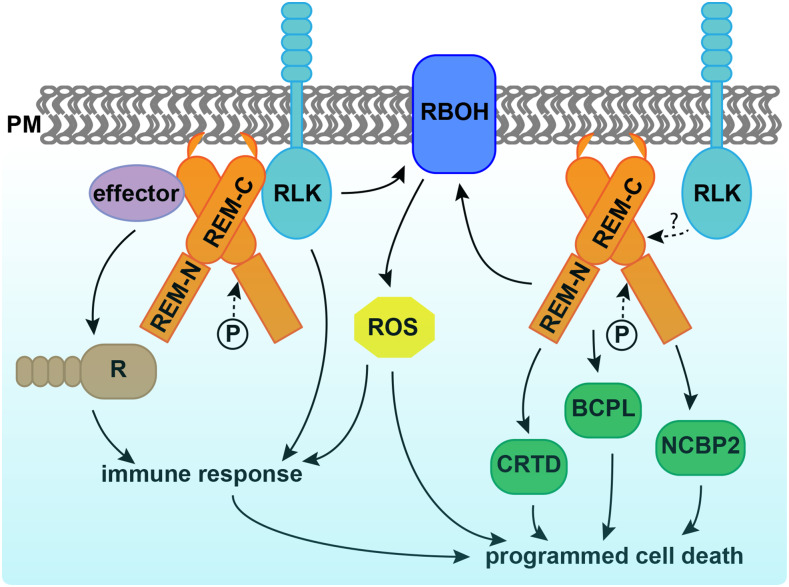
Programmed cell death (PCD) is a genetically controlled process triggered by developmental or environmental cues. Plant-microbe interactions often lead to PCD in plant host cells, triggered by the hypersensitive response. Plants may recognize microbes through receptor-like kinases in the plasma membrane or detect pathogen effectors through resistance (R) proteins in the cytoplasm. Both pathways activate immune responses and trigger PCD (Fig. 1).
Figure 1.
REMs participate in plant-microbe interaction and PCD. REMs polymerize and physically interact with receptor-like kinases (RLK) and pathogen effectors; the latter activate R proteins and trigger immunity. The C terminus of REM (REM-C) is required for plasma membrane (PM) localization and protein interaction, while phosphorylation occurs at the N terminus (REM-N). REMs induce reaction oxygen species (ROS) and up-regulate protein abundance of CYSTEINE-RICH AND TRANSMEMBRANE DOMAIN-CONTAINING PROTEIN A-LIKE (CRTD), BLUE COPPER PROTEIN-LIKE (BCPL), NUCLEAR CAP-BINDING PROTEIN SUBUNIT 2 (NCBP2), and RBOHB in tobacco, which all contribute to programmed cell death. Whether REM induction of cell death is triggered by RLKs is not known.
In this issue of Plant Physiology, Cai et al. (2020) describe a new role for remorin (REM) in regulating PCD. REMs are plant-specific proteins localized on the inner side of the plasma membrane and plasmodesmata. REMs accumulate in detergent-insoluble fractions of the plasma membrane called lipid rafts (Raffaele et al., 2009). REMs have a highly conserved C terminus with a coiled-coil domain and a divergent N terminus, the sequence of which permits them to be classified into six groups (Raffaele et al., 2007). The roles of REMs in plant-microbe interaction have been demonstrated in various species (Fig. 1). Potato (Solanum tuberosum) and tobacco (Nicotiana benthamiana) group I REMs physically interact with virus movement proteins and inhibit virus movement (Raffaele et al., 2009; Fu et al., 2018). Arabidopsis (Arabidopsis thaliana) group IV REMs increase susceptibility to viruses, as the atrem4.1 atrem4.2 double mutant is more resistant to geminivirus infection (Son et al., 2014). In legumes, orthologs in Medicago truncatula and Lotus japonicus of a group II REM are required for nodulation and interact with symbiotic receptor-like kinases (Lefebvre et al., 2010; Tóth et al., 2012).
Cai et al. (2020) report that transgenic tomato (Solanum lycopersicum) overexpressing a group I REM, SlREM1, was more susceptible to the necrotrophic fungus Botrytis cinerea. Additionally, Agrobacterium tumefaciens-mediated transient expression of SlREM1 in tobacco leaves induced PCD, as assayed by Trypan Blue staining and cell electrolyte leakage. Overexpression of other group I REMs, SlREM1.4, SlREM1.5, SlREM1.6, and SlREM1.7, but not SlREM1.2/SlREM1.3, in tobacco leaves resulted in similar symptoms, suggesting at least partially conserved roles for group I SlREMs in PCD induction. Consistent with previous reports that REMs form homooligomers and heterooligomers (Lefebvre et al., 2010; Tóth et al., 2012; Son et al., 2014; Fu et al., 2018), SlREM1 physically interacted with itself and SlREM1.4, SlREM1.5, SlREM1.6, and SlREM1.7. However, no interaction between SlREM1 and SlREM1.2/ SlREM1.3 was detected.
Cai et al. (2020) further performed quantitative proteomics analysis using tobacco leaves with or without SlREM1 overexpression. In the proteins up-regulated by SlREM1 overexpression, the authors identified a set of proteins involved in PCD regulation, including the known plasma membrane NADPH oxidase NtRBOHB. Quantitative real-time PCR showed that higher abundance of NtRBOHB is attributable to a higher transcript level. Consistent with the molecular data, reactive oxygen species including hydrogen peroxide and superoxide were more abundant in leaves overexpressing SlREM1. In addition, the authors identified three novel cell death regulators through the proteomics analysis, including CYSTEINE-RICH AND TRANSMEMBRANE DOMAIN-CONTAINING PROTEIN A-LIKE, BLUE COPPER PROTEIN-LIKE, and NUCLEAR CAP-BINDING PROTEIN SUBUNIT 2. Transient expression of these proteins in tobacco leaves enhanced cell death, confirming their positive roles in PCD induction (Fig. 1).
To reveal the functional domains that are required for REM function, Cai et al. (2020) generated a series of mutated forms of SlREM1 and transiently expressed them in tobacco leaves. They concluded that amino acids 173 to 187 at the C terminus are essential for the plasma membrane localization of SlREM1 and induction of cell death. Additionally, the 35 amino acids at the C terminus of SlREM1 were necessary for interaction with SlREM1.4 (Cai et al., 2020). These results are consistent with previous findings in other species, including StREM1.3 in potato (Perraki et al., 2012), LjSYMREM1 in L. japonicus (Tóth et al., 2012), and NbREM1 and NbREM4 in tobacco (Fu et al., 2018; Albers et al., 2019). Together, these results demonstrate that the conserved C terminus of REMs is responsible for plasma membrane localization and protein interactions.
Lastly, Cai et al. (2020) investigated the effect of phosphorylation on SlREM function. REMs were originally identified as phosphorylated proteins, and they are phosphorylated by various protein kinases in vitro (Tóth et al., 2012; Son et al., 2014; Albers et al., 2019). Cai et al. (2020) identified five phosphorylated sites of SlREM1 expressed in tobacco leaves using mass spectrometry. Interestingly, all phosphorylated sites were localized in the N terminus of the protein, which is consistent with earlier findings in LjSYMREM1 (Tóth et al., 2012) and NbREM4 (Albers et al., 2019). Both the phosphorylation-inactive and -active forms of SlREM1 exhibited decreased efficiency in PCD induction. The active form of SlREM1 also failed to interact with the native SlREM1 in a yeast two-hybrid assay, suggesting that these sites are necessary for SlREM1 function. It would be interesting to further dissect how phosphorylation of SlREM1 regulates the protein activity in plants.
Earlier studies suggest that REMs participate in upstream signaling during plant-microbe interaction, as they physically interact with receptor-like kinases and pathogen effectors. Cai et al. (2020) have provided evidence that REMs up-regulate PCD regulators at transcript and protein levels and induce PCD, a downstream defense mechanism. Further studies may reveal whether REMs directly participate in PCD induction by interacting with PCD signaling proteins or through the plant defense response.
References
- Albers P, Üstün S, Witzel K, Kraner M, Börnke F(2019) A remorin from Nicotiana benthamiana interacts with the Pseudomonas type-III effector protein HopZ1a and is phosphorylated by the immune-related kinase PBS1. Mol Plant Microbe Interact 32: 1229–1242 [DOI] [PubMed] [Google Scholar]
- Cai J, Chen T, Wang Y, Qin G, Tian S(2020) SlREM1 triggers cell death by activating an oxidative burst and other regulators. Plant Physiol 183: 717–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Xu Y, Li C, Li Y, Wu J, Zhou X(2018) Rice stripe virus interferes with S-acylation of remorin and induces its autophagic degradation to facilitate virus infection. Mol Plant 11: 269–287 [DOI] [PubMed] [Google Scholar]
- Lefebvre B, Timmers T, Mbengue M, Moreau S, Hervé C, Tóth K, Bittencourt-Silvestre J, Klaus D, Deslandes L, Godiard L, et al. (2010) A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc Natl Acad Sci USA 107: 2343–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraki A, Cacas JL, Crowet JM, Lins L, Castroviejo M, German-Retana S, Mongrand S, Raffaele S(2012) Plasma membrane localization of Solanum tuberosum remorin from group 1, homolog 3 is mediated by conformational changes in a novel C-terminal anchor and required for the restriction of Potato virus X movement. Plant Physiol 160: 624–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Bayer E, Lafarge D, Cluzet S, German Retana S, Boubekeur T, Leborgne-Castel N, Carde JP, Lherminier J, Noirot E, et al. (2009) Remorin, a Solanaceae protein resident in membrane rafts and plasmodesmata, impairs Potato virus X movement. Plant Cell 21: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Mongrand S, Gamas P, Niebel A, Ott T(2007) Genome-wide annotation of remorins, a plant-specific protein family: Evolutionary and functional perspectives. Plant Physiol 145: 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S, Oh CJ, An CS(2014) Arabidopsis thaliana remorins interact with SnRK1 and play a role in susceptibility to Beet Curly Top Virus and Beet Severe Curly Top Virus. Plant Pathol J 30: 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Stratil TF, Madsen EB, Ye J, Popp C, Antolín-Llovera M, Grossmann C, Jensen ON, Schüssler A, Parniske M, et al. (2012) Functional domain analysis of the remorin protein LjSYMREM1 in Lotus japonicus. PLoS ONE 7: e30817. [DOI] [PMC free article] [PubMed] [Google Scholar]