Abstract
Background
Youth who experience puberty earlier than their peers are at heightened risk for substance use during adolescence. However, little is known about whether pubertal timing exacerbates effects of relevant early risk factors, such as family substance use history, as predicted by the “accentuation hypothesis”. Using longitudinal data from youth with and without a family history of alcohol use disorder (AUD FHx), we evaluated whether pubertal timing intensifies preexisting familial risk effects on late adolescent substance use.
Methods
Participants were 568 males and 245 females from the Michigan Longitudinal Study. Pubertal timing was indexed by fitting mixed-effects linear models to repeated measures of self-reported Tanner stage. Multilevel models then tested: (a) whether AUD FHx predicted pubertal timing, and (b) whether AUD FHx, pubertal timing, or their interaction predicted alcohol and marijuana use at ages 16–18.
Results
AUD FHx was unrelated to pubertal timing in either males or females. In males, alcohol and marijuana use in late adolescence were predicted by AUD FHx and timing, but not their interaction. In females, AUD FHx predicted alcohol-related outcomes, but there were no main or interaction effects of timing.
Conclusions
Pubertal timing does not moderate the link between AUD FHx and late adolescent substance use, in contrast to the accentuation hypothesis. In males, measures of pubertal maturation and familial risk provide unique information for prediction of use. Females displayed no link between pubertal timing and use, which may suggest different risk pathways, or may have been due to the female sample’s smaller size.
Keywords: alcohol use disorder, puberty, familial risk factors, adolescence, substance abuse, growth models
. Introduction
Puberty is a major event in youths’ psychosocial development. The timing of puberty relative to one’s peers, in particular, can impact risk for detrimental behavioral outcomes, and one of the most well-replicated associations concerns earlier maturation and increased risk for substance use problems in adolescence (Beltz et al., 2014; Senia et al., 2018; Ullsperger and Nikolas, 2017).
However, it is unclear whether pubertal timing interacts with early life risk factors for substance use problems, such as family history of addiction. It is well-established that children of individuals with substance use disorders are at elevated risk of developing such disorders themselves (Kendler et al., 1997; Sher et al., 1991), and pubertal timing may interact with this potent risk factor. The “accentuation” hypothesis (Caspi and Moffitt, 1991), which posits that puberty intensifies preexisting individual differences, suggests that early maturation may disproportionately increase risk for youth who already have developmental vulnerabilities. Although this hypothesis is difficult to test directly because longitudinal data are essential to assess the temporal ordering of the relationships it posits (Ge and Natsuaki, 2009), interaction effects between early timing and contextual risk factors have been documented (Negriff and Susman, 2011). Furthermore, recent longitudinal analyses suggest that, at least for boys, early pubertal maturation accentuates the link between early childhood behavior problems and adolescent substance use (Beltz et al., 2019). However, this hypothesis has not been empirically evaluated with respect to familial risk for substance use problems, despite its relevance for interpreting effects of pubertal timing on adolescent use.
We explicitly assessed this hypothesis using pubertal development and substance use data from a large longitudinal study of youth with and without a family history of alcohol use disorder (AUD FHx). By indexing timing with growth models (Beltz et al., 2014), which leverage multiple data points for measurement, adjust for missing data, and provide parallel timing measures across sexes (Berebaum et al., 2015), we directly tested whether timing moderates the effects of AUD FHx on substance use. Although we primarily focused on testing the accentuation hypothesis, we also evaluated the related hypothesis that childhood stress, such as that related to AUD FHx, leads to earlier puberty (Negriff et al., 2015; Belsky et al., 1991).
2. Methods
Data were from the Michigan Longitudinal Study (MLS: Zucker et al., 1996, 2000), an ongoing prospective study of youth with (FH+) and without (FH−) AUD FHx. Youth from both backgrounds were recruited from the same neighborhoods at ages 3–5. Assessments were conducted in three-year waves, with pubertal status assessed at three waves (roughly ages 10–16). Brief annual assessments, which included self-reported substance use, were also conducted through participants’ 20s. All participants, and parents of minor participants, provided written informed consent. Initial data collection targeted male youth, although female siblings were followed thereafter. Recruitment for later cohorts targeted both sexes, and a recent extension is following children of the original cohort. Hence, the sample contains sets of individuals from the same families and has more males than females. Substance use data have been previously reported (Wong et al., 2004; Buu et al., 2012; Martz et al., 2016), but reporting of puberty data is novel.
2.1. Sample
For inclusion in this report, individuals needed complete data on pubertal status and age from at least one wave, data on AUD FHx, and at least one (of three) substance use outcomes (detailed below) during ages 16–18. Descriptive data from males (N=568) and females (N=245) who met these criteria are displayed in Table 1.
Table 1.
Demographic variables and descriptive statistics for male and female samples.
| Males | Females | |
|---|---|---|
| Overall N | 568 | 245 |
| FH+/FH− (%FH+) | 439/129 (77%) | 194/51 (79%) |
| Number of unique families | 386 | 207 |
| Race/Ethnicity | 89% Caucasian; 5% African American 3% Bi-racial 3% Hispanic/Caucasian |
84% Caucasian; 7% African American 4% Bi-racial 5% Hispanic/Caucasian |
| % 3 Tanner Ratings | 48% | 54% |
| % 2 Tanner Ratings | 34% | 34% |
| % 1 Tanner Rating | 18% | 11% |
| Age at mid-puberty (SD) | 12.63 (.50) | 12.22 (.34) |
| Average Drink Volume 16–18 (SD) | 168.54 (404.10) | 104.63 (211.47) |
| Average Binge Freq. 16–18 (SD) | 18.94 (43.84) | 10.56 (28.55) |
| Average Marijuana Freq. 16–18 (SD) | 35.23 (84.07) | 19.95 (62.86) |
| N Drink Volume | 557 | 235 |
| N Binge Freq. | 563 | 244 |
| N Marijuana Freq. | 542 | 230 |
Notes: Overall N = total number of subjects; FH+ = positive family history for alcohol use disorder; FH− = no family history for alcohol use disorder; % X Tanner Ratings = percentage of sample with X number of self-report pubertal status ratings; SD = standard deviation; drink volume = average yearly number of alcoholic drinks; binge freq. = average yearly number of binge-drinking days; marijuana freq. = average yearly number of marijuana use days; N drink volume = number of subjects with available data for the drink volume; N binge freq. = number with available data for binge freq.; N marijuana freq. = number with available data for marijuana freq.
We maximized information available for indexing pubertal timing in mixed-effects growth models by utilizing data from all MLS participants who had at least one wave of valid pubertal status data (675 males, 314 females). We did not exclude family members from these models or model members separately because previous work (Beltz et al., 2014) suggests separate models for siblings produce roughly identical parameters. We accounted for nesting of siblings in the main inferential analyses (no parent-child pairs were present in this sub-sample) using random-intercept multilevel models.
2.2. Measures
2.1.1. Pubertal status and timing.
The Naomi Morris Scale of Physical Development (Morris and Udry, 1980) is a self-report measure in which youth select which of five line drawings, corresponding to Tanner stages (Marshall and Tanner, 1969; 1970), most closely resembles their own development in two aspects of puberty: pubic hair, reflecting adrenarche; and genital (boys) and breast development (girls), reflecting gonadarche. Data were collected at three waves, when youth were ages 10.56(SD=.94), 13.56(SD=.95), and 16.56(SD=.97). Following others (Beltz et al., 2014; Castellanos-Ryan et al., 2013), we averaged participants’ responses to the two items at each wave to obtain a general measure of pubertal status.
SAS 9.4 PROC NLMIXED was used to fit linear mixed-effects growth curve models treating intercept as a random effect (SAS Institute Inc., Cary, NC). With a maximum of three observations per participant (43%, 34%, and 23% of MLS participants had 3, 2, or 1 observations, respectively), more complex models (e.g., logistic or linear with random slopes: Beltz et al., 2014; Marceau et al., 2011) were not feasible. The model is represented as
where g1 is the slope, g0i is the intercept for individual i, and rit is the residual for individual i at wave t, which has a normal distribution with 0 mean and constant variance. Following Beltz et al. (2014), we calculated age at mid-puberty (Tanner stage 3) as (3 − g0i)/ g1 for each individual as a measure of timing.
Although estimates of pubertal tempo can be obtained from linear growth models, these estimates have questionable validity due in part to their strong correlations with linear timing estimates (Beltz et al., 2014). Therefore, tempo was not considered.
2.2.2. Substance use.
The Drinking and Drug History Form (Zucker et al., 1990) is a self-report measure of past-year substance use that youth completed annually. We focused on three outcomes: drink volume (total past-year alcoholic drinks), binge frequency (binge-drinking days), and marijuana frequency (marijuana use days). We focused on measurements from ages 16–18 so that pubertal timing was not confounded with use. Participants’ responses were averaged across all available ages to obtain estimates of average yearly drink volume, binge frequency, and marijuana frequency during late adolescence.
2.3. Prediction of Substance Use Outcomes
We used multilevel models in SPSS with siblings nested within families and a variance components error structure. Following others (Beltz et al., 2014; Marceau et al., 2011), models were run separately for males and females because puberty is a qualitatively different process in each sex (Beltz, Beery and Becker, 2019). Models were also run separately for each substance use outcome, which were square-root transformed due to positive skew (skew prior to transform: 2.82– 4.92; post-transform: 1.45–2.74). The first model (Model 1) assessed whether AUD FHx (0=FH+,1=FH−) predicted pubertal timing in order to determine whether these risk factors were independent. The second (Model 2A) assessed whether AUD FHx and timing predicted each substance use outcome, and the third (Model 2B) assessed whether both predictors as well as their interaction (computed by multiplying the AUD FHx variable by the grand-mean centered pubertal timing variable) predicted use. AIC (Akaike, 1973) was used to determine whether Model 2B displayed better relative fit than the more parsimonious Model 2A. Following selection of the model with the lowest AIC, p-values were used to assess significance.
3. Results
Detailed results of multilevel models, as well as Pearson correlations between all variables (which provide rough approximations of effect sizes for the relationships discussed below), are contained in Supplementary Materials1. In Model 1, there was no significant relationship between AUD FHx and pubertal timing for either males (b=0.040,t=0.782,p=.435) or females (b=0.029,t=0.554,p=.580), suggesting the predictors were independent.
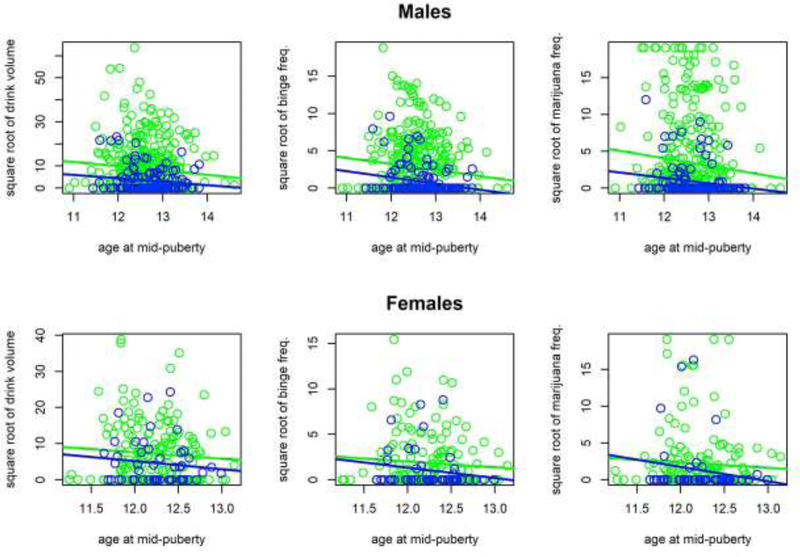
In models predicting substance use (2A/2B), the pattern of results differed between males and females. For males, AIC preferred Model 2A over Model 2B for drink volume (2A=4162.46; 2B=4164.43), binge frequency (2A=3013.57; 2B=3015.55), and marijuana frequency (2A=3282.18; 2B=3284.15). In the selected models, substance use outcomes were predicted by AUD FHx (drink volume: b=−5.215,t=−4.777,p<.001; binge frequency: b=−1.870,t=−4.849,p<.001; marijuana frequency: b=−2.631,t=−4.964,p<.001) and earlier pubertal timing (drink volume: b=−1.987,t=−2.319,p=.021; binge frequency: b=−0.748,t=−2.537,p=.011; marijuana frequency: b=− 0.965,t=−2.270,p=.024). Consistent with model selection, the interaction term from Model 2B was not significant for any outcome (all |t|≤0.169, p≥.866). Therefore, results suggest that AUD FHx and earlier pubertal timing are predictors of late adolescent substance use in males, but their interaction is not. This can be seen in the scatterplots in Figure 1; later timing is linked to lower substance use similarly (i.e., in parallel) for both FH+ and FH− males, with FH+ males having consistently higher use overall.
Figure 1.
Scatterplots and simple linear regression lines (displayed for ease of interpretation) of relationships between pubertal timing (age at mid-puberty) and late adolescent substance use outcomes for individuals with (green) and without (blue) a family history of alcohol use disorder. Results for males are on top, and results for females are on bottom. Outcomes are square-root transformed and are, from left to right, average drink volume per year, average frequency of binge drinking (days) per year, and average frequency of marijuana use (days) per year.
For females, AIC preferred Model 2A over Model 2B for drink volume (2A=1627.75; 2B=1629.70), binge frequency (2A=1189.61; 2B=1191.28), and marijuana frequency (2A=1292.58; 2B=1294.14). In the selected models, AUD FHx predicted drink volume (b=−2.933,t=−2.317,p=.022) and binge frequency (b=−0.973,t=−2.114,p=.036), but not marijuana frequency (b=−1.069,t=−1.670,p=.097), and pubertal timing did not predict any substance use outcome (drink volume: b=−1.924,t=−1.333,p=.184; binge frequency: b=−0.470,t=−0.947,p=.345; marijuana frequency: b=−1.051,t=−1.360,p=.175). Consistent with model selection, interaction terms from Model 2B were not significant (all |t|≤0.670, p≥.503). The scatterplots in Figure 1 suggest trends similar to the pubertal timing and AUD FHx effects found in males, but a lower overall base rate of substance use. Furthermore, the r values reported in Supplementary Materials1 indicate these effects are of comparable size between females and males, suggesting that the lack of statistical significance in females is due to sample size.
4. Discussion
We used growth modeling of pubertal development in a longitudinal study of youth with and without AUD FHx to evaluate whether pubertal timing accentuates the effects of familial risk factors on late adolescent substance use. There was little indication that pubertal timing moderates the effects of AUD FHx on substance use, although inferences depended upon sex.
In males, both AUD FHx and earlier pubertal timing, but not their interaction, predicted greater alcohol and marijuana use in late adolescence. Therefore, early pubertal timing heightens risk in males similarly regardless of AUD FHx, indicating that biosocial family risk factors, although they increase risk in general, do not provide a precondition for early timing to increase males’ level of risk. In females, AUD FHx affected alcohol-related outcomes, but there were no statistically-significant main or interaction effects of pubertal timing on substance use, possibly suggesting different risk pathways in the sexes. However, as females displayed qualitative trends similar to the small effects found in males (Figure 1; Supplemental Tables 1–2), it is possible that features of our female sample, which was less than half the size of the male sample and had lower overall substance use rates (Table 1), may have prevented detection of small effects. Thus, it is premature to make claims about sex differences.
Despite previous findings that AUD FHx is linked to childhood stressors (Sher et al., 1997), our analyses suggest that AUD FHx is not related to early pubertal timing. This finding is inconsistent with the hypothesis that childhood stress may lead to earlier puberty (Negriff et al., 2015; Belsky et al., 1991), at least with regard to stress linked to AUD FHx.
Findings should be considered in the context of the study’s strengths and limitations. Strengths include the high-risk and community-recruited sample, use of longitudinal data and state-of-the-art modeling analyses to index pubertal timing, and use of substance use outcomes from late adolescence, which allowed effects of timing to be assessed independent of pubertal status effects (see: Berenbaum et al., 2015). Limitations include the relatively low number of pubertal status observations available (1–3 vs. 6–7 in: Beltz et al., 2014; Marceau et al., 2011), small size of the female sample, and lack of family nesting in the puberty measurement models.
Taken together, the current findings suggest that, at least in males, AUD FHx and early pubertal timing are independent predictors of substance use in late adolescence and do not interact to enhance risk, providing evidence against an accentuation hypothesis (Caspi and Moffitt, 1991).
Supplementary Material
Highlights.
Pubertal timing was unrelated to family history of alcohol use disorder
In males, adolescent substance use was predicted by early timing and familial risk
Pubertal timing did not moderate familial risk effects on males’ substance use
Acknowledgements
Collection of the MLS data used in this project was supported by NIAAA grant R01/R37 AA07065 and R01 AA025790. Alexander Weigard was supported by NIAAA T32 AA007477. Jillian Hardee was supported by NIAAA K01 AA024804.
Role of Funding Source
Collection of the Michigan Longitudinal Study data used in this project was supported by NIAAA grants R01 AA07065 and R01 AA025790. AW was supported by NIAAA T32 AA007477. JH was supported by NIAAA K01 AA024804.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: 10.1016/j.drugalcdep.2020.107955.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H, 1973. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika, 60, 255–265. [Google Scholar]
- Beltz AM, Corley RP, Bricker JB, Wadsworth SJ, and Berenbaum SA, 2014. Modeling pubertal timing and tempo and examining links to behavior problems. Dev. Psychol, 50, 2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Corley RP, Wadsworth SJ, DiLalla LF, and Berenbaum SA, 2019. Does puberty affect the development of behavior problems as a mediator, moderator, or unique predictor? Dev. Psychopathol, 1–13. [DOI] [PubMed] [Google Scholar]
- Beltz AM, Beery AK, and Becker JB, 2019. Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology, 44, 2155–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, and Draper P, 1991. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Dev., 62, 647–670. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM, and Corley R, 2015. The importance of puberty for adolescent development: conceptualization and measurement. In Advances in Child Development and Behavior (Vol. 48, pp. 53–92). JAI. [DOI] [PubMed] [Google Scholar]
- Buu A, Wang W, Schroder SA, Kalaida NL, Puttler LI, and Zucker RA, 2012. Developmental emergence of alcohol use disorder symptoms and their potential as early indicators for progression to alcohol dependence in a high risk sample: A longitudinal study from childhood to early adulthood. J. Abnorm. Psychol, 121, 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, and Séguin JR, 2013. Pubertal development, personality, and substance use: A 10-year longitudinal study from childhood to adolescence. J. Abnorm. Psychol, 122, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, and Moffitt TE, 1991. Individual differences are accentuated during periods of social change: the sample case of girls at puberty. J. Pers. Soc. Psychol, 61, 157. [DOI] [PubMed] [Google Scholar]
- Ge X, and Natsuaki MN, 2009. In search of explanations for early pubertal timing effects on developmental psychopathology. Curr. Dir. Psychol. Sci, 18, 327–331. [Google Scholar]
- Graber JA, 2013. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm. Behav, 64, 262–269. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, and Kessler RC, 1997. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. Br. J. Psychiatry, 170, 541–548. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, and Susman EJ, 2011. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Dev. Psychol, 47, 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, and Tanner JM, 1969. Variations in pattern of pubertal changes in girls. Arch. Dis. Child, 44, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, and Tanner JM, 1970. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child, 45, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, and Heitzeg MM, 2016. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry, 73, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah FK, Bayer JK, Wake M, Carlin JB, Allen NB, and Patton GC, 2013. Early puberty and childhood social and behavioral adjustment. J. Adolesc. Health, 53, 118–124. [DOI] [PubMed] [Google Scholar]
- Morris NM, and Udry JR, 1980. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc, 9, 271–280. [DOI] [PubMed] [Google Scholar]
- Negriff S, Saxbe DE, and Trickett PK, 2015. Childhood maltreatment, pubertal development, HPA axis functioning, and psychosocial outcomes: An integrative biopsychosocial model. Dev. Psychobiol, 57, 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negriff S, & Susman EJ, 2011. Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. J Res Adolesc, 21, 717–746. [Google Scholar]
- Senia JM, Donnellan MB, and Neppl TK, 2018. Early pubertal timing and adult adjustment outcomes: Persistence, attenuation, or accentuation?. J.Adolesc, 65, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Gershuny BS, Peterson L, and Raskin G, 1997. The role of childhood stressors in the intergenerational transmission of alcohol use disorders. J. Stud. Alcohol, 58, 414–427. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, and Brent EE, 1991. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J. Abnorm. Psychol, 100, 427. [DOI] [PubMed] [Google Scholar]
- Ullsperger JM, and Nikolas MA, 2017. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk?. Psychol. Bull, 143, 903. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, and Zucker RA, 2004. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol. Clin. Exp. Res, 28, 578–587 [DOI] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, and Sanford K, 1996. Other evidence for at least two alcoholisms II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Dev. Psychopathol. 8, 831–848. [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, and Ellis DA, 2000. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy In: Fitzgerald HE, Lester BM, and Zucker RA (Eds), Children of Addiction: Research, Health and Policy Issues. (pp. 109–141). New York: Routledge Falmer Publishers. [Google Scholar]
- Zucker RA, Fitzgerald HE, and Noll RB, 1990. Drinking and drug history Unpublished Manuscript, Michigan State University. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.