Abstract
Context
Prenatal exposures and lifestyle factors are important for metabolic health.
Objective
Determine how prenatal exposures to maternal obesity and/or gestational diabetes mellitus (GDM) and childhood lifestyle factors independently contribute to child insulin sensitivity.
Design and Participants
Ninety children aged 7 to 11 years (56% girls, 60% exposed to GDM), born at Kaiser-Permanente Southern California, completed an oral glucose tolerance test (OGTT) as part of the BrainChild Study. Matsuda insulin sensitivity index (ISI) was used to estimate insulin sensitivity. Participants completed two 24-hour dietary recalls, and daily energy intake (EI), dietary added sugar, and total sugar were calculated. The 3-day physical activity recall determined the average minutes per day of moderate to vigorous physical activity (MVPA) and the average minutes per day spent sedentary. Maternal prepregnancy body mass index (BMI) and GDM status were extracted from electronic medical records.
Main Outcome Measure
Matsuda-ISI.
Results
Linear regression showed that children who spent more time in MVPA had better ISI (β = 0.33; P = 0.001), and results remained after adjustment for maternal prepregnancy BMI, GDM exposure, child age, sex, daily EI, dietary added sugar (β = 0.34; P = 0.001), and further adjustment for child adiposity (β = 0.29; P = 0.001). Time spent sedentary, maternal prepregnancy BMI, GDM exposure, dietary added sugar, total sugar, and EI were not associated with ISI.
Conclusions
Physical activity was the only predictor of ISI at this age, suggesting that engaging in physical activity during childhood is beneficial for insulin sensitivity and may ameliorate future risk for metabolic disease.
Keywords: child insulin sensitivity; gestational diabetes mellitus; maternal obesity; physical activity; sedentary behavior, diet
Over the past few decades, the number of youth impacted by type 2 diabetes has increased substantially (1). Growing evidence suggests that prenatal exposure to gestational diabetes mellitus (GDM) and/or maternal obesity are contributing factors to the rising rates of type 2 diabetes in children and adolescents (2–4). One of the major determinants of type 2 diabetes is reduced insulin sensitivity, which requires a compensatory increase in insulin secretion in order to maintain normal glucose levels (5).
Prior studies have shown that offspring exposed to GDM in utero exhibit reduced insulin sensitivity as early as birth (6, 7). Additional studies have observed that reduced insulin sensitivity in GDM-exposed offspring is present during late childhood (8–10) and adulthood (2). Children born to mothers with obesity during pregnancy are also at higher risk for insulin resistance and type 2 diabetes (3, 4, 11). While some studies have observed an association between in utero exposure to maternal obesity and reduced insulin sensitivity in offspring at birth (11, 12) and during adulthood (3), only a few have examined associations between in utero exposure to maternal obesity and insulin sensitivity during childhood, and they have found conflicting results (8, 9, 13).
In addition to prenatal exposure factors, lifestyle factors also play an important role in insulin sensitivity during childhood (14–17). Higher energy intake (EI) or excessive sugar intake, particularly in the form of sugar-sweetened beverages, are both associated with reduced insulin sensitivity during adolescence and increased type 2 diabetes risk, especially among overweight/obese youth (14, 15, 18–20). Sedentary lifestyles during childhood may also be related to reduced insulin sensitivity (16), and longitudinal studies have shown that children who maintain high levels of physical activity have improved insulin sensitivity and a reduced risk of developing type 2 diabetes (17, 21).
There is a significant gap in the literature in understanding the independent predictors of prenatal and postnatal factors on insulin sensitivity during childhood. This is the first study to investigate how both prenatal factors and early childhood lifestyle factors influence insulin sensitivity in a well-characterized cohort of healthy, typically developing elementary-aged children. Therefore, in addition to investigating the impact of in utero exposure to GDM or maternal obesity on child insulin sensitivity, an additional goal was to investigate the role that modifiable lifestyle factors, including physical activity levels and EI, particularly dietary total and added sugar, contribute to insulin sensitivity at an early age when potential interventions could be targeted.
Methods
Participants
From 2014 to 2018, children between the ages of 7 to 11 years old were recruited from Kaiser Permanente Southern California (KPSC) to participate in the BrainChild Study, a study on the impact of intrauterine exposure to metabolic disorders on brain appetite pathways. Kaiser Permanente Southern California is a large healthcare organization that uses an integrated electronic medical record (EMR) system. The BrainChild cohort is a unique cohort of children born at KPSC with documented data on measures of maternal prepregnancy height and weight and oral glucose testing results during pregnancy. Children were excluded if they were born to mothers diagnosed with diabetes pre-existing pregnancy or if the children had a history of neurological, psychiatric, metabolic, or other significant medical disorders and/or use of medications known to alter metabolism (ie, glucocorticoids). Each participating Institutional Review Board approved this study (University of Southern California #HS-14-00034 and KPSC #10282). Participants’ parents gave written informed consent and children provided written informed assent.
Prenatal exposures: maternal gestational diabetes and prepregnancy obesity
Using electronic medical records, each mother’s GDM status was extracted. Diagnosis of GDM was based on laboratory glucose values confirming a plasma glucose level ≥ 11.10 mmol/L from a 50-g glucose challenge tests or at least 2 plasma glucose values meeting or exceeding the following values on the 100-g or 75-g oral glucose tolerance test (OGTT): fasting, 5.27 mmol/L; 1 hour, 9.99 mmol/L; 2 hours, 8.60 mmol/L; and 3 hours, 7.77 mmol/L (22). Maternal prepregnancy body mass index (BMI) (kg/m2) was calculated from maternal height (cm) and weight (kg) measurements closest to the last menstrual period from the EMR. Only measurements from 180 days before the last menstrual period to 90 days after the last menstrual period were used.
In-person visits
The study included 2 in-person visits. The first visit was to the Clinical Research Unit of the USC Diabetes and Obesity Research Institute following a 12-hour overnight fast. Trained staff members measured each child’s height to the nearest 0.1 cm using a stadiometer and each child’s weight to the nearest 0.1 kg using a calibrated digital scale. Body mass index was calculated using the standard formula: weight (kg) divided by height (m2). Body mass index z-scores and BMI percentiles (age- and sex-specific standard deviation scores) were determined based on Center for Disease Control (CDC) standards (23). Participants were given the option of having Tanner Staging assessed by a physical exam (24, 25) and/or by a validated sex-specific assessment questionnaire for children and parents, containing both illustrations and explanatory text (26). Fifty-two participants (58%) opted for both the physical exam and questionnaire. Thirty-eight participants (42%) opted for self-reported puberty status only. The correlation between Tanner Staging assessed by physical exam and by questionnaire was 0.91. A 24-hour dietary recall and a self-reported 3-day physical activity recall (3DPAR) were obtained (27, 28). Additionally, children also completed an OGTT during this visit. Plasma glucose and insulin were assayed from blood samples collected at 0, 30, 60, 90, and 120 minutes before and after the participant consumed a glucose load (1.75 g/kg; maximum dose of 75 grams) (Glucola; Azer Scientific, Morgantown, Pennsylvania). Plasma glucose was measured using the enzymatic electrode YSI analyzer machine, YSI 2300 STAT PLUS (YSI Inc., Yellow Springs, Ohio). Plasma insulin was measured using a human insulin ELISA kit (Millipore, Billerica, Massachusetts). Insulin sensitivity was estimated using the Matsuda insulin sensitivity index (29) (Supplemental Information (SI) Fig. 1, which can be found in a digital repository) (30). Children returned for a second in-person visit during which MRI scans of the brain were performed and an additional 24-hour dietary recall was obtained. MRIs were collected as part of the larger BrainChild Study but are not included in this manuscript.
Physical activity assessment
Physical activity was assessed using the 3DPAR (27, 31). A trained staff member asked participants, with the input of their parents, to recall their activities from 7:00 am to 12:00 am in 30-minute increments for the previous 3 days. Activities were classified based on a reference sheet with 73 activities and classified based on the activities that best matched the participant’s response. The participant then was asked to rate the intensity of each activity; the intensity levels ranged from light, moderate, hard, to very hard. The activities were categorized as either moderate to vigorous physical activity (MVPA) or sedentary behavior (SB) based on the activity’s metabolic equivalent (MET) determined by the compendium of energy expenditure (27, 31). Activities with METs ≥ 3 were classified as MVPA. Nonsleep activities with METs ≤ 1.5 and > 1.0 were classified as SB, which also included sedentary activities during school hours. Time spent sleeping or napping was categorized as METs = 1.0. Examples of moderate to vigorous physical activities included bike riding or swimming. Examples of SB included watching television or playing video games. The final output was the number of 30-minute increments spent in either MVPA or in SB for each of the 3 days converted to average minutes per day spent in MVPA or SB. The 3DPAR has previously been used in pediatric populations and validated against accelerometers (3–34).
Diet intake assessment
Diet was assessed using two 24-hour dietary recalls, which were performed during in-person visits as part of the BrainChild Study, collected on nonconsecutive days. Using the multiple-pass method for dietary recall, a trained staff member asked the participant, with the input of the participant’s parent, to recall what food and beverages they had consumed over a 24-hour period prior to the visit. Participants were asked to start with the first item they ate or drank and to list all food and/or beverages consumed 24 hours after their first meal or drink. The trained staff member then went through 3 other “passes” to complete the quantity of food and/or beverages consumed as well as to include missing or forgotten food or beverages. Use of the multiple-pass 24-hour dietary recall method—and interviewing both the parent and child together—has been shown to improve the accuracy of dietary data collection in children (28, 35, 36). Once the dietary recalls were collected, the recalls were analyzed using the Nutritional Data System for Research software v.2018 (Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, Mississippi) (37). The variables used were average daily EI in kilojoules, percent of EI from added sugar by available carbohydrate, and percent of EI from total sugar. Dietary recalls were then assessed for outliers using a similar approach to Davis et al (19). Participant weight was regressed onto EI, and residuals were extracted and standardized. Residual z-scores > 3 or > -3 were flagged as outliers and removed from subsequent analyses. Using this method, 1 participant was identified as an outlier and was not included in final analyses, and 2 other participants had 1 of their recalls removed.
Statistical analysis
Participant descriptive statistics (means, frequencies) were performed. Linear regression was used to analyze the relationships between the predictor variables (MVPA and SB [min/day], maternal prepregnancy BMI (kg/m2), maternal GDM status [yes/no], daily EI [kJ], and dietary total and added sugar) and the outcome variable, ISI. Additionally, linear regression was used to analyze the relationships between prenatal factors (ie, maternal prepregnancy BMI and maternal GDM status) as the predictor variables with postnatal factors, MVPA, SB, daily EI, and dietary total and added sugar as the outcome variables. For continuous predictors, standardized regression coefficients were reported such that the regression coefficients represent changes in ISI per standard deviation of change in each predictor variable. The standardized regression coefficients are unit-free and directly comparable across different predictors with different measurement units. Time in MVPA and ISI were not normally distributed, and a square-root transformation normalized the distribution and was applied for the regression analysis. A priori covariates included in each linear regression analysis were child age in years, sex, and BMI z-score. Each linear regression model also simultaneously included the other exposure variables of interest (ie, time spent in MVPA, time spent in SB, maternal prepregnancy BMI, maternal GDM, daily EI, and dietary total and added sugar), with the exception of models containing time spent in MVPA and time spent in SB and models containing dietary added sugar and total sugar. Time spent in MVPA and time spent in SB were highly inversely correlated with a spearman rank of 0.62 (P < 0.0001); therefore, these variables were not modeled together. The same was true for dietary added sugar and total sugar (r = 0.67, P <0 .0001). P-values < 0.05 were interpreted as statistically significant. SAS 9.4 statistical software (SAS Institute, Cary, North Carolina) was used for all statistical analyses.
Results
Participants’ characteristics
Of the 114 children enrolled in the study, 90 children completed the OGTT and had adequate dietary data (SI Fig. 2) (30). The demographics of participants who did not complete the OGTT did not differ significantly from participants who did, except a greater frequency of girls were unable to complete the OGTT compared to boys (SI Table 1) (30). The demographics of participants who completed the OGTT are shown in Table 1. Mean ± SD age was 8.32 ± 0.85 years old, 93% of the children were prepubertal (Tanner Stage < 2), and 56% were girls. Maternal prepregnancy BMI ranged from 18.97 to 50.38 kg/m2, 22 (25%) were normal weight, 29 (32%) were overweight, and 39 (43%) were obese. Fifty-two (59%) mothers had GDM. Child BMI z-scores, ISI, dietary intake, and PA levels are also shown in Table 1.
Table 1.
Participant demographics (N = 90)
| Variable | N (%) or Mean (SD) | Range |
|---|---|---|
| Age (years) | 8.32 (0.85) | 7.19–11.23 |
| Sex | Girls: 50 (56%) Boys: 40 (44%) | |
| BMI z-score | 0.64 (1.07) | -1.78–2.64 |
| BMI category | Healthy-weight: 59 (66%) Overweight: 13 (14%) Obese: 18 (20%) | |
| Tanner stage | Stage 1: 84 (93%) Stage 2: 5 (6%) Stage 3: 1 (1%) | |
| Matsuda insulin sensitivity index | 10.87 (6.17) | 0.94–26.57 |
| Daily energy intake (kJ) | 7374.34 (1652.64) | 3452.18–11314.96 |
| Dietary added sugar (%) | 15.03 (6.53) | 2.65–39.92 |
| Total sugars (%) | 23.56 (6.26) | 8.56–42.09 |
| Time in MVPA (min/day) | 134.90 (101.22) | 0–430.00 |
| Time in SB (min/day) | 607.30 (129.30) | 270.00–850.00 |
| Maternal prepregnancy BMI (kg/m 2) | 30.17 (7.15) | 18.97–50.38 |
| GDM exposure | GDM-exposed: 54 (60%) Unexposed: 36 (40%) |
Associations between prenatal and postnatal factors
Maternal prepregnancy BMI was not associated with daily EI in either unadjusted, β = 0.09; P = 0.39 or fully adjusted models, β = 0.06; P = 0.56 (SI Table 2) (30). There was also no association between maternal prepregnancy BMI and dietary added sugar (unadjusted, β = 0.05; P = 0.66; fully adjusted, β = 0.07; P = 0.51) or dietary total sugar (unadjusted, β = 0.11; P = 0.28; fully adjusted, β = 0.19; P = 0.093); although, there was a trend in the expected direction with dietary total sugar. Maternal prepregnancy BMI was not associated with time spent in MVPA (unadjusted, β = -0.03; P = 0.77; fully adjusted, β = 0.003; P = 0.98) or time spent in SB (unadjusted, β = -0.03; P = 0.76; fully adjusted, β = -0.05; P = 0.65).
There was no association between GDM exposure and daily EI (unadjusted, β = 0.35; P = 0.11; fully adjusted, β = 0.34; P = 0.11), (SI Table 3) (30). Gestational diabetes mellitus exposure was also not associated with dietary added sugar (unadjusted, β = 0.24; P = 0.27; fully adjusted, β = 0.21; P = 0.37), total sugar (unadjusted, β = 0.12; P = 0.60; fully adjusted, β = 0.12; P = 0.60), with time spent in MVPA (unadjusted β = 0.14; P = 0.53; fully adjusted, β = -0.04; P = 0.85) or time spent in SB (unadjusted, β = -0.02; P = 0.91; fully adjusted, β = 0.08; P = 0.72).
Association between Matsuda ISI and predictor variables
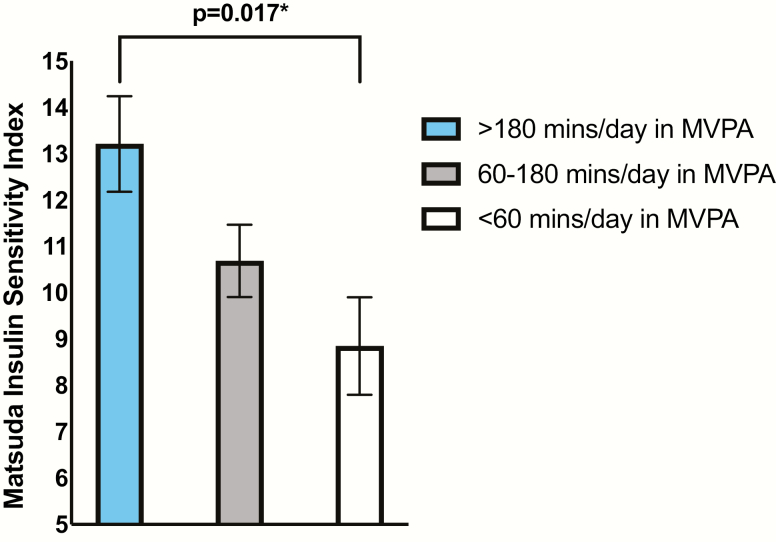
There was a positive association between time spent in MVPA and ISI (β = 0.34; P = 0.001) (Table 2). Adjusting for child age, sex, maternal prepregnancy BMI, maternal GDM status, daily EI, and dietary total sugar did not change this association (β = 0.33; P = 0.001). The association remained after further adjusting for child BMI z-score (β = 0. 29; P = 0.001). Additionally, to visually demonstrate this significant association, least-square means (LSmeans) of ISI by terciles of time spent in MVPA were compared using analysis of covariance, adjusted for child age, sex, maternal prepregnancy BMI, maternal GDM status, daily EI, dietary total sugar, and child BMI z-score (Fig. 1). Children in the upper tercile of reported time spent in MVPA had significantly better insulin sensitivity than children in the lower tercile (LSmean upper tercile: 13.21 SE = 1.03; LSmean lower tercile: 8.85, SE = 1.05; P = 0.017). The LSmean ISI of children in the middle tercile did not differ significantly from children in the lower tercile (LSmean middle tercile: 10.69, SE = 0.78; P = 0.39). Although time spent in SB was not significantly associated with ISI, the trend was in the expected direction (unadjusted, β = -0.18; P = 0.087; fully adjusted, β = -0.14; P = 0.11). Daily EI was not associated with ISI (unadjusted, β = 0.17; P = 0.12; fully adjusted, β = 0.12; P = 0.21), and neither was dietary added sugar (unadjusted, β = -0.05; P = 0.67; fully adjusted, β = -0.01; P = 0.88) or total sugar intake (unadjusted, β = -0.003; P = 0.97; fully adjusted, β = 0.02; P = 0.82).
Table 2.
Summary of unadjusted and adjusted linear regression models, with Matsuda ISI as the outcome variable
| Predictor Variables | Beta (95% CI) | P-value | Covariates |
|---|---|---|---|
| Time in MVPA | 0.34a (0.14, 0.54) | 0.001* | Unadjusted |
| 0.33a (0.14, 0.53) | 0.001* | Child age, sex, prepregnancy BMI, EI, GDM exposure, total sugar | |
| 0.29a (0.12, 0.45) | 0.001* | +BMI z-score | |
| Time in SB | -0.18a (-0.39, 0.02) | 0.087 | Unadjusted |
| -0.17a (-0.37, 0.03) | 0.11 | Child age, sex, prepregnancy BMI, EI, GDM exposure, total sugar | |
| -0.14a (-0.32, 0.03) | 0.11 | +BMI z-score | |
| Daily energy intake | 0.17a (-0.04, 0.37) | 0.12 | Unadjusted |
| 0.06a (-0.15, 0.27) | 0.60 | Child age, sex, prepregnancy BMI, GDM exposure, total sugar, MVPA | |
| 0.12a (-0.07, 0.30) | 0.21 | +BMI z-score | |
| Dietary added sugar | -0.05a (-0.26, 0.16) | 0.67 | Unadjusted |
| 0.04a (-0.15, 0.24) | 0.67 | Child age, sex, prepregnancy BMI, EI, GDM exposure, MVPA | |
| -0.01a (-0.18, 0.16) | 0.88 | +BMI z-score | |
| Total Sugar | -0.003a (-0.210, 0.210) | 0.97 | Unadjusted |
| 0.12a (-0.08, 0.32) | 0.24 | Child age, sex, prepregnancy BMI, EI, GDM exposure, MVPA | |
| 0.02a (-0.25, 0.19) | 0.82 | +BMI z-score | |
| Maternal prepregnancy BMI | -0.14a (-0.35, 0.07) | 0.20 | Unadjusted |
| -0.18a (-0.37, 0.02) | 0.076 | Child age, sex, EI, GDM exposure, total sugar, MVPA | |
| -0.03a (-0.21, 0.14) | 0.72 | +BMI z-score | |
| GDM exposure | 0.14 (-0.29, 0.56) | 0.53 | Unadjusted |
| 0.14 (-0.26, 0.54) | 0.48 | Child age, sex, EI, prepregnancy BMI, total sugar, MVPA | |
| 0.23 (-0.11, 0.58) | 0.19 | +BMI z-score |
Abbreviations: EI, energy intake; GDM, gestational diabetes mellitus; MVPA, moderate to vigorous physical activity; SB, sedentary behavior.
*Denotes P-value < 0.05.
a Standardized regression coefficient.
Figure 1.
LSmeans of insulin sensitivity by terciles of moderate to vigorous physical activity. Visual demonstration of the significant relationship between the ISI and time spent in MVPA. Adjusted for child age, sex, dietary added sugar, daily EI, maternal prepregnancy BMI, GDM status, and child BMI z-score. *Denotes P-value < 0.05.
There was a negative trend in the relationship between maternal prepregnancy BMI and child ISI (β = -0.18; P = 0.076), but adjusting for child adiposity eliminated this relationship (β = -0.03; P = 0.72) (Table 2). Gestational diabetes mellitus exposure was not associated with ISI (unadjusted, β = 0.14; P = 0.53; fully adjusted, β = 0.23; P = 0.19).
Discussion
To our knowledge, this is the first study to investigate the unique contributions of both prenatal exposures and postnatal lifestyle factors to insulin sensitivity in healthy children. Time spent in MVPA was the only predictor significantly associated with child insulin sensitivity at this early age, and its contribution to insulin sensitivity was independent of other prenatal and postnatal factors. We observed a negative trend between maternal prepregnancy BMI and child insulin sensitivity; however, this was not independent of child BMI z-score. Our findings indicate that physical activity and a modifiable lifestyle factor had stronger associations with children’s insulin sensitivity compared to in utero programming mechanisms of maternal obesity and GDM, dietary factors (EI, sugar intake), and time spent sedentary.
Similar to other studies, time spent in MVPA was more strongly associated with child insulin sensitivity than time spent sedentary (38). Several studies in adults have illuminated the benefits of engaging in physical activity for improving insulin sensitivity (see the review by Bird and Hawley (39)). Potential mechanisms by which engaging in physical activity positively impacts insulin sensitivity include facilitating greater glucose transporter type 4 (GLUT4) receptor translocation to the cell surface for glucose uptake into muscles (40), increasing skeletal muscle capillary recruitment (41), and decreasing circulating tumor-necrosis factor-α concentrations and other inflammatory cytokines (42). Our results suggest that the benefits of MVPA on insulin sensitivity are significant during childhood, and our study adds to the existing literature by showing an independent contribution of MVPA on insulin sensitivity from an array of prenatal and postnatal factors.
Animal studies have shown that excessive EI can lead to insulin resistance (43); however, findings in humans are mixed. For example, Donin et al (15) observed that EI was associated with insulin resistance during childhood, whereas other studies have found null associations (44). Similarly, we did not observe an association between insulin sensitivity and daily EI. Additionally, we did not observe a relationship between added or total sugar consumption and child insulin sensitivity. There have been discrepancies in the literature on the associations between sugar intake and insulin sensitivity during childhood (14, 15, 18, 20, 44). While studies have observed associations between dietary sugar, particularly sugar-sweetened beverages, with reduced insulin sensitivity in adolescents (14, 18), associations with total sugar were not observed in younger children (15, 44). Moreover, the associations between sugar intake and reduced insulin sensitivity were observed predominately in overweight/obese youth (19, 20). In our cohort, 93% of children were prepubescent and the majority were healthy-weight. Together with prior studies, these findings suggest the possibility that the association between high consumption of sugar and insulin resistance may be influenced by age, puberty, and/or adiposity and that this association may become more prominent during adolescence, when large changes in body composition and insulin resistance typically occurs (see the review by Kelsey and Zeitler (45)). Moreover, other studies have found that sugar intake during childhood was not associated with insulin resistance, while other dietary components were (15, 44). Therefore, it is also possible that other components of the participants’ diet, such as a higher fiber content, may have had beneficial effects on insulin sensitivity (46). Detailed nutritive studies are needed to determine if increased EI, specific macronutrients, and/or overall diet quality are associated with decreased insulin sensitivity during childhood.
A number of prior studies have shown that children exposed to GDM in utero have an increased risk of developing obesity, insulin resistance, and type 2 diabetes (8–10, 13, 47–49), whereas recent findings from the Project Viva study showed no significant association between GDM exposure and insulin resistance in their large cohort of children in early adolescence (50). The recent Hyperglycemia and Adverse Pregnancy Outcome follow up study, which included over 4000 children aged 10–14 years, found that offspring of mothers with GDM (untreated) had lower insulin sensitivity than offspring of mothers without GDM, and the results remained significant after adjusting for a number of confounding variables (10). However, in exploratory analyses to assess Tanner Stage–specific associations between maternal GDM and child metabolic outcomes, GDM exposure was not found to associated with insulin sensitivity in children who were Tanner Stage 1 (prepubertal) (10). As mentioned by the Hyperglycemia and Adverse Pregnancy Outcome investigators, these results may suggest that associations between GDM exposure and insulin sensitivity are stronger after the onset of puberty (10). In line with those findings, prior work has demonstrated an age-related difference in the relationship between GDM exposure and child insulin sensitivity whereby the relationship between GDM exposure and child insulin resistance does not appear to manifest until late childhood (8). Therefore, it is possible that we did not observe an association between GDM exposure and child insulin sensitivity due to the relatively young age range and prepubertal status of our cohort, and this relationship may become more apparent as these children traverse through puberty. The relatively small sample size in our study can only allow us to detect relatively large associations.
Similarly, age and pubertal status may also be a factor in the lack of evidence for an association between maternal prepregnancy BMI and child insulin sensitivity. Several studies have found an association between maternal prepregnancy BMI and offspring insulin resistance at birth (11) and during adulthood (3). However, there are limited studies that have observed this relationship during childhood (8, 9, 13). Further, it is unclear whether the relationship between maternal prepregnancy BMI and offspring insulin resistance is independent of the offspring’s adiposity (8, 9, 13). Similar to Sauder et al (9), we also observed that there was no relationship between child insulin sensitivity and maternal prepregnancy BMI after controlling for child adiposity. Based on these results, it is evident that more longitudinal studies are needed to determine how prenatal exposure to maternal obesity and/or GDM influence offspring insulin sensitivity across the lifespan.
Strengths and Limitations
Our unique cohort had several strengths. In addition to a thorough assessment of the lifestyle behaviors of our participants, we had objective measures of the mother’s GDM status and prepregnancy BMI, which were both obtained from electronic medical records. We performed OGTTs and used the Matsuda ISI to estimate insulin sensitivity, which is strongly correlated with the gold-standard method using the hyperinsulinemic-euglycemic insulin clamp technique (29, 51). However, our study had some limitations. The relatively small size of our cohort limited our ability to detect small associations between child exposures and insulin sensitivity. Moreover, we were limited in investigating potential interactions between prenatal and postnatal factors, which a larger sample size could potentially address. While the 3DPAR is a valid measurement for assessing time spent in MVPA and SB during childhood (31), there is the possibility that participants over or underreported their physical activity levels. Children in our cohort reported greater time spent in MVPA than the average of 88 minutes per a day reported by the National Health and Nutritional Examination Survey (NHANES) for elementary-aged children (52). Future studies should incorporate accelerometer devices to provide an objective measurement of time spent in MVPA and time spent sedentary. Further, although the 24-hour dietary recall method is considered a validated recall method to obtain detailed information about dietary intake, including total EI and sugar consumption (28), the data are subject to recall bias. However, children in our cohort reported consuming a similar EI as well as dietary added sugar intake to nationally reported averages (53, 54), and the data was rigorously assessed for plausibility.
Conclusions
Time spent in MVPA was the only predictor of child insulin sensitivity, and its effects remained significant after adjusting for the child’s dietary intake, BMI z-score, and prenatal exposures to GDM and maternal obesity. These findings are encouraging and suggest that engaging in MVPA during childhood may be beneficial for insulin sensitivity, independent of diet, BMI, or prenatal exposures to maternal obesity or GDM. Thus, engaging in MVPA during childhood may be 1 strategy to mitigate the adverse effects of prenatal exposures to maternal obesity and/or GDM on future risk for insulin resistance and type 2 diabetes. Longitudinal studies and intervention trials are necessary to test this possibility.
Acknowledgments
Financial Support: This work was supported by an American Diabetes Association Pathway Accelerator Award (#1-14-ACE-36) (PI: K.A.P) and in part by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases R01DK116858 (PIs: K.A.P, A.H.X), K01DK115638 (PI: SL), the National Institute Of Mental Health F31MH115640 (PI: J.M.A), and National Cancer Institute T32CA009492. A Research Electronic Data Capture, REDCap, database was used for this study, which is supported by the Southern California Clinical and Translational Science Institute (SC CTSI) through NIH UL1TR001855.
Glossary
Abbreviations
- 3DPAR
3-day physical activity recall;
- CDC
Center for Disease Control
- EI
energy intake
- EMR
electronic medical record
- GDM
gestational diabetes mellitus
- ISI
insulin sensitivity index
- KPSC
Kaiser Permanente Southern California
- MET
metabolic equivalent
- MVPA
moderate to vigorous physical activity
- SB
sedentary behavior
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated and analyzed during the current study are available from the corresponding author (K.A.P.), on reasonable request.
References
- 1. Dabelea D, Mayer-Davis EJ, Saydah S, et al. ; SEARCH for Diabetes in Youth Study Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clausen TD, Mathiesen ER, Hansen T, et al. . High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–346. [DOI] [PubMed] [Google Scholar]
- 3. Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46(6):434–438. [DOI] [PubMed] [Google Scholar]
- 4. Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. . Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31(7):1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Festa A, Williams K, D’Agostino R Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55(4):1114–1120. [DOI] [PubMed] [Google Scholar]
- 6. Zhang F, Xiao X, Liu D, Dong X, Sun J, Zhang X. Increased cord blood angiotensin II concentration is associated with decreased insulin sensitivity in the offspring of mothers with gestational diabetes mellitus. J Perinatol. 2013;33(1):9–14. [DOI] [PubMed] [Google Scholar]
- 7. Luo ZC, Delvin E, Fraser WD, et al. . Maternal glucose tolerance in pregnancy affects fetal insulin sensitivity. Diabetes Care. 2010;33(9):2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boerschmann H, Pflüger M, Henneberger L, Ziegler AG, Hummel S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care. 2010;33(8):1845–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sauder KA, Hockett CW, Ringham BM, Glueck DH, Dabelea D. Fetal overnutrition and offspring insulin resistance and β-cell function: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabet Med. 2017;34(10):1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lowe WL Jr, Scholtens DM, Kuang A, et al. ; HAPO Follow-up Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Group HSCR. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index: HAPO - BMI and perinatal outcomes. BJOG Int J Obstet Gynaecol. 2010;117(5):575–584. [DOI] [PubMed] [Google Scholar]
- 12. Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maftei O, Whitrow MJ, Davies MJ, Giles LC, Owens JA, Moore VM. Maternal body size prior to pregnancy, gestational diabetes and weight gain: associations with insulin resistance in children at 9-10 years. Diabet Med. 2015;32(2):174–180. [DOI] [PubMed] [Google Scholar]
- 14. Bremer AA, Auinger P, Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents. Arch Pediatr Adolesc Med. 2009;163(4):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donin AS, Nightingale CM, Owen CG, et al. . Dietary energy intake is associated with type 2 diabetes risk markers in children. Diabetes Care. 2014;37(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nightingale CM, Rudnicka AR, Donin AS, et al. . Screen time is associated with adiposity and insulin resistance in children. Arch Dis Child. 2017;102(7):612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson M, Benedetti A, Barnett TA, Mathieu ME, Deladoëy J, Gray-Donald K. Influence of adiposity, physical activity, fitness, and screen time on insulin dynamics over 2 years in children. JAMA Pediatr. 2016;170(3):227–235. [DOI] [PubMed] [Google Scholar]
- 18. Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123(3):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis JN, Alexander KE, Ventura EE, et al. . Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am J Clin Nutr. 2007;86(5):1331–1338. [DOI] [PubMed] [Google Scholar]
- 20. Wang JW, Mark S, Henderson M, et al. . Adiposity and glucose intolerance exacerbate components of metabolic syndrome in children consuming sugar-sweetened beverages: QUALITY cohort study. Pediatr Obes. 2013;8(4):284–293. [DOI] [PubMed] [Google Scholar]
- 21. Savoye M, Caprio S, Dziura J, et al. . Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37(2):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Diabetes Association. Standards of medical care in diabetes - 2012. Diabetes Care. 2012;35(SUPPL. 1). doi: 10.2337/dc12-s011. [DOI] [PubMed] [Google Scholar]
- 23. CDC. About Child & Teen BMI | Healthy Weight.2018. ProMED-mail website. https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html. Accessed November 27, 2018.
- 24. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasmussen AR, Wohlfahrt-Veje C, Tefre de Renzy-Martin K, et al. . Validity of self-assessment of pubertal maturation. Pediatrics. 2015;135(1):86–93. [DOI] [PubMed] [Google Scholar]
- 27. Ainsworth BE, Haskell WL, Herrmann SD, et al. . 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 28. Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–1144. [DOI] [PubMed] [Google Scholar]
- 29. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 30. Alves J. Data from: contributions of prenatal exposures and child lifestyle to insulin sensitivity. Open Science Framework. Deposited 16 November 2019. doi: 10.17605/OSF.IO/TWXKM. [DOI]
- 31. Pate RR, Ross R, Dowda M, Trost SG, Sirard JR. Validation of a 3-day physical activity recall instrument in female youth. Pediatr Exerc Sci. 2003;15(3):257–265. [Google Scholar]
- 32. Powell KE, Roberts AM, Ross JG, Phillips MA, Ujamaa DA, Zhou M. Low physical fitness among fifth- and seventh-grade students, Georgia, 2006. Am J Prev Med. 2009;36(4): 304–310. [DOI] [PubMed] [Google Scholar]
- 33. Dollman J, Stanley R, Wilson A. The concurrent validity of the 3-day physical activity recall in australian youth. Pediatr Exerc Sci. 2015;27(2):262–267. [DOI] [PubMed] [Google Scholar]
- 34. Hearst M, Sirard J, Lytle L, Dengel D, Berrigan D. Comparison of three measures of physical activity and associations with blood pressure, HDL and body composition in a sample of adolescents. J Phys Act Health. 2012;9(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moshfegh AJ, Rhodes DG, Baer DJ, et al. . The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. [DOI] [PubMed] [Google Scholar]
- 36. Foster E, Bradley J. Methodological considerations and future insights for 24-hour dietary recall assessment in children. Nutr Res. 2018;51:1–11. [DOI] [PubMed] [Google Scholar]
- 37. Schakel SF, Buzzard IM, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Compos Anal. 1997;10(2):102–114. [Google Scholar]
- 38. Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A; International Children’s Accelerometry Database (ICAD) Collaborators Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307(7):704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2016;2(1):1– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thorell A, Hirshman MF, Nygren J, et al. . Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol. 1999;277(4):E733–E741. [DOI] [PubMed] [Google Scholar]
- 41. Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes. 2001;50(12):2659–2665. [DOI] [PubMed] [Google Scholar]
- 42. Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295(3):E586–E594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50(12):2786–2791. [DOI] [PubMed] [Google Scholar]
- 44. Casazza K, Dulin-Keita A, Gower BA, Fernández JR. Relationships between reported macronutrient intake and insulin dynamics in a multi-ethnic cohort of early pubertal children. Int J Pediatr Obes. 2009;4(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelsey MM, Zeitler PS. Insulin resistance of puberty. Curr Diab Rep. 2016;16(7):1– 6. [DOI] [PubMed] [Google Scholar]
- 46. Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism. 2006;55(7):871–878. [DOI] [PubMed] [Google Scholar]
- 47. Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33(2):402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Page KA, Romero A, Buchanan TA, Xiang AH. Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. J Pediatr. 2014;164(4):807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chandler‐Laney PC, Bush NC, Granger WM, Rouse DJ, Mancuso MS, Gower BA. Overweight status and intrauterine exposure to gestational diabetes are associated with children’s metabolic health. Pediatr Obes. 2012;7(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gingras V, Rifas-Shiman SL, Derks IPM, Aris IM, Oken E, Hivert MF. Associations of gestational glucose tolerance with offspring body composition and estimated insulin resistance in early adolescence. Diabetes Care. 2018;41(12):e164–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henderson M, Rabasa-Lhoret R, Bastard JP, et al. . Measuring insulin sensitivity in youth: How do the different indices compare with the gold-standard method? Diabetes Metab. 2011;37(1):72–78. [DOI] [PubMed] [Google Scholar]
- 52. Belcher BR, Berrigan D, Dodd KW, Emken BA, Chou C-P, Spuijt-Metz D. Physical activity in US youth: impact of race/ethnicity, age, gender, & weight status. Med Sci Sports Exerc. 2010;42(12):2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ervin RB, Ogden CL. Trends in intake of energy and macronutrients in children and adolescents from 1999–2000 through 2009–2010. NCHS Data Brief. 2013; 113:1– 8. [PubMed] [Google Scholar]
- 54. Ervin RB, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005–2008. NCHS Data Brief. 2012; 87:8. [PubMed] [Google Scholar]