Abstract
Hypoxia/reoxygenation (H/R) is one of the main causes of coronary artery disease (CAD), which is primarily induced by damage to coronary artery endothelial cells (CAECs). Glycyrrhizic acid (GA) is a natural and abundant pentacyclic triterpenoid glycoside of the licorice root extract, and it has been reported to elicit protective effects against hypoxia, inflammation and apoptosis in ischemic myocardium; therefore, GA may serve as a promising therapeutic agent for ischemia-associated CAD. In the present study, the protective effects of GA against H/R-induced injury in CAECs were investigated. Treatment with GA during H/R maintained cell viability and decreased H/R-induced cell apoptosis in human CAECs. In addition, H/R-mediated induction of intracellular and mitochondrial reactive oxygen species (ROS) was significantly decreased by GA exposure. Similar to ROS scavengers, GA treatment not only exhibited protective effects, but also maintained the mitochondrial membrane potential after H/R and inhibited H/R-induced mitochondrial dysfunction, including deficits in ATP synthesis, mitochondrial DNA copy number and mitochondrial transcriptional activity. Furthermore, GA decreased autophagy/mitophagy, and its protective effect against H/R was abolished by autophagy promotion. Collectively, the results suggested that GA exhibited protective effects against H/R-induced CAEC injury by decreasing ROS accumulation and maintaining mitochondrial homeostasis. Further investigation into the precise mechanisms underlying the decrease in ROS accumulation induced by GA is required.
Keywords: hypoxia/reoxygenation, coronary artery endothelial cells, glycyrrhizic acid, mitochondrial homeostasis, reactive oxygen species
Introduction
Ischemia/reperfusion (I/R) and hypoxia/reoxygenation (H/R) injuries are harmful to endothelial cells in the arterial system, and are the primary causes of cardiac artery disease (CAD) (1). Reperfusion and reoxygenation induce reactive oxygen species (ROS) accumulation and mitochondrial dysfunction, leading to apoptotic cell death (2). Therefore, therapeutic drugs and molecules that decrease or prevent reperfusion/reoxygenation injury have been investigated, which has improved the knowledge of the pathophysiology of the processes and has exhibited encouraging results in preclinical trials (3,4).
Glycyrrhizic acid (GA), a triterpene saponin glycoside, is the primary bioactive component of the root extract of Glycyrhiza glabra (licorice) (5). Although it is clinically used as an antiulcer, antiallergic, antioxidant, antiviral and anticancer agent (6), several studies have also demonstrated its potential protective effects against I/R- and H/R-induced endothelial injury (6,7). Cai et al (8), reported that GA elicits protective effects against myocardial I/R injury by regulating oxidative stress and inflammatory reactions via the transcriptional modification of high-mobility group box 1 and mitogen-activated protein kinase in rats (8). However, whether GA exhibits protective effects against I/R- and H/R-induced injury in coronary artery endothelial cells (CAECs) is not completely understood.
Mitochondria are essential eukaryotic organelles that are the primary source of cellular energy and participate in essential physiological processes, including cell signaling and apoptosis (5-7). During I/R or H/R, ROS accumulation decreases the mitochondrial permeability transition, decreases the mitochondrial membrane potential and alters mitochondrial homeostasis, which particularly affects myocardial and endothelial cells (9). The accumulation of excessive ROS critically damages mitochondria, resulting in damage to DNA, lipids and proteins (10). Damaged mitochondria subsequently undergo mitophagy, which results in decreased ATP production, impaired calcium buffering and ultimately, apoptosis (4,11). ROS accumulation also promotes autophagy/mitophagy to remove the damaged mitochondria, leading to further mitochondrial dysfunction (12), which has been reported to be closely associated with H/R-induced CAD.
To evaluate the effects of GA, a model of H/R injury was constructed with human CAECs (HCAECs) using a hypoxia/reoxygenation system. The present study aimed to investigate whether GA affected ROS accumulation and subsequent mitochondrial dysfunction; therefore, indicating whether GA may display protective effects against H/R-induced CAD.
Materials and methods
Cell culture and establishment of the H/R model
HCAECs were purchased from iCell Bioscience, Inc. (www.icellbioscience.com/cellDetail/914/0/-1) and cultured in Endothelial Cell Medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) at 37˚C with 5% CO2.
To mimic ischemia, hypoxia should be induced in oxygen-free and nutrition-free conditions; therefore, HCAECs were cultured with pure nitrogen for 30 min at 37˚C to expel the air and subsequently, pure nitrogen gas was used to fill the culture vessels and hypoxia chamber (Corning Inc.). Subsequently, HCAECs were cultured in hypoxic solution in the hypoxia chamber for 4 h at 37˚C. Endothelial cell medium (cat. no. CC-3162; Lonza Group, Ltd.) containing 10% FBS (cat. no. 10099; Thermo Fisher Scientific, Inc.) was pre-maintained in a hypoxia chamber at 37˚C for 24 h. Following hypoxia induction, the medium was replaced with oxygenated culture medium supplemented with 10% FBS and the culture vessels were transferred into a normoxic incubator at 37˚C with 5% CO2 for 2 h of reoxygenation. To evaluate the effect of GA on H/R, 50, 100, 150 or 200 µM GA was added to culturing medium immediately after H/R exposure and incubated for 1 h at 37˚C. After 4, 8 or 12 h, cell viability was measured by performing CCK-8 assay as described below.
To scavenge total ROS, 10 µM NAC was added into endothelial cell medium containing 10% FBS together with H/R exposure. To scavenge mitochondrial ROS, 1 µM MitoQ10 (Sigma-Aldrich; Merck KGaA) was added into medium together with H/R exposure.
To inhibit autophagy/mitophagy, 100 µmol/l rapamycin was added into endothelial cell medium containing 10% FBS together with H/R exposure. To inhibit LC3B-II degradation, 20 µmol/l chloroquine (Sigma-Aldrich; Merck KGaA) was added into endothelial cell medium containing 10% FBS together with H/R exposure.
Evaluation of cell viability
Cells (5x103) were seeded into a 96-well plate and incubated overnight 37˚C. To evaluate cell viability after hypoxia treatment for 4, 8 or 12 h, 10 µl Cell Counting Kit-8 (CCK-8) solution (Shanghai Shenggong Biology Engineering Technology Serve, Ltd.) was added to each well and incubated at 37˚C for 2 h. The absorbance of each well was measured at a wavelength of 450 nm using a Multiskan spectrum microplate reader (Thermo Fisher Scientific, Inc.).
Apoptosis assay
Apoptotic cells were detected using the Annexin V/PI Apoptosis kit (BioVision, Inc.) following the manufacturer's protocol. Briefly, cell suspensions were made using 0.25% trypsin and the cell concentration was modified to 1x106 cells/ml. Subsequently, cells (5x105) were stained with 5 µl Annexin V-FITC and 10 µl propidium iodide (PI) for 10 min in darkness at room temperature. Apoptotic cells were detected using a 3 laser Navios flow cytometer (Beckman Coulter, Inc.). The Annexin V-FITC positive/PI negative (early stage apoptosis) and Annexin V-FITC positive/PI positive (late stage apoptosis) subpopulations were considered as apoptotic cells. Data were analyzed using FlowJo software (version 10.5.2; FlowJo LLC).
Detecting intracellular and mitochondrial ROS levels in HCAECs
To measure intracellular and mitochondrial ROS levels, 2',7'-dichlorofluorescin diacetate (DCFH-DA) and MitoSOX™ staining were performed, respectively. Nuclei were counterstained with DAPI to a final concentration of 5 µg/ml (Beyotime Institute of Biotechnology) at room temperature for 5 min. Cells were washed 3 times with ice-cold PBS. Subsequently, cells were incubated with 1 ml serum-free medium containing 10 µM DCFH-DA probe or 5 µM MitoSOX™ in the dark at 37˚C for 30 min with gentle shaking every 5 min. Cells were washed 3 times with ice-cold PBS. Green fluorescence was observed using an X71 (U-RFL-T) fluorescence microscope (Olympus Corporation) at an excitation wavelength of 488 nm and magnification, x40.
JC-1 staining
JC-1 staining (Thermo Fisher Scientific, Inc.) was used to investigate the mitochondrial membrane potential. Cells were stained with 15 µg/ml JC-1 at 37˚C for 30 min in dark. Subsequently, cells were washed 3 times with PBS. Stained cells were observed using an X71 (U-RFL-T) fluorescence microscope (Olympus Corporation) at a magnification, x100.
ATP measurement
To assess ATP production, the ATP Bioluminescent assay kit (Sigma-Aldrich; Merck KGaA) was used according to the manufacturer's protocol. Cells were washed 3 times with ice-cold PBS and subsequently lysed using a reaction buffer containing 0.22 M sucrose, 0.12 M mannitol, 40 mM tricine (pH 7.5) and 1 mM EDTA. ATP production was measured using an Optocomp I BG-1 luminometer (GEM Biomedical, Inc.).
Measurement of telomere length and mitochondrial DNA (mtDNA) copy number
Total DNA (including nuclear and mitochondrial genomic DNA) was extracted using a TIANamp total DNA kit (Tiangen Biotech Co., Ltd.) and directly employed as a template for PCR. The mtDNA copy number was then assessed by qPCR using SYBRGreen master mix (Thermo Fisher Scientific, Inc.) and the ABI7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following primer pairs were used for qPCR: 200 nM mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 1 (ND1) forward, 5'-CCCTAAAACCCGCCACATCT-3' and reverse, 5'-GAGCGATGGTGAGAGCTAAGGT-3'; and 167 nM cytoglobin (HGB) forward, 5'-GTGCACCTGACTCCTGAGGAGA-3' and reverse, 5'-CCTTGATACCAACCTG-CCCAG-3'. The following thermocycling conditions were used for qPCR: Initial denaturation, 98˚C for 30 sec, followed by 40 cycles of 98˚C for 10 sec and 60˚C for 60 sec. mtDNA copy numbers were normalized to the nuclear single-copy gene HGB. The relative mtDNA copy number was calculated using the 2-ΔΔCq method (13).
Mitochondrial transcriptional activity
Total RNA was extracted from cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Total RNA was reverse transcribed into cDNA using the RT-for-PCR kit (Qiagen, Inc.) in accordance with the manufacturer's protocol. Subsequently, qPCR was performed using SYBRGreen master mix (Thermo Fisher Scientific, Inc.) and the ABI7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following primer pairs were used for qPCR: Mitochondrially encoded cytochrome c oxidase I forward, 5'-ATGCGGCCATAGGTTCTGC-3' and reverse, 5'-TCCTCAAGATGTCTCAGTTCCAT-3'; ND1 forward, 5'-TCGTCATAATCTGTCCCTACACA-3' and reverse, 5'-CGGCTTCGGCTCTTAGCAAA-3'; β-actin forward, 5'-CATGTACGTTGCTATCCAGGC3' and reverse, 5'-CTCCTTAATGTCACGCACGAT-3'. The following thermocycling conditions were used for qPCR: Initial denaturation at 98˚C for 30 sec, followed by 45 cycles of 98˚C for 10 sec and 60˚C for 60 sec. mRNA expression levels were normalized to the internal reference gene β-actin. Relative expression levels were calculated using 2-ΔΔCq method (13).
Western blotting
Total protein was extracted using the SoniConvert™ system (DocSense) and RIPA buffer (Sigma-Aldrich; Merck KGaA). Total protein was denatured at 100˚C for 10 min and was quantitatively measured using a bicinchoninic acid assay kit (Sigma-Aldrich; Merck KGaA) in accordance with the manufacturer's protocol. Total protein (20 µg) were separated by 8-16% SDS-PAGE and transferred to nitrocellulose membranes. After blocking in 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) at room temperature for 30 min, the membranes were incubated with primary antibodies targeted against microtubule associated protein 1 light chain 3B (LC3B; cat. no. ab48394; 1:1,000; Abcam), p62 (cat. no. ab109012; 1:2,000; Abcam) and β-actin (cat. no. ab8226; 1:5,000; Abcam) at room temperature for 1 h. After three washes with PBS-T, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (cat. no. ab7090; 1:5,000; Abcam) at room temperature for 1 h. Protein bands were visualized using a chemiluminescence kit (Thermo Fisher Scientific, Inc.). Protein expression levels were quantified using Image J software (version 1.51; National Institutes of Health) with β-actin as the loading control.
Statistical analysis
Data are presented as the mean ± standard error of the mean and analyzed using SPSS software (version 16.0; SPSS, Inc.) All experiments were repeated 3 times independently. Comparisons among multiple groups were analyzed using one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
GA exerts protective effects against H/R-induced cellular injury in HCAECs
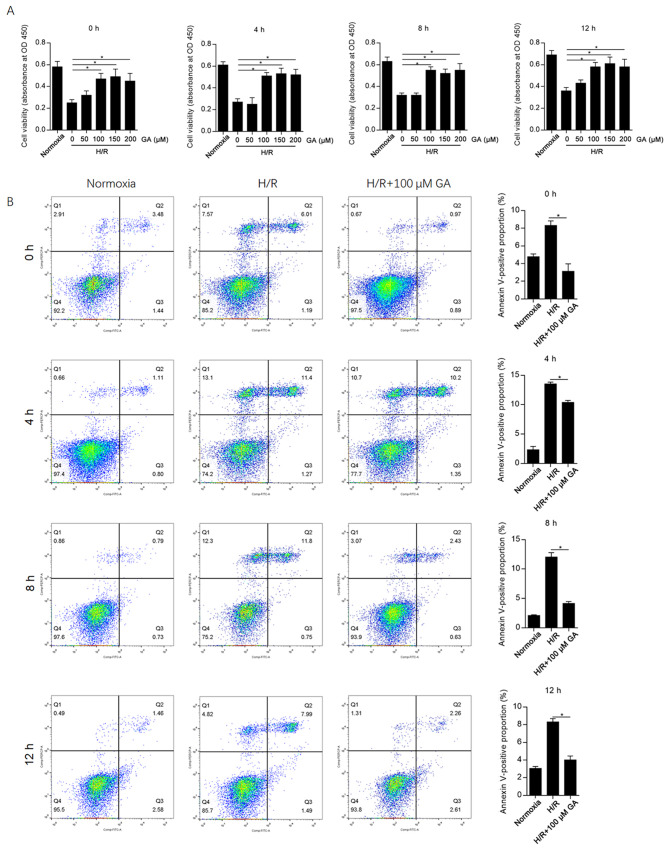
To evaluate the effect of GA on H/R-induced cellular injury in HCAECs, different concentrations of GA (0-200 µM) were added to the medium during H/R induction, and the CCK-8 assay was subsequently performed after 4, 8 or 12 h. The results suggested that 100, 150 and 200 µM GA significantly decreased H/R-induced cell injury at each time point compared with the 0 µM GA group (Fig. 1A). At each time point, there were no significant differences between the protective effects of 100 and 200 µM GA; therefore, 100 µM GA was used for subsequent experiments.
Figure 1.
GA exhibits protective effects against H/R-induced cell injury in human coronary artery endothelial cells. (A) The viability of H/R-induced cells treated with different concentrations of GA was assessed using the Cell Counting Kit-8 assay. (B) Following H/R treatment with or without 100 µM GA for 0, 4, 8 or 12 h, Annexin V-FITC/PI double labelling followed by flow cytometry was performed to assess the proportion of apoptotic cells. *P<0.05. GA, glycyrrhizic acid; H/R, hypoxia/reoxygenation; PI, propidium iodide; OD, optical density.
Considering that H/R induces cell injury and decreases cell viability mainly by inducing apoptosis (13), apoptotic cell death following GA incubation was assessed at different time points. GA incubation significantly decreased the Annexin V-FITC+/PI- and Annexin V-FITC+/PI+ subpopulations at each time point compared with the H/R group (Fig. 1B). The highest proportion of apoptotic cells was observed at 4 h after H/R induction, indicating that H/R-induced cell injury peaked at this time point.
GA decreases total and mitochondrial ROS levels and consequently decreases H/R-induced apoptosis
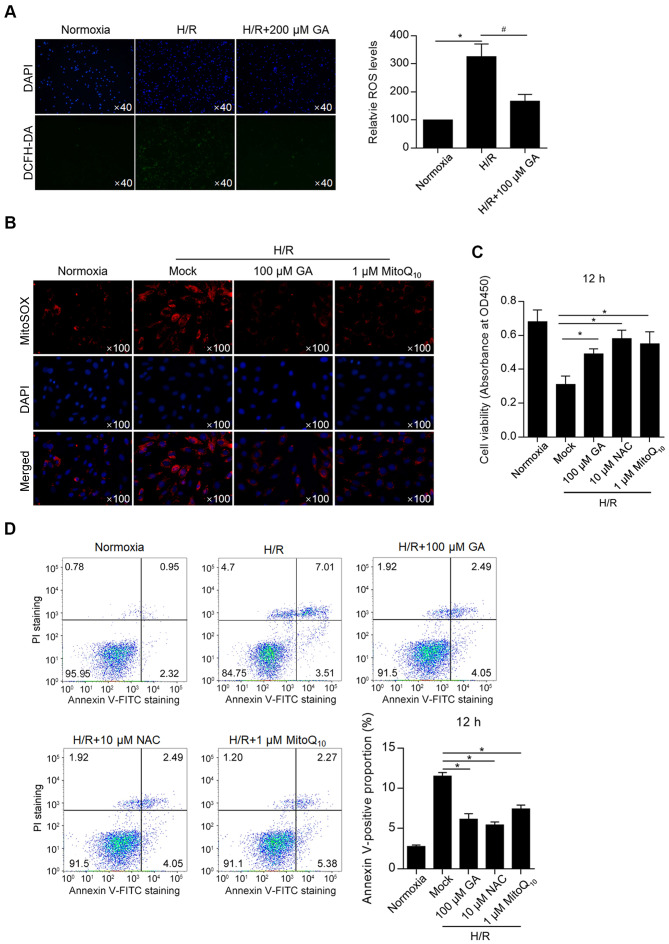
Considering that H/R induces intracellular and mitochondrial ROS accumulation (14-17), whether GA altered H/R-induced ROS accumulation was investigated and the rate of apoptosis was assessed. By detecting intracellular ROS (with DCFH-DA labeling) and mitochondrial ROS (with MitoSOX™ Red), total ROS and mitochondrial ROS levels after GA treatment were measured. H/R-induced ROS accumulation was significantly decreased by incubation with 100 µM GA (Fig. 2A). In addition, H/R-induced mitochondrial ROS accumulation was decreased by incubation with 100 µM GA, which exhibited similar effects compared with treatment with 1 µM MitoQ10 (Fig. 2B), indicating that GA and MitoQ10 elicited similar effects.
Figure 2.
GA exerts protective effects against H/R by decreasing ROS accumulation. (A) Intracellular and (B) mitochondrial accumulation was assessed. (C) Cell viability was assessed using the Cell Counting Kit-8 assay. (D) Cell apoptosis was assessed by Annexin V-FITC/PI double staining. *P<0.05. #P<0.05. GA, glycyrrhizic acid; H/R, hypoxia/reoxygenation; ROS, reactive oxygen species; PI, propidium iodide; NAC, N-acetylcysteine.
Subsequently, the effects of GA on cell viability and apoptosis were measured. A CCK-8 assay was performed and the results revealed that GA treatment significantly increased the H/R-induced reduction of cell viability, which was an effect similar to that of NAC and MitoQ10 treatments (Fig. 2C). Annexin-FITC/PI double staining followed by flow cytometry was performed to detect apoptosis. Treatment with GA, N-acetylcysteine (NAC) or MitoQ10 decreased H/R-induced cell apoptosis (Fig. 2D). The results indicated that H/R-induced ROS accumulation was important for inducing apoptosis, and that GA treatment potentially displayed a protective effect against cell apoptosis by decreasing ROS accumulation.
GA treatment maintains the mitochondrial membrane potential and mitochondrial function
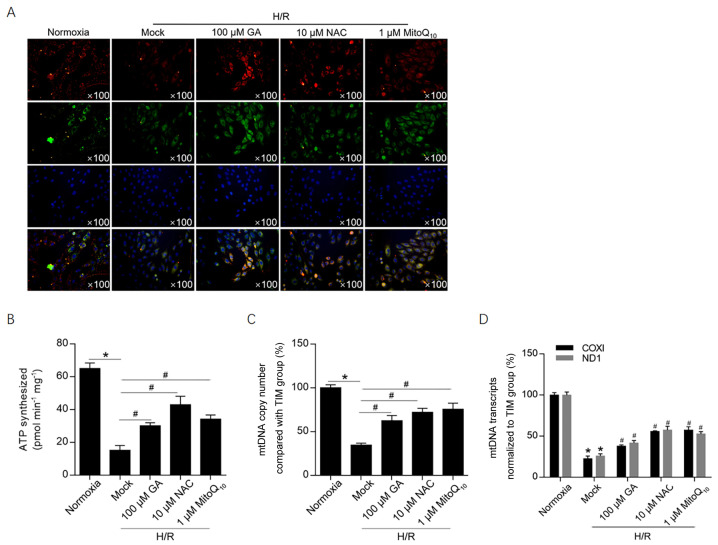
To investigate the effects of H/R-induced ROS accumulation on the mitochondrial membrane potential, JC-1 staining was performed. The mitochondrial membrane potential was notably decreased following H/R induction, as suggested by a decrease in red fluorescence compared with the normoxia group (Fig. 3A). Treatment with 100 µM GA, 10 µM NAC or 1 µM MitoQ10 markedly increased red fluorescence compared with the H/R group, which indicated that the 3 treatments maintained the mitochondrial membrane potential following H/R induction. To further investigate mitochondrial function, ATP synthesis, mtDNA copy number and mitochondrial transcriptional activity were assessed (Fig. 3B-D). Treatment with GA or NAC significantly protected cells against H/R-induced mitochondrial dysfunction, decreasing ATP synthesis and normalizing transcriptional activity to that of the internal reference gene (β-actin). In addition, the relatively high mtDNA copy number following GA treatment was normalized to HGB, which is a nuclear single-copy gene. The results revealed that GA treatment was potentially associated with promotion of mitochondria biogenesis or inhibition of mitophagy.
Figure 3.
GA maintains the mitochondrial membrane potential potentially via decreasing mitochondrial ROS accumulation. (A) JC-1 staining was performed to investigate the effect of GA on the mitochondrial membrane potential. Multi-aggregates are stained red and mono-aggregates are stained green. Following treatment with 100 µM GA, 10 µM NAC or 1 µM MitoQ10, (B) ATP synthesis, (C) mtDNA copy number and (D) mtDNA transcriptional activity were assessed. *P<0.05 vs. normoxia group. #P<0.05 vs. H/R + mock group. GA, glycyrrhizic acid; ROS, reactive oxygen species; NAC, N-acetylcysteine; mtDNA, mitochondrial DNA; H/R, hypoxia/reoxygenation; TIM, Rho guanine nucleotide exchange factor 5; COXI, mitochondrially encoded cytochrome c oxidase I; ND1, mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 1.
GA decreases autophagy/mitophagy and autophagy induction abolishes its protective effects
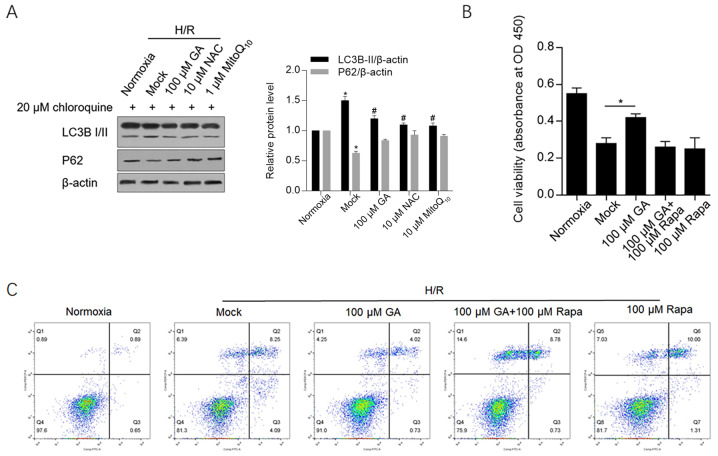
To determine whether GA treatment maintained mitochondrial function via autophagy/mitophagy, markers of autophagy/mitophagy, including LC3B and p62, were detected by western blotting. H/R-induced LC3B-II expression was significantly decreased by GA, NAC or MitoQ10 treatment. Similarly, the expression of p62, which is downregulated during autophagy/mitophagy, was also markedly maintained by treatment with GA, NAC or MitoQ10 following H/R induction (Fig. 4A).
Figure 4.
GA decreases mitophagy/autophagy, exhibiting protective effects against H/R. (A) Following H/R induction, LC3B-I/II and p62 protein expression levels were detected by western blotting. *P<0.05 vs. normoxia group; #P<0.05 vs. the H/R+mock group. The (B) Cell Counting Kit-8 and (C) apoptosis assays were performed to assess cell viability following H/R induction. *P<0.05 vs. the H/R+mock group. GA, glycyrrhizic acid; H/R, hypoxia/reoxygenation; LC3B, microtubule associated protein 1 light chain 3B; NAC, N-acetylcysteine; OD, optical density; Rapa, rapamycin.
To further clarify whether GA exerted protective effects by inhibiting autophagy/mitophagy, cell viability and apoptosis were analyzed in cells treated with GA and rapamycin, an autophagy-inducing compound. Rapamycin exposure abolished GA-induced protective effects on cell viability and apoptosis, which indicated that the protective effects of GA were associated with a decrease in autophagy/mitophagy (Fig. 4B and C).
Collectively, the results suggested that GA decreased H/R-induced ROS accumulation, maintained the mitochondrial membrane potential and inhibited mitophagy/autophagy, which protected cells against H/R-induced injury.
Discussion
In the present study, the protective effects of GA against H/R-induced decreases in cell viability and increases in cell apoptosis in HCAECs were investigated. Treatment with GA significantly inhibited H/R-induced ROS accumulation, maintained the mitochondrial membrane potential and mitochondrial homeostasis, and prevented mitochondrial dysfunction following H/R.
I/R and H/R are common causes of tissue and cell damage due to a lack of blood supply or oxygen. Unfortunately, restoration of the blood or oxygen supply leads to further oxidative damage and inflammatory effects by inducing oxidative stress (1). Certain endothelial cells in different organs are particularly sensitive to ischemia and hypoxia due to their direct contact with the circulation, and damage to these cells can result from an oxidative stress imbalance arising from the inflammatory response (18). I/R- and H/R-induced ROS accumulation damages mitochondria, decreases the mitochondrial membrane potential, damages DNA, lipids and proteins, and promotes mitophagy (16). Considering the critical effects of ROS in promoting cell injury following H/R, scavenging ROS is a promising treatment strategy. N-n-butyl haloperidol iodide (F2) has been reported to exert protective effects against myocardial I/R injury by downregulating H/R-induced ROS levels, inactivating JNK and downregulating early growth response 1 protein expression in H9c2 cells (19). Metformin, a first-line drug for the management of type 2 diabetes, has been reported to protect cardiomyocytes against H/R injury by inhibiting ROS generation and inflammatory responses (20). Collectively, the aforementioned studies indicated that GA might protect against H/R-induced injury by regulating ROS levels.
In the present study, H/R induced intracellular and mitochondrial ROS accumulation, and treatment with GA significantly decreased ROS levels. Further investigation is required to determine whether ROS generation or accumulation is affected by GA. To distinguish mitochondrial ROS from intracellular ROS, 0.5 µM MitoSOX with an excitation wavelength of 365 nm was used. H/R induced intracellular and mitochondrial ROS accumulation; however, whether the observed increase in mitochondrial ROS levels was amplified by intracellular ROS accumulation, as has been previously reported, was not investigated in the present study (21,22). Although H/R-induced intracellular ROS accumulation directly interacts with mitochondrial proteins and lipids to accelerate mitochondrial dysfunction (23), in the present study, scavengers of intracellular and mitochondrial ROS decreased the rate of apoptosis following H/R, suggesting that mitochondrial ROS directly induced apoptosis. A decrease in the percentage of Annexin V+/PI- and Annexin V+/PI+ cells following treatment with GA in H/R-induced cells was also observed, which suggested that necrosis had also occurred; however, the effects of GA on necrosis require further investigation.
An excess of mitochondrial ROS leads to mitophagy and subsequent apoptosis, which is accompanied by a decrease in ATP production and collapse of the mitochondrial membrane potential (24,25). It has also been reported that GA exerts a protective role against hypoxia-induced mitochondrial damage by regulating mitochondria (26). Following H/R, GA treatment inhibited the decrease in ATP production, the collapse of the mitochondrial membrane potential and the decrease in mtDNA copy number. By detecting the hallmarks of autophagy/mitophagy, it was further revealed that GA treatment inhibited ROS-induced autophagy/mitophagy and subsequent cell apoptosis by decreasing ROS levels. The effects of GA on the viability of cells following H/R induction was analyzed using the CCK-8 assay. Future studies should perform time-lapse live-cell experiments to reveal the effects of GA on myocardial cells and H/R-induced necrosis.
The present study had several limitations. Firstly, H/R-induced mitochondrial ROS accumulation may induce endothelial injury; however, whether the observed increase in mitochondrial ROS levels was amplified by intracellular ROS was not investigated in the present study. Secondly, the exact mechanism underlying GA-induced reductions in ROS accumulation was not identified in the present study and requires further investigation.
Acknowledgements
The authors would like to thank Mrs Yun Bai (Third Military Medical University, Chongqing) for language editing the manuscript.
Funding
The present study was supported by the Starting Scientific Foundation of Zunyi Medical University (grant no. SS20180601ZF).
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QT designed the study. YC, WX and XK performed the cellular experiments and analyzed the data. JZ and YX collected the data, wrote the manuscript and performed the flow cytometry experiments. DL contributed to designing the study and writing the manuscript, and also provided supervision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Jimenez R, Garcia-Prieto J, Sanchez-Gonzalez J, Agüero J, López-Martín GJ, Galán-Arriola C, Molina-Iracheta A, Doohan R, Fuster V, Ibáñez B. Pathophysiology underlying the bimodal edema phenomenon after myocardial ischemia/reperfusion. J Am Coll Cardiol. 2015;66:816–828. doi: 10.1016/j.jacc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Rezende PC, Ribas FF, Serrano CJ, Hueb W. Clinical significance of chronic myocardial ischemia in coronary artery disease patients. J Thorac Dis. 2019;11:1005–1015. doi: 10.21037/jtd.2019.02.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 5.Blackstone NW. The impact of mitochondrial endosymbiosis on the evolution of calcium signaling. Cell Calcium. 2015;57:133–139. doi: 10.1016/j.ceca.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai X, Wang X, Li J, Chen S. Protective effect of glycyrrhizin on myocardial ischemia/reperfusion injury-induced oxidative stress, inducible nitric oxide synthase and inflammatory reactions through high-mobility group box 1 and mitogen-activated protein kinase expression. Exp Ther Med. 2017;14:1219–1226. doi: 10.3892/etm.2017.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valls-Lacalle L, Barba I, Miro-Casas E, Alburquerque-Béjar JJ, Ruiz-Meana M, Fuertes-Agudo M, Rodríguez-Sinovas A, García-Dorado D. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc Res. 2016;109:374–384. doi: 10.1093/cvr/cvv279. [DOI] [PubMed] [Google Scholar]
- 10.Xu M, Bi X, He X, Yu X, Zhao M, Zang W. Inhibition of the mitochondrial unfolded protein response by acetylcholine alleviated Hypoxia/Reoxygenation-Induced apoptosis of endothelial cells. Cell Cycle. 2016;15:1331–1343. doi: 10.1080/15384101.2016.1160985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets. 2009;20:588–593. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 2015;116:1477–1490. doi: 10.1161/CIRCRESAHA.116.303790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KD, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Cao X, Wang X, Ling Y, Song X, Yang P, Liu Y, Liu L, Wang L, Guo J, Chen A. Comparison of the degree of autophagy in neonatal rat cardiomyocytes and H9c2 cells exposed to hypoxia/reoxygenation. Clin Lab. 2014;60:809–814. doi: 10.7754/clin.lab.2013.130521. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Shi G, Zheng J, Tang Z, Gao P, Lv Y, Guo F, Jia Q. The protective effects of N-n-butyl haloperidol iodide on myocardial ischemia-reperfusion injury in rats by inhibiting Egr-1 overexpression. Cell Physiol Biochem. 2007;20:639–648. doi: 10.1159/000107547. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Chen G, Zhong S, Zheng F, Gao F, Chen Y, Huang Z, Cai W, Li W, Liu X, et al. N-n-butyl haloperidol iodide ameliorates cardiomyocytes hypoxia/reoxygenation injury by extracellular calcium-dependent and -independent mechanisms. Oxid Med Cell Longev. 2013;2013(912310) doi: 10.1155/2013/912310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: Implications for pharmacological cardioprotection. Am J Physiol Heart Circ Physiol. 2018;315:H1341–H1352. doi: 10.1152/ajpheart.00028.2018. [DOI] [PubMed] [Google Scholar]
- 18.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Liao H, Zhong S, Gao F, Chen Y, Huang Z, Lu S, Sun T, Wang B, Li W, et al. Effect of N-n-butyl haloperidol iodide on ROS/JNK/Egr-1 signaling in H9c2 cells after hypoxia/reoxygenation. Sci Rep. 2015;5(11809) doi: 10.1038/srep11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu M, Ye P, Liao H, Chen M, Yang F. Metformin protects h9c2 cardiomyocytes from high-glucose and hypoxia/reoxygenation injury via inhibition of reactive oxygen species generation and inflammatory responses: Role of AMPK and JNK. J Diabetes Res. 2016;2016(2961954) doi: 10.1155/2016/2961954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol. 2017;174:1670–1689. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajeddine N. How do reactive oxygen species and calcium trigger mitochondrial membrane permeabilisation? Biochim Biophys Acta. 2016;1860:1079–1088. doi: 10.1016/j.bbagen.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Lin Y, Tang X, Zhang P, Liu B, Liu Y, Miao F. Helix B surface peptide protects cardiomyocytes against hypoxia/reoxygenation-induced apoptosis through mitochondrial pathways. J Cardiovasc Pharmacol. 2016;67:418–426. doi: 10.1097/FJC.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 25.Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011;6(e28154) doi: 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du JK, Cong BH, Yu Q, Wang H, Wang L, Wang CN, Tang XL, Lu JQ, Zhu XY, Ni X. Upregulation of microRNA-22 contributes to myocardial ischemia-reperfusion injury by interfering with the mitochondrial function. Free Radic Biol Med. 2016;96:406–417. doi: 10.1016/j.freeradbiomed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Xu CL, Liang CH, Sun WX, Chen J, Chen X. Glycyrrhizic acid ameliorates myocardial ischemic injury by the regulation of inflammation and oxidative state. Drug Des Devel Ther. 2018;12:1311–1319. doi: 10.2147/DDDT.S165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.