Abstract
Influence of lidocaine on rats with cerebral ischemia-reperfusion injury (CIRI) was studied to explore its mechanism of action. A total of 30 Sprague-Dawley rats were randomly divided into control group and model group, and the rat model of CIRI was prepared by the suture-occluded method in the model group. Then the rats in the model group were randomly assigned into the model group (n=10) and the lidocaine group (n=10). The neurological function score of rats was evaluated, and the levels of serum B-cell lymphoma-2 (Bcl-2) and Bcl-2 associated X protein (Bax) in rats were determined using ELISA. TUNEL assay was performed to detect the neuronal apoptosis in the brain of rats. The messenger ribonucleic acid (mRNA) and protein expression levels of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) were measured via RT-PCR and western blotting, respectively. Compared with those in the control group, the rats in the model group had an elevated neurological function score, a raised level of Bcl-2, but a reduced level of Bax in the serum, an obviously increased rate of neuronal apoptosis in the brain and decreased mRNA and protein levels of cAMP and PKA in cerebral tissues. The rats in lidocaine group had a lower neurological function score, a lower level of Bcl-2, but a higher level of Bax in the serum, an evidently lower rate of neuronal apoptosis in the brain and higher mRNA and protein levels of cAMP and PKA in cerebral tissues than those in the model group. Lidocaine can improve the neurological function of rats with CIRI and inhibit neuronal apoptosis in the brain, and its mechanism of action may be related to the activation of the cAMP/PKA signaling pathway.
Keywords: cerebral ischemia-reperfusion injury, cAMP/PKA signaling pathway, lidocaine, cell apoptosis
Introduction
In humans the brain is the most sensitive organ to oxygen, the cerebral tissue hypoxia or ischemia causes local damage to cerebral tissues, and their function fails to be restored, further leading to more severe cerebral dysfunction known as cerebral ischemia-reperfusion injury (CIRI) (1-3). CIRI is a complex physiological and pathological process, but it has complicated and various pathogeneses mainly involving inflammatory responses, Ca2+ overload, excessive accumulation of free radicals, and excitatory amino acid toxicity (4,5). Currently, there is no pathogenesis that can elucidate the cause of CIRI. However, increasing number of literature has found that neuronal apoptosis is closely associated with the development and progression of CIRI, and hence, this indicates that neuronal apoptosis in the brain could be a potential treatment regimen for CIRI.
Rall and Sutherland first discovered the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway in the 1990s, and they found through experiments that cAMP, also known as adenosine 3',5'-cyclic monophosphate, is condensed from an adenosine triphosphate by removing two phosphates (6). PKA is one of the crucial downstream target genes regulated by cAMP and the most thoroughly researched protein kinase as well, and it exerts varying physiological functions in different tissues (7,8). Large bodies of literature have proved that neuronal apoptosis modulated by the cAMP/PKA signaling pathway, a vital neuronal transduction pathway, is of great significance for organisms to exert cognitive, learning and memory functions, and that this pathway has received growing attention from researchers.
Lidocaine, a local anesthetic, can take effect only at 1-3 min after administration, with the anesthetic effect lasting for 1-3 h, and clinically, it is also used to treat arrhythmia and serves as the preferred drug for ventricular tachycardia and tremor (9,10). The incidence rate of adverse reactions to lidocaine is ~6%, with the major nervous, cardiovascular and allergic manifestations. Lidocaine has an exact neuroprotective effect, but there are few studies on whether it can exert the protective effect on the CIRI model rats through regulating the cAMP/PKA signaling pathway.
Therefore, the rat CIRI model was established using the suture-occluded method in this experiment to study the influence of lidocaine on neuronal apoptosis in the brain of rats and explore its mechanism of action, thereby providing an experimental basis for the clinical treatment of CIRI with lidocaine.
Materials and methods
Reagents
Lidocaine was purchased from Sigma-Aldrich; Merck KGaA, enzyme-linked immunosorbent assay (ELISA) kit from eBioscience, TRIzol solution from Shanghai Shiyi Biotech Inc., terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit from Nanjing KeyGen Biotech Co., Ltd., bicinchoninic acid (BCA) protein assay kit from Thermo Fisher Scientific, Inc., polymerase chain reaction (PCR) SuperMix kit from Beijing TransGen Biotech Co., Ltd., cAMP, PKA and β-actin primers from Invitrogen; Thermo Fisher Scientific, Inc. and rabbit anti-cAMP, PKA and β-actin primary antibodies and horseradish peroxidase (HRP)-labeled secondary antibodies from Beijing Biosynthesis Biotechnology Co., Ltd.
Instruments
Fluorescence inverted microscope was provided by Olympus, low-temperature refrigerator by Qingdao Haier Co., Ltd., fluorescence quantitative PCR instrument by Applied Biosystems, thermostatic water bath by Grant, low-temperature centrifuge by Shenzhen Anke High-tech Co., Ltd., and western blotting electrophoresis apparatus and transfer instrument by Bio-Rad Laboratories.
Rats
A total of 30 healthy male Sprague-Dawley rats weighing 200-240 g, aged 12-weeks were purchased from the Laboratory Animal Center of Capital Medical University (Beijing, China), and they were fed with standard granulated rat food in clean environment and allowed to move freely. This study was approved by the Animal Ethics Committee of Capital Medical University Animal Center.
Methods
Preparation of rat CIRI models
In this experiment, the rat models were prepared with reference to Longa's suture-occluded method, and the rats in model group fasted for water and food 12 h preoperatively. After being anesthetized using 10% chloral hydrate via intraperitoneal injection at a dose of 300-350 mg/kg, the rats were fixed in the supine position on an operating table, and the neck was cut open in the middle to expose the common carotid artery, external carotid artery and internal carotid artery. No rat exhibited signs of peritonitis after the administration of 10% chloral hydrate. Then the proximal ends of the external and common carotid arteries were ligated, and a V-shaped incision was made at ~0.2 mm from the common carotid artery. Subsequently, a nylon fishing line was inserted to cause partial occlusion of the common carotid artery in the brain of rats, with ~8 mm of the thread left outside, followed by suture of the wound. After ischemia for 2 h, the thread was pulled out. After modeling, the rats in lidocaine group were intraperitoneally injected with 10 mg/kg lidocaine, while those in control group with an equal dose of normal saline. Finally, the neurological function score of rats in each group was evaluated and recorded 24 h later based on Zea Longa's scoring criteria. Cervical dislocation (after being anesthetized using 10% chloral hydrate at a dose of 300 mg/kg) was used as the method of euthanasia. No rat exhibited signs of peritonitis after the administration of 10% chloral hydrate. We verified that the experimental animals (weighed 200-240 g) were dead by observing the breathing and heartbeat.
Determination of levels of serum B-cell lymphoma-2 (Bcl-2) and Bcl-2 associated X protein (Bax) in rats via ELISA
Serum was collected from each group of rats, let stand for 1 h, and centrifuged at 2,500 x g at 4˚C for 10 min, and the supernatant was aspirated. Each well was added with the standard to plot the standard curves, and then with 30 µl of buffer, 20 µl each of standard and sample, separately, and 50 µl of antibody solution. The fluid was removed, and the resulting sample and standard were cleaned by washing 3 times, adding 100 µl of enzyme solution, and then reacted for 30 min, washed again, and reacted with 100 µl of substrate solution for 15 min. The reaction was terminated by adding 100 µl of stop solution. Finally, the absorbance was measured using a microplate reader to calculate the content of Bcl-2 and Bax.
Evaluation of neuronal apoptosis in the brain of rats via TUNEL staining
The cerebral tissues of rats were embedded in paraffin and sectioned, and the sections were transparentized using xylene, soaked in ethanol solution at descending concentrations each for 1 min, fixed in 4% paraformaldehyde, penetrated using 0.2% Triton solution for 15 min, added with TUNEL staining solution, incubated in the dark for 30 min, and added with antifade mounting medium. Finally, the staining was observed under a fluorescence microscope.
Measurement of messenger ribonucleic acid (mRNA) levels in the cerebral tissues of rats via reverse transcription (RT)-PCR
The cerebral tissues of rats in each group were harvested, lysed with 1 ml of TRIzol solution and centrifuged at 10,500 x g at 4˚C for 10 min, and the supernatant was obtained. According to the instructions of RT-PCR, complementary deoxyribose nucleic acid (cDNA) was synthesized through RT, and then, PCR amplification was performed, with the information of added primers is shown in Table I. Subsequently, 3% gel electrophoresis was carried out. Finally, the bands were observed under a gel imager.
Table I.
Primer sequences of cAMP and PKA.
| Gene name | Sequence |
|---|---|
| cAMP | 5'-AGGTCCTCAGCTACAAGGAAG-3' |
| 5'-TCTTGAAGTCACAATCCTCTGGT-3' | |
| PKA | 5'-CTAAAGCAGATCGAGCACACTC-3' |
| 5'-GCCACCAGCTACATACTCCA-3' | |
| β-actin | 5'-CTCCATCCTGGCCTCGCTGT-3' |
| 5'-GCTGTCACCTTCACCGTTCC-3' |
cAMP, cyclic adenosine monophosphate; PKA, protein kinase A.
Determination of cAMP and PKA protein expression levels in cerebral tissues of rats using western blotting
The cerebral tissues of rats were lysed using RIPA lysis buffer containing protease inhibitor, and the concentration of proteins was determined using BCA kit. The prepared samples were loaded separately for sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) to separate proteins, and then the proteins were transferred onto a polyvinylidene fluoride (PVDF) membranes (Millipore), and blocked in 5% skim milk powder for 1 h, followed by incubation with the corresponding rabbit anti-cAMP, PKA and β-actin primary antibodies at 4˚C overnight. Subsequently, the HRP-labeled secondary antibodies were added, and the bands were detected using the chemiluminescence method. Finally, the optical density was analyzed using ImageJ software (NIH).
Statistical analysis
Statistical Product and Service Solutions (SPSS) 20.0 software (IBM, Corp.) was used for statistical analysis. Measurement data were expressed as mean ± standard deviation, and two-sample t-test was performed for means. P<0.05 indicates that the difference is statistically significant.
Results
The successful rat CIRI model
The rats in control group moved normally, and compared with those in control group, the rats in model group toppled rightward and circled to the right, with a higher neurological function score. In comparison with those in model group, few rats in lidocaine group circled to the right, with an obvious improvement in behavioral symptoms and a substantial decline in the neurological function score (Table II).
Table II.
Neurobehavioral score of rats.
aP<0.05, model group vs. control group;
bP<0.05, lidocaine group vs. model group.
Lidocaine decreases the level of Bcl-2, but increases that of Bax in the serum of CIRI rats
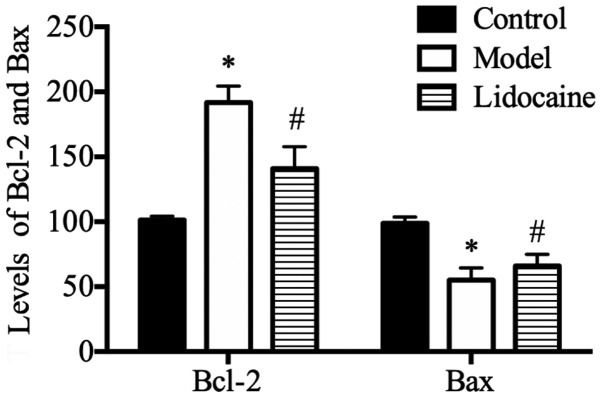
According to the ELISA results (Fig. 1), the rats in model group had a higher level of Bcl-2, but an obviously lower level of Bax in the serum than those in the control group (P<0.05), and compared with those in the model group, the level of Bcl-2 was lower, but that of Bax was evidently increased in the serum of rats in lidocaine group (P<0.05).
Figure 1.
Levels of serum Bcl-2 and Bax in rats determined using ELISA. *P<0.05, model group vs. control group; and #P<0.05, lidocaine group vs. model group. ELISA, enzyme-linked immunosorbent assay.
Lidocaine inhibits neuronal apoptosis in the brain of CIRI rats
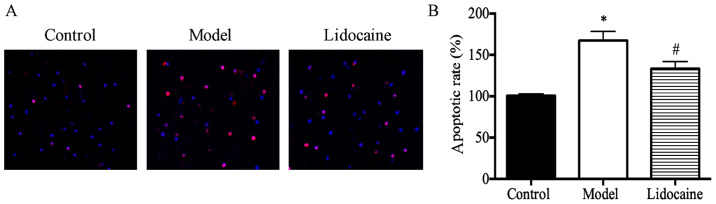
It was found through the analysis of TUNEL staining results (Fig. 2A) that the rate of neuronal apoptosis in the brain of rats in the model group was substantially higher than that in the control group (P<0.05), and that the rate in lidocaine group was obviously lower than that in the model group (P<0.05) (Fig. 2B), suggesting that lidocaine can inhibit the neuronal apoptosis in the brain of CIRI rats.
Figure 2.
Neuronal apoptosis in the brain of rats evaluated using TUNEL assay. (A) TUNEL staining results (x20). (B) Cell apoptosis rate. *P<0.05, model group vs. control group; and #P<0.05, lidocaine group vs. model group. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Lidocaine increases the mRNA levels of cAMP and PKA in the cerebral tissues of CIRI rats
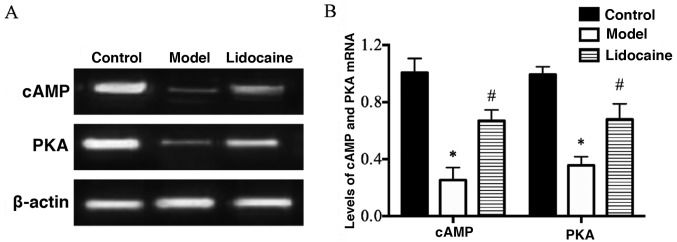
According to the results of RT-PCR (Fig. 3A), the mRNA levels of cAMP and PKA in the cerebral tissues of rats in the model group were decreased markedly compared with those in the control group (P<0.05), and their mRNA levels in lidocaine group were remarkably increased in comparison with those in the model group (P<0.05) (Fig. 3B), implying that lidocaine promotes the mRNA expression of cAMP and PKA in the brain of CIRI rats.
Figure 3.
mRNA levels of cAMP and PKA in the cerebral tissues of rats detected via RT-PCR. (A) RT-PCR bands. (B) Statistical graphs of the bands. *P<0.05, model group vs. control group; and #P<0.05, lidocaine group vs. model group. cAMP, cyclic adenosine monophosphate; PKA, protein kinase A.
Lidocaine elevates protein levels of cAMP and PKA in the cerebral tissues of CIRI rats
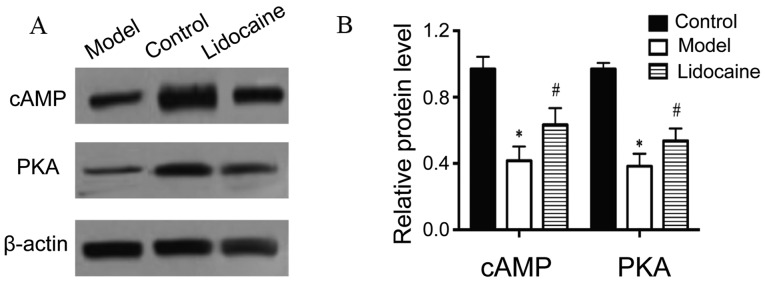
Based on the results of western blotting (Fig. 4A), the protein levels of cAMP and PKA in the cerebral tissues of rats in the model group declined notably in comparison with those in the control group (P<0.05), and the protein levels in lidocaine group increased distinctly compared with those in the model group (P<0.05) (Fig. 4B), illustrating that lidocaine accelerates the protein expression of cAMP and PKA in the brain of CIRI rats.
Figure 4.
Protein levels of cAMP and PKA in the cerebral tissues of rats by western blotting. (A) Western blotting bands. (B) Statistical graphs of the bands. *P<0.05, model group vs. control group; and #P<0.05, lidocaine group vs. model group. cAMP, cyclic adenosine monophosphate; PKA, protein kinase A.
Discussion
CIRI is an ischemic cerebrovascular disease that accounts for ~80-90% of the total cases, and its disability and mortality rates are extremely high, seriously endangering human health and posing heavy burden on the society and family (11). In cerebral ischemia, brain neurons have energy metabolism disorders and acidosis, so that the homeostasis in local cerebral tissues is damaged, and after restoration of brain blood supply, cerebral function cannot return to the original state, with progressive impairment (12,13). CIRI is a complex pathological process, and a study found that neuronal apoptosis in the brain is an important cause of reperfusion injury (14). Therefore, how to protect brain neuronal cells from IRI is a hotspot of research.
Lidocaine (N-diethylaminoacetyl-2,6-dimethylaniline and molecular formula of C14H22N2O), is a common drug clinically used for anesthesia (15). Increasing number of studies have discovered that lidocaine can resist arrhythmia, prevent excessive inflammatory responses, alleviate acute lung injury, inhibit premature ejaculation and protect the brain (16,17). Modern pharmacology of lidocaine, especially its protective effect on cerebral tissues, has attracted increasingly extensive attention from researchers. A previous study reported that lidocaine can block the Na-K channel, and reduce the concentrations of intracellular Na and K, thereby protecting the hypoxic neurons (18). However, whether lidocaine can be used to treat CIRI remains unclear, and its mechanism is rarely reported.
Hence, in the present study, the rat CIRI model was first prepared by the suture-occluded method, and 24 h later, the neurological function score of rats was statistically analyzed in each group. According to the statistics, the rats in the model group had an obviously raised neurological function score and the symptom of circling to the right, which were greatly decreased and alleviated after treatment with lidocaine. Then the expression of pro-apoptotic and anti-apoptotic factors in the serum of rats were detected using ELISA, and as shown in Fig. 1, compared with those in the model group, the expression of pro-apoptotic factor Bax was substantially promoted by lidocaine, and that of anti-apoptotic factor Bcl-2 was inhibited. Subsequently, neuronal apoptosis in the brain of rats was detected via TUNEL staining in each group, and the results showed that lidocaine considerably suppressed the neuronal apoptosis in the brain. The regulatory mechanism of lidocaine was further explored, as it was known through literature that the cAMP/PKA signaling pathway plays a vital role in inhibiting neuronal apoptosis. Once cells are stimulated externally, the signaling molecules bind to the receptors on the surface of cell membranes to form complexes, and then activate adenylate cyclases. Ultimately, cAMP is generated, and it enters the nucleus and directly activates RNA polymerases to promote the mRNA transcription of the target genes (19,20). Therefore, the mRNA and protein levels of cAMP and PKA in the cerebral tissues of rats were determined using RT-PCR and western blotting, in this study, and it was revealed that compared with the model group, lidocaine group exhibited obviously raised mRNA and protein levels of cAMP and PKA in the cerebral tissues of rats (Figs. 3 and 4), suggesting that the mechanism by which lidocaine protects the cerebral tissues of CIRI rats may be related to the activation of the cAMP/PKA signaling pathway.
In conclusion, the results of this study demonstrate that lidocaine substantially improves the neurological function injury and promote the repair of neurological function in CIRI rats, and that it can also inhibit the neuronal apoptosis in the brain of rats by the mechanism of action that is probably associated with the activation of the cAMP/PKA signaling pathway, providing a novel experimental basis for the treatment of CIRI with lidocaine.
Acknowledgements
Not applicable.
Funding
This study was supported by Beijing University of Chinese Medicine Scientific Research Subject (2016-JYB-JSMS-038).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YL, JZh, GL and AW designed the study and performed the experiments, YL and JZa established the animal models, JZh and FZ collected the data, GL and AW analyzed the data, YL, JZh, GL and AW prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Capital Medical University Animal Center (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
References
- 1.Yang Z, Weian C, Susu H, Hanmin W. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol. 2016;771:145–151. doi: 10.1016/j.ejphar.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Wu XJ, Sun XH, Wang SW, Chen JL, Bi YH, Jiang DX. Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPARγ. Eur Rev Med Pharmacol Sci. 2018;22:5688–5696. doi: 10.26355/eurrev_201809_15836. [DOI] [PubMed] [Google Scholar]
- 3.Deng H, Zuo X, Zhang J, Liu X, Liu L, Xu Q, Wu Z, Ji A. A lipoic acid protects against cerebral ischemia/reperfusion-induced injury in rats. Mol Med Rep. 2015;11:3659–3665. doi: 10.3892/mmr.2015.3170. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Qiao L, Xu W, Wang X, Li H, Xu W, Chu K, Lin Y. Paeoniflorin attenuates cerebral ischemia-induced injury by regulating Ca(2+)/CaMKII/CREB signaling pathway. Molecules. 2017;22(359) doi: 10.3390/molecules22030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu W, Gao D, Jin W, Liu S, Qi S. Propofol prevents oxidative stress by decreasing the ischemic accumulation of succinate in focal cerebral ischemia-reperfusion injury. Neurochem Res. 2018;43:420–429. doi: 10.1007/s11064-017-2437-z. [DOI] [PubMed] [Google Scholar]
- 6.Forman MB, Gillespie DG, Cheng D, Jackson EK. A novel adenosine precursor 2',3'-cyclic adenosine monophosphate inhibits formation of post-surgical adhesions. Dig Dis Sci. 2014;59:2118–2125. doi: 10.1007/s10620-014-3139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ould Amer Y, Hebert-Chatelain E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim Biophys Acta Bioenerg. 2018;1859:868–877. doi: 10.1016/j.bbabio.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang L. Neuronal cAMP/PKA signaling and energy homeostasis. Adv Exp Med Biol. 2018;1090:31–48. doi: 10.1007/978-981-13-1286-1_3. [DOI] [PubMed] [Google Scholar]
- 9.Berk T, Silberstein SD. The use and method of action of intravenous lidocaine and its metabolite in headache disorders. Headache. 2018;58:783–789. doi: 10.1111/head.13298. [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1016/j.pmn.2019.06.008. Lancaster RJ, Wren K, Hudson A, Leavitt K, Albala M and Tischaefer D: Intravenous lidocaine for chronic neuropathic pain a systematic review addressing nursing care. Pain Manag Nurs: Jul 30, 2019 (Epub ahead of print). doi: 10.1016/j.pmn.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Zuo G, Zhang D, Mu R, Shen H, Li X, Wang Z, Li H, Chen G. Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Mol Brain. 2018;11(9) doi: 10.1186/s13041-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao SL, Lin YW, Hsieh CL. Neuronal regeneration after electroacupuncture treatment in ischemia-reperfusion-injured cerebral infarction rats. Biomed Res Int. 2017;2017(3178014) doi: 10.1155/2017/3178014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao X, Bao W, Hong X, Jiang H, Yu Z. Identification and functional analysis of differentially expressed genes associated with cerebral ischemia/reperfusion injury through bioinformatics methods. Mol Med Rep. 2018;18:1513–1523. doi: 10.3892/mmr.2018.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaidikar L, Byna B, Thakur SR. Neuroprotective effect of punicalagin against cerebral ischemia reperfusion-induced oxidative brain injury in rats. J Stroke Cerebrovasc Dis. 2014;23:2869–2878. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology. 2017;126:729–737. doi: 10.1097/ALN.0000000000001527. [DOI] [PubMed] [Google Scholar]
- 16.Kirk LM, Brown SD, Luu Y, Ogle A, Huffman J, Lewis PO. Beyond-use dating of lidocaine alone and in two ‘magic mouthwash’ preparations. Am J Health Syst Pharm. 2017;74:e202–e210. doi: 10.2146/ajhp160214. [DOI] [PubMed] [Google Scholar]
- 17.Seah DS, Herschtal A, Tran H, Thakerar A, Fullerton S. Subcutaneous lidocaine infusion for pain in patients with cancer. J Palliat Med. 2017;20:667–671. doi: 10.1089/jpm.2016.0298. [DOI] [PubMed] [Google Scholar]
- 18.Arsyad A, Dobson GP. Lidocaine relaxation in isolated rat aortic rings is enhanced by endothelial removal: Possible role of Kv, KATP channels and A2a receptor crosstalk. BMC Anesthesiol. 2016;16(121) doi: 10.1186/s12871-016-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deb DK, Bao R, Li YC. Critical role of the cAMP-PKA pathway in hyperglycemia-induced epigenetic activation of fibrogenic program in the kidney. FASEB J. 2017;31:2065–2075. doi: 10.1096/fj.201601116R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Pan S, Zhang HT. Interaction of Cdk5 and cAMP/PKA signaling in the mediation of neuropsychiatric and neurodegenerative diseases. Adv Neurobiol. 2017;17:45–61. doi: 10.1007/978-3-319-58811-7_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.