=32) | Standard deviation | P-value |
|---|---|---|---|---|---|
| Sex (women/men) % | 64/36 | - | 43.75/56.25 | - | 0.05 |
| Age (years) | 44.07 | 11.19 | 48.15 | 8.39 | 0.05 |
| Weight (kg) | 75.95 | 19.93 | 96.27 | 18.82 | <0.01 |
| Height (m) | 1.67 | 0.08 | 1.7 | 0.08 | 0.03 |
| BMI (kg/m2) | 27.32 | 6.79 | 33.2 | 4.86 | <0.01 |
| Waist circumference (cm) | 89.67 | 17.18 | 107.83 | 14.72 | <0.01 |
| Hip circumference (cm) | 103.16 | 13.48 | 115.78 | 14.11 | <0.01 |
| Waist/hip circumference | 0.86 | 0.1 | 0.93 | 0.06 | <0.01 |
| SBP (mmHg) | 119.57 | 13.08 | 132.5 | 12.67 | <0.01 |
| DBP (mmHg) | 74.59 | 8.9 | 82.94 | 8.9 | <0.01 |
| Glycaemia (mg/dl) | 88.6 | 8.51 | 98.28 | 9.29 | <0.01 |
| HbA1c (%) | 5.29 | 0.33 | 5.49 | 0.41 | <0.01 |
| Triglycerides (mg/dl) | 85.31 | (60, 104) | 95.25 | (84.25, 189.5) | <0.01 |
| Cholesterol (mg/dl) | 182.34 | 37.11 | 190 | 37.05 | 0.3 |
| LDL-cholesterol (mg/dl) | 108.87 | 32.36 | 118.5 | 35.96 | 0.14 |
| HDL-cholesterol (mg/dl) | 56.84 | 14.16 | 42.56 | 9.01 | <0.01 |
The presence of the metabolic syndrome was associated with statistically significant higher values of serum insulin, HOMA-IR, leptin and leptin/adiponectin ratio and statistically significant lower values of adiponectin and adiponectin/HOMA-IR ratio (Table IV).
Table IV.
Inflammation and adipocyte biomarkers in the subjects with/without metabolic syndrome.
| Parameter | Without metabolic syndrome (=128) | Standard deviation | With metabolic syndrome (n=32) | Standard deviation | P-value |
|---|---|---|---|---|---|
| Insulin (µU/ml) | 8.95 | (5.32, 11.7) | 12.89 | (6.62, 14.97) | <0.01 |
| HOMA-IR | 1.97 | (1.15, 2.69) | 3.16 | (1.61, 3.82) | <0.01 |
| hsCRP (mg/dl) | 0.4 | (0.09, 0.46) | 0.41 | (0.13, 0.46) | 0.28 |
| Leptin (ng/ml) | 15.22 | (4.76, 21.98) | 21.4 | (5.30, 28.36) | 0.14 |
| Adiponectin (ng/ml) | 13.27 | (8.49, 16.74) | 7.79 | (6.03, 8.01) | <0.01 |
| Adiponectin/HOMA-IR | 11.77 | (3.81, 15.61) | 4.23 | (1.50, 5.13) | <0.01 |
| Leptin/adiponectin | 1.64 | (0.27, 2.57) | 3.18 | (0.57, 3.87) | <0.01 |
The parameters that correlated statistically significantly with metabolic syndrome were HOMA-IR, adiponectin, adiponectin/HOMA-IR ratio and leptin/adiponectin ratio. Adiponectin and adiponectin/HOMA-IR ratio correlated with all the components of the metabolic syndrome. In multivariate regression analysis of adiponectin was the only biochemical marker that correlated with metabolic syndrome. Also, adiponectin correlated with abdominal obesity, low HDL-cholesterol and raised blood pressure. HOMA-IR correlated with low HDL-cholesterol and raised glycaemia (Table V).
Table V.
Correlation between inflammation and adipocyte biomarkers and metabolic syndrome and its components.
| Univariate regression | ||||||
|---|---|---|---|---|---|---|
| Parameter | Metabolic syndrome | Abdominal obesity | Raised triglycerides | Low HDL | Increased blood pressure | Increased glycaemia |
| HOMA-IR | 0.0002 | 0.0001 | 0.09 | 0.001 | 0.004 | 0.0001 |
| Leptin (ng/ml) | 0.05 | 0.0001 | 0.91 | 0.17 | 0.039 | 0.01 |
| Adiponectin (ng/ml) | 0.0001 | 0.0001 | 0.005 | 0.0001 | 0.0001 | 0.01 |
| hsCRP (mg/dl) | 0.87 | 0.0002 | 0.79 | 0.05 | 0.26 | 0.72 |
| Adiponectin/HOMA-IR | 0.0005 | 0.0001 | 0.03 | 0.0005 | 0.03 | 0.006 |
| Leptin/adiponectin | 0.0007 | 0.0001 | 0.41 | 0.01 | 0.0001 | 0.03 |
| Multivariate regression adjusted by age and sex | ||||||
| Sex | 0.5187 | 0.0238 | 0.0975 | 0.9951 | 0.3345 | 0.0645 |
| Age | 0.1250 | 0.0039 | 0.2134 | 0.2032 | 0.0074 | 0.6219 |
| hsCRP (mg/dl) | 0.1756 | 0.7135 | 0.9193 | 0.2979 | 0.7130 | 0.5728 |
| HOMA-IR | 0.1229 | 0.6734 | 0.4094 | 0.0133 | 0.5291 | 0.0102 |
| Leptin (ng/ml) | 0.8549 | 0.0815 | 0.6002 | 0.1475 | 0.2036 | 0.8684 |
| Adiponectin (ng/ml) | 0.0003 | 0.0000 | 0.1290 | 0.0037 | 0.0343 | 0.1203 |
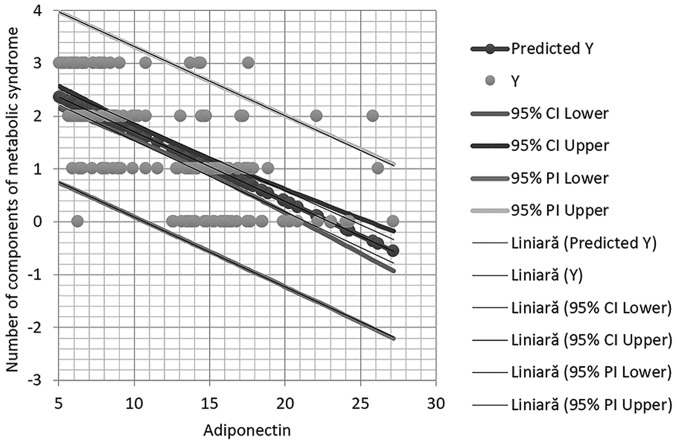
It should be indicated that in multivariate regression analysis adiponectin remained statistically significantly associated with high blood pressure (Fig. 2).
Figure 2.
Correlation between adiponectin levels and the number of components of metabolic syndrome.
Discussion
Increased prevalence worldwide predisposes to insulin resistance, which is the central key of metabolic syndrome. The aetiology of the metabolic syndrome is complex, being involved in genetic mechanisms and environmental factors that predispose to it (19-21). Therapeutic intervention may be a preventive measure, at first disease should be recorded to register and monitor the chronic patients in order to assess their needs and improve their care (22-24).
The present study confirmed that lower adiponectin levels are negatively associated with the presence of obesity and metabolic syndrome, a finding reported by previous studies (25). Adiponectin was associated with the presence of all the components of metabolic syndrome: abdominal obesity, hypertriglyceridemia, low HDL-cholesterol, high blood pressure and high glycaemia. Studies report that adiponectin levels are lower with increasing number of metabolic syndrome components (25). A study performed on a large population including 2,471 men and 3,463 women of Korean origin confirmed that adiponectin levels are associated with metabolic syndrome phenotype and all its components (26); similarly to the present study, all these individuals were persons not suffering of diabetes mellitus. Also, increased levels of serum adiponectin were associated with higher number of metabolic syndrome components.
The usefulness of these findings consists in the metabolic action of adiponectin, it is important to mention that low levels of adiponectin are not only a consequence of increased adiposity, but also adiponectin has substantial beneficial roles in many metabolic pathways and therefore obese individuals do not benefit of the favourable effects of adiponectin. Firstly, adiponectin activated APPL1 signalling pathway. APPL1 has many roles, among them is activating the insulin receptor substrate proteins, therefore adiponectin stimulates the activity of insulin pathway and reduces insulin resistance (27). On the other hand, adiponectin activates AMPK signalling pathway increasing fat oxidation and energy expenditure (28,29). An increase of adiponectin can be obtained with the help of pharmacotherapy, by administration of thiazolidinediones, or by aerobic exercise (17). There is also a negative relationship between adiponectin levels and hypertension. Experimentally, in mice with reduced levels of adiponectin, the existence of endothelial dysfunction was found with alteration of vasodilatation and increased transformation of macrophages into foam cells, therefore adiponectin is associated with decreased atherosclerosis (16). In the present study adiponectin was negatively associated with increased blood pressure, systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg.
Adiponectin/HOMA-IR ratio and leptin/adiponectin ratio were statistically significantly associated with metabolic syndrome in the present study. Similar findings have been reported by Ding et al (30), who demonstrated that adiponectin/HOMA-IR is a better predictor of metabolic syndrome than adiponectin or HOMA-IR alone. In the present study the degree of correlation in univariate regression between adiponectin, HOMA-IR, adiponectin/HOMA-IR and leptin/adiponectin ratio and the presence of metabolic syndrome was comparable.
No relationship between hs-CRP and metabolic syndrome was found in this study. Also, except for obesity, hs-CRP did not correlate with any components of metabolic syndrome. Results from literature are contradictory regarding the association between hs-CRP and metabolic syndrome and its components. There are studies that reported that patients with metabolic syndrome had values of hs-CRP 4 times higher than patients without metabolic syndrome and that hs-CRP was associated with metabolic syndrome and all its components (31). However, other scientists reported that hs-CRP has limited capacity to predict metabolic syndrome (32).
Leptin/adiponectin ratio was a strong predictor of metabolic syndrome revealed in the present study. Leptin has higher levels in patients with obesity mainly because of leptin resistance. Also, it was demonstrated that high levels of leptin are associated with increased insulin secretion which further exacerbates obesity and increases leptin levels (33). Leptin increases insulin resistance and has proinflammatory effects, adiponectin increases insulin sensitivity and decreases inflammatory response, therefore because of their antagonistic actions the leptin/adiponectin ratio is a good predictor of diabetes risk and of metabolic syndrome (34).
Although individuals included in the study were not suffering of diabetes mellitus type 2, the metabolic syndrome group are at high risk for progression towards this disease, showing statistically significant higher levels of HOMA-IR index, leptin and lower adiponectin. Adiponectin is genetically linked with diabetes mellitus type 2, it was proven that one of the loci of diabetes mellitus susceptibility is 3q27, in this region the gene responsible for adiponectin synthesis is also located (35).
Given these findings, adiponectin emerges as a target molecule for reducing the risk of metabolic syndrome, type 2 diabetes mellitus and atherosclerotic disease. Research is currently conducted to determine the measures, pharmacological or non-pharmacological, that may increase the circulating levels of adiponectin (19,36-38). Physical effort appears to be associated with an increase in serum adiponectin levels; one week of aerobic exercise was reported to lead to a significant increase in adiponectin in obese men (39). Complex experiments involving administration of adiponectin using adenoviruses as vectors in obese mice, with low blood adiponectin levels, demonstrated that adiponectin reduces obesity related hypertension (40).
Increased leptin/adiponectin ratio is strongly influenced by radical measures such as bariatric surgery. Severely obese type 2 diabetes mellitus patients that underwent Roux-en-Y gastric bypass had significantly lower leptin levels and significantly higher adiponectin levels compared to baseline values (41), these modifications of adipokines could contribute to the remission of glycaemic misbalance frequently observed in this category of patients.
In conclusion, adiponectin appears to be the hallmark molecule negatively associated with metabolic syndrome and its components. The derived variables such as leptin/adiponectin ratio or adiponectin/HOMA-IR gain statistical significance mainly because of the markedly decreased levels of adiponectin in metabolic syndrome. HsCRP is associated only with obesity, not with the metabolic syndrome. Therefore, assessment of adiponectin in population could help identify patients with high risk of diabetes mellitus and cardiovascular disease.
Acknowledgements
Not applicable.
Funding
This study was supported by the project ‘SmartDoct - High quality programs for PhD students and postdoctoral researchers of the University of Oradea for increasing the relevance of research and innovation in the context of the regional economy’, ID/Project code: 123008, co-financed by the European Social Fund through the Human Capital Operational Program 2014-2020.
Availability of data and materials
At the private medical offices where the data were collected.
Authors' contributions
DCZ, CV, DU, OF and CP selected the patients, analyzed and interpreted the patient data regarding BMI and biochemical markers. OB, DMT, CCD and SB made substantial contributions to the conception of the work and interpretation of data; also, they drafted the manuscript and were major contributors in writing the manuscript. All authors read and approved the final manuscript to be published. All the authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The research was performed according the WMA Declaration of Ethics, Helsinki - Medical Research Involving Human Principles for Subjects; the study was also approved by the Ethics Commission of the Council of Clinical County Emergency Hospital of Oradea (Romania). All subjects gave their written consent for the participation in the study and the medical practitioner gave written consent for the selection of the subjects from the patient lists.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(12) doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adambekov S, Yi Y, Fabio A, Miljkovic I, Edwards RP, Lopa S, Linkov F. Metabolic syndrome in endometrial cancer patients: Systematic review. Metab Syndr Relat Disord. 2019;17:241–249. doi: 10.1089/met.2018.0106. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. IDF Epidemiology Task Force Consensus Group. [DOI] [PubMed] [Google Scholar]
- 6.Rajput R, Rajput M, Mishra S, Ahlawat P. Prevalence of metabolic syndrome in prediabetes. Metab Syndr Relat Disord. 2019;17:406–410. doi: 10.1089/met.2019.0010. [DOI] [PubMed] [Google Scholar]
- 7.Popa AR, Bungau S, Vesa CM, Bondar AC, Pantis C, Maghiar O, Dimulescu (Nica) IA, Nistor Cseppento DC, Rus M. Evaluating the efficacy of the treatment with benfotiamine and alpha-lipoic acid in distal symmetric painful diabetic polyneuropathy. Rev Chim. 2019;70:3108–3114. [Google Scholar]
- 8.Xu H, Li X, Adams H, Kubena K, Guo S. Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci. 2018;20(E128) doi: 10.3390/ijms20010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: Molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 10.Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Voeks J, Zakai NA, Jenny NS, Brown TM, Safford MM, LeWinter M, Howard G, Cushman M. Metabolic syndrome, C-reactive protein, and mortality in U.S. Blacks and Whites: The reasons for geographic and racial differences in stroke (REGARDS) study. Diabetes Care. 2014;37:2284–2290. doi: 10.2337/dc13-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, de Lemos JA. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori Y, Otabe S, Dina C, Yasuda K, Populaire C, Lecoeur C, Vatin V, Durand E, Hara K, Okada T, et al. Genome-wide search for type 2 diabetes in Japanese affected sib-pairs confirms susceptibility genes on 3q, 15q, and 20q and identifies two new candidate loci on 7p and 11p. Diabetes. 2002;51:1247–1255. doi: 10.2337/diabetes.51.4.1247. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 17.Sanjari M, Khodashahi M, Gholamhoseinian A, Shokoohi M. Association of adiponectin and metabolic syndrome in women. J Res Med Sci. 2011;16:1532–1540. [PMC free article] [PubMed] [Google Scholar]
- 18.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 19.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(E1321) doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Daim MM, El-Tawil OS, Bungau SG, Atanasaov AG. Applications of antioxidants in metabolic disorders and degenerative diseases: Mechanistic approach. Oxid Med Cell Longev. 2019;2019(4179676) doi: 10.1155/2019/4179676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Daim MM, Zakhary NI, Aleya L, Bungau SG, Bohara RA, Siddiqi NJ. Aging, metabolic, and degenerative disorders: Biomedical value of antioxidants. Oxid Med Cell Longev. 2018;2018(2098123) doi: 10.1155/2018/2098123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popa AR, Vesa CM, Uivarosan D, Jurca CM, Isvoranu G, Socea B, Stanescu AMA, Iancu MA, Scarneciu I, Zaha DC. Cross-sectional study regarding the association between sweetened beverages intake, fast-food products, body mass index, fasting blood glucose and blood pressure in the young adults from North-western Romania. Rev Chim. 2019;70:156–160. [Google Scholar]
- 23.Grigore O, Mihailescu AI, Solomon I, Boda D, Caruntu C. Role of stress in modulation of skin neurogenic inflammation. Exp Ther Med. 2019;17:997–1003. doi: 10.3892/etm.2018.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghita MA, Caruntu C, Lixandru D, Pitea A, Batani A, Boda D. The quest for novel biomarkers in early diagnosis of diabetic neuropathy. Curr Proteomics. 2017;14:86–99. [Google Scholar]
- 25.Gabor-Harosa FM, Stan OP, Daina L, Mocean F. Proposed model for a Romanian register of chronic diseases in children. Comput Methods Programs Biomed. 2016;130:198–204. doi: 10.1016/j.cmpb.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Koh SB, Yoon J, Kim JY, Yoo BS, Lee SH, Park JK, Choe KH. Relationships between serum adiponectin with metabolic syndrome and components of metabolic syndrome in non-diabetic Koreans: ARIRANG study. Yonsei Med J. 2011;52:234–241. doi: 10.3349/ymj.2011.52.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Frankenberg AD, do Nascimento FV, Gatelli LE, Nedel BL, Garcia SP, de Oliveira CSV, Saddi-Rosa P, Reis AF, Canani LH, Gerchman F. Major components of metabolic syndrome and adiponectin levels: A cross-sectional study. Diabetol Metab Syndr. 2014;6(26) doi: 10.1186/1758-5996-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deepa SS, Zhou L, Ryu J, Wang C, Mao X, Li C, Zhang N, Musi N, DeFronzo RA, Liu F, et al. APPL1 mediates adiponectin-induced LKB1 cytosolic localization through the PP2A-PKCzeta signaling pathway. Mol Endocrinol. 2011;25:1773–1785. doi: 10.1210/me.2011-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014(658913) doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding YS, Guo SX, Ma RL, Li SG, Guo H, Zhang JY, Zhang M, Liu JM, He J, Yan YZ, et al. Association of metabolic syndrome with the adiponectin to homeostasis model assessment of insulin resistance ratio. Mediators Inflamm. 2015;2015(607364) doi: 10.1155/2015/607364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huffman FG, Gomez GP, Zarini GG. Metabolic syndrome and high-sensitivity C-reactive protein in Cubans. Ethn Dis. 2009;19:115–120. [PubMed] [Google Scholar]
- 32.den Engelsen C, Koekkoek PS, Gorter KJ, van den Donk M, Salomé PL, Rutten GE. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: A cross-sectional analysis. Cardiovasc Diabetol. 2012;11(25) doi: 10.1186/1475-2840-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdella NA, Mojiminiyi OA, Moussa MA, Zaki M, Al Mohammedi H, Al Ozairi ES, Al Jebely S. Plasma leptin concentration in patients with type 2 diabetes: Relationship to cardiovascular disease risk factors and insulin resistance. Diabet Med. 2005;22:278–285. doi: 10.1111/j.1464-5491.2004.01405.x. [DOI] [PubMed] [Google Scholar]
- 34.López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18:37–45. doi: 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi M, Arita Y, Yamagata K, Matsukawa Y, Okutomi K, Horie M, Shimomura I, Hotta K, Kuriyama H, Kihara S, et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord. 2000;24:861–868. doi: 10.1038/sj.ijo.0801244. [DOI] [PubMed] [Google Scholar]
- 36.Ilie MA, Caruntu C, Tampa M, Georgescu SR, Matei C, Negrei C, Ion RM, Constantin C, Neagu M, Boda D. Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions. Exp Ther Med. 2019;18:916–925. doi: 10.3892/etm.2019.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghiţă MA, Căruntu C, Rosca AE, Căruntu A, Moraru L, Constantin C, Neagu M, Boda D. Real-time investigation of skin blood flow changes induced by topical capsaicin. Acta Dermatovenerol Croat. 2017;25:223–227. [PubMed] [Google Scholar]
- 38.Caruntu C, Boda D, Constantin C, Caruntu A, Neagu M. Catecholamines increase in vitro proliferation of murine B16F10 melanoma cells. Acta Endocrinol (Copenh) 2014;10:545–558. [Google Scholar]
- 39.Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27:629–630. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 41.Unamuno X, Izaguirre M, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, et al. Increase of the Adiponectin/leptin ratio in patients with obesity and type 2 diabetes after Roux-en-Y gastric bypass. Nutrients. 2019;11(2069) doi: 10.3390/nu11092069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
At the private medical offices where the data were collected.