Abstract
Advances in algal biology have built on gas exchange measurements by MIMS in the fields of photosynthesis, biofuel production, and climate research.
Dear Editor,
Microalgae are continuously shaping Earth’s atmosphere through oxygenic photosynthesis, and nowadays, half of the photosynthesis is attributed to microbial photosynthesis (Field et al., 1998; Behrenfeld et al., 2005). While algal photosynthesis contributes to offsetting the CO2 footprint, it also produces nitric oxide (N2O), a potent greenhouse gas. In some ecological niches microalgae can produce hydrogen (H2), a promising energy carrier; therefore microalgae are actively explored for their potential as a platform for production of renewable energy. Measuring gas exchange between algae and the atmosphere, and understanding biological mechanisms underlying photosynthetic CO2 capture, and O2, H2, or N2O production, holds great promise—not only to better evaluate the reciprocal effects of global changes on oceanic carbon sinks, but also to explore the limits of biomass and biofuel productivity of algae. Membrane inlet mass spectrometry (MIMS) was initially developed to measure O2 exchange between algal cells and the surrounding liquid medium (Hoch and Kok, 1963), and its use has since been extended to other gases including H2 and more recently, N2O (Burlacot et al., 2020). Here we review recent breakthroughs allowed by MIMS in dissecting molecular mechanisms of gas exchange in microalgae (Fig. 1) and provide perspectives on how MIMS will be crucial to address future challenges in algal research.
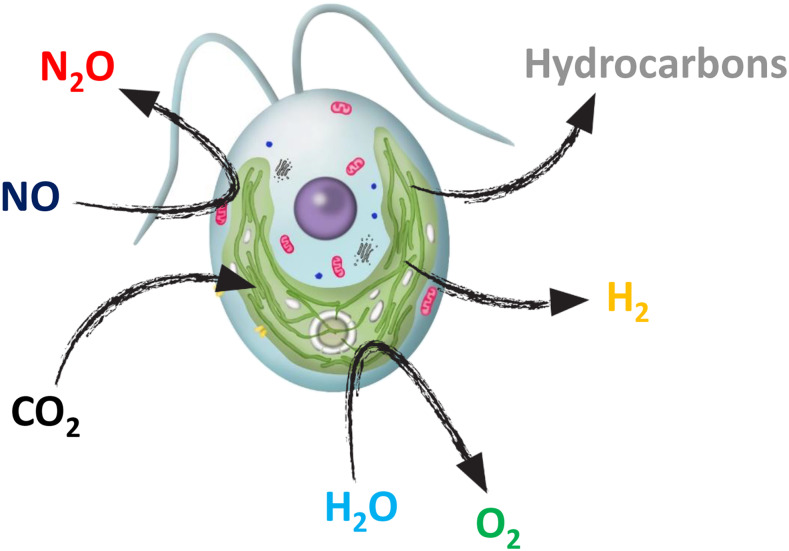
Figure 1.
Schematic view of gas exchange between microalgae and its surrounding medium illustrated in the model species C. reinhardtii.
DECIPHERING PHOTOSYNTHETIC OXYGEN UPTAKE MECHANISMS
MIMS was initially developed to monitor O2-consuming processes during photosynthesis (Hoch and Kok, 1963). The use of 18O labeling made it possible to differentiate O2-consuming processes from O2-generating processes. While O2 is produced from water splitting by PSII, various mechanisms participate in O2 consumption including mitochondrial respiration, chlororespiration, PSI-driven reduction, and photorespiration (Curien et al., 2016). Measuring O2 exchange by MIMS has contributed to the discovery of several factors involved in O2 consumption, including the plastid terminal oxidase (Cournac et al., 2000) and flavodiiron proteins (FLVs; Helman et al., 2003). Recently, the use of MIMS has also helped to reveal the occurrence of redox communication between mitochondria and chloroplasts (Dang et al., 2014; Bailleul et al., 2015), and between chloroplasts and peroxisomes (Kong et al., 2018). Intriguingly, although FLVs are recognized as major factors in light-dependent O2 uptake in cyanobacteria (Helman et al., 2003; Allahverdiyeva et al., 2013; Santana-Sanchez et al., 2019) and green microalgae (Chaux et al., 2017), they are absent from diatoms where interaction between chloroplasts and mitochondria is critical (Bailleul et al., 2015; Flori et al., 2017). The use of MIMS will be crucial in establishing the significance of such diverse bioenergetic mechanisms among algal species in relation to their ecological niches and environmental constraints.
MEASURING CO2 GAS EXCHANGE AND THE CARBON-CONCENTRATING MECHANISM
Many microalgae and cyanobacteria have the ability to improve the affinity of photosynthesis for dissolved inorganic carbon (DIC) when grown at limiting DIC levels (Badger et al., 1980; Reinfelder, 2011). This property is due to the induction of a carbon-concentrating mechanism, which involves, among other components, active bicarbonate pumping and carbonic anhydrases (Mackinder et al., 2017; Mackinder, 2018). MIMS has been used to elucidate different aspects of carbon-concentrating mechanisms, including (1) the disequilibrium between bicarbonate and CO2 pools resulting from this active pumping (Badger and Price, 1989; Sültemeyer and Rinast, 1996; Sültemeyer et al., 1998), (2) in vivo activity of carbonic anhydrases by following 18O exchange between CO2 and water (Tansik et al., 2015; Tolleter et al., 2017), and (3) more recently, the direct assessment of apparent affinity of photosynthesis for DIC (Douchi et al., 2019).
MIMS AND BIOFUEL RESEARCH
The study of microalgae has been greatly boosted by biofuel research, as microalgae naturally produce energy-rich compounds such as H2, reserve lipids, or hydrocarbons (Stephens et al., 2010). Secretion of H2 or short-chain hydrocarbons by photosynthetic cells avoids energy-costly steps of harvesting and extraction. H2 is produced by microalgae through a coupling between hydrogenase (H2ase) and the photosynthetic electron transport chain. By in situ-monitoring of H2 exchange performed in a time-resolved manner, MIMS contributed to the identification of biological bottlenecks of H2 production. These include the photosynthetic control triggered by the Proton Gradient Regulatory protein Like-1-mediated cyclic electron flow (Tolleter et al., 2011), competition for electrons with the Calvin cycle (Milrad et al., 2018), and competition with the FLV-mediated O2 photoreduction (Burlacot et al., 2018). Lately, MIMS has been used in the development of a very promising method for H2 photoproduction based on the intermittent illumination of microalgae (Kosourov et al., 2018; Jokel et al., 2019).
MIMS has also contributed to the study of hydrocarbon synthesis by microalgae. It enabled the detection of CO2 produced by the Fatty-Acid Photodecarboxylase (FAP), therefore demonstrating its decarboxylase activity and further establishing that FAP is a photoenzyme using blue photons as substrates (Sorigué et al., 2017). Taken together, MIMS should be useful in future studies aimed at characterizing the bottlenecks of volatile compound production by FAPs and H2ase at the both enzyme and cellular levels.
PHOTOREDUCTION OF NO INTO N2O
N2O is a potent greenhouse gas responsible for 6% of the Earth’s radiative force although it is present at a concentration 1,000 times lower than that of CO2 (IPCC, 2013). Recently, N2O production by the photosynthetic chain has been demonstrated using real-time MIMS measurements in axenic cultures of Chlamydomonas reinhardtii (Burlacot et al., 2020). NO was shown to be produced during nitrogen assimilation and reduced into N2O by FLVs in a light-dependent manner (Burlacot et al., 2020). These results open new perspectives for the study of mechanisms regulating the intracellular concentration of NO, an important signaling molecule in plants (Farnese et al., 2016). They also provide insights into the possible consequences of algal blooms and large-scale algae cultivation on global warming.
ADVANTAGES AND DRAWBACKS
When compared to other methods like gas chromatography or specific gas electrodes, MIMS has the great advantage of simultaneously measuring the concentrations of various gases in a time-resolved manner by selecting mass peaks specific to the gases of interest. This flexibility largely explains the recent success of MIMS (Ketola and Lauritsen, 2016), but it must be kept in mind that MIMS is demanding from a technical point of view (Shevela et al., 2018). Proper use of MIMS requires a suitable experimental setup (including efficient temperature control and limitation of gas leaks), as well as appropriate calibration and data processing procedures (Bailleul et al., 2017). The choice of the type of membrane, in particular its material (usually silicon or polytetrafluoroethylene) and thickness, is critical because the membrane permeability and selectivity affects the sensitivity of the setup and the relative enrichment of specific gases (Beckmann et al., 2009). Furthermore, the diffusion of gases through the membrane from the liquid sample to the vacuum pump of the mass spectrometer creates a gas consumption that needs to be corrected to determine gas exchange rates related to biological processes (Kotiaho and Lauritsen, 2002; Bailleul et al., 2017). Depending on the targeted application, a compromise must be found among membrane permeability (which affects both sensitivity and gas consumption), cell or enzyme concentration (which affects rates of gas exchange), and the volume of the reaction vessel (which affects gas consumption kinetics). Ultimately, the sensitivity of the MIMS is generally limited by (1) the permeability of the membrane and (2) gas leakage of the setup, which can reduce the use of the technique in highly diluted samples, such as found in the natural environment (Chatton et al., 2017).
PERSPECTIVES
This decade has seen an increasing use of MIMS in addressing biological questions in algal research. This has helped to push the limits of the types of biological questions that we can address in photosynthetic microorganisms at various scales. Coupling MIMS measurements with chlorophyll fluorescence has proven to be of interest to study gas exchange beyond the theoretical limits of MIMS (Burlacot et al., 2018) and should allow deeper understanding of photosynthesis. Further coupling to absorption change measurements (Bailleul et al., 2010) should provide new insights into mechanisms regulating photosynthesis and their interactions in response to environmental constraints. While several O2 photoreduction mechanisms have been observed in algae (Curien et al., 2016), their physiological significance and their relevance in natural environments remain largely unexplored. Recently, the miniaturization of MIMS allowed measuring in situ O2 exchange rates in samples from the north Pacific (Ferrón et al., 2016) or in planktonic blooms from the north Atlantic (Bailleul et al., 2017), starting thus a new era for expanding our laboratory models to natural environments.
MIMS has now emerged as a key technique to study cellular mechanisms involved in the exchange of gas molecules with the medium, paving the way toward better understanding of the interaction among algae, aquatic ecosystems, and the atmosphere. In situ measurements of O2 and CO2 exchange in oceanic samples will provide a better understanding of the physiological relevance of mechanisms regulating photosynthesis under natural conditions. In another perspective, it will be of great interest to uncover the distribution of recently discovered N2O production pathways in aquatic ecosystems (Burlacot et al., 2020) and further estimate their global influence on the climate through measuring N2O production in oceanic hotspots.
Acknowledgments
The authors thank Dr. Solène Moulin for preparing Figure 1.
Footnotes
This work was supported by the French Agence Nationale de la Recherche (grant nos. OTOLHYD and PHOTOALKANE) and CEA (Irtelis PhD studentship to A.B.).
Articles can be viewed without a subscription.
References
- Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM(2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110: 4111–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Kaplan A, Berry JA(1980) Internal inorganic carbon pool of Chlamydomonas reinhardtii. Plant Physiol 66: 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD(1989) Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol 89: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B, Berne N, Murik O, Petroutsos D, Prihoda J, Tanaka A, Villanova V, Bligny R, Flori S, Falconet D, et al. (2015) Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524: 366–369 [DOI] [PubMed] [Google Scholar]
- Bailleul B, Cardol P, Breyton C, Finazzi G(2010) Electrochromism: A useful probe to study algal photosynthesis. Photosynth Res 106: 179–189 [DOI] [PubMed] [Google Scholar]
- Bailleul B, Park J, Brown CM, Bidle KD, Lee SH, Falkowski PG(2017) Direct measurements of the light dependence of gross photosynthesis and oxygen consumption in the ocean. Limnol Oceanogr 62: 1066–1079 [Google Scholar]
- Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W(2009) On-line mass spectrometry: Membrane inlet sampling. Photosynth Res 102: 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrenfeld MJ, Boss E, Siegel DA, Shea DM(2005) Carbon-based ocean productivity and phytoplankton physiology from space. Global Biogeochem Cycl 19: GB1006 [Google Scholar]
- Burlacot A, Richaud P, Gosset A, Li-Beisson Y, Peltier G(2020) Algal photosynthesis converts nitric oxide into nitrous oxide. Proc Natl Acad Sci USA 117: 2704–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlacot A, Sawyer A, Cuiné S, Auroy-Tarrago P, Blangy S, Happe T, Peltier G(2018) Flavodiiron-mediated O2 photoreduction links H2 production with CO2 fixation during the anaerobic induction of photosynthesis. Plant Physiol 177: 1639–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton E, Labasque T, de La Bernardie J, Guihéneuf N, Bour O, Aquilina L(2017) Field continuous measurement of dissolved gases with a CF-MIMS: Applications to the physics and biogeochemistry of groundwater flow. Environ Sci Technol 51: 846–854 [DOI] [PubMed] [Google Scholar]
- Chaux F, Burlacot A, Mekhalfi M, Auroy P, Blangy S, Richaud P, Peltier G(2017) Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant Physiol 174: 1825–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse E-M, Kuntz M, Peltier G(2000) Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. 275: 17256–17262 [DOI] [PubMed] [Google Scholar]
- Curien G, Flori S, Villanova V, Magneschi L, Giustini C, Forti G, Matringe M, Petroutsos D, Kuntz M, Finazzi G(2016) The water to water cycles in microalgae. Plant Cell Physiol 57: 1354–1363 [DOI] [PubMed] [Google Scholar]
- Dang KV, Plet J, Tolleter D, Jokel M, Cuiné S, Carrier P, Auroy P, Richaud P, Johnson X, Alric J, Allahverdiyeva Y, Peltier G(2014) Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 26: 3036–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douchi D, Liang F, Cano M, Xiong W, Wang B, Maness P-C, Lindblad P, Yu J(2019) Membrane-inlet mass spectrometry enables a quantitative understanding of inorganic carbon uptake flux and carbon concentrating mechanisms in metabolically engineered cyanobacteria. Front Microbiol 10: 1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA(2016) When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrón S, del Valle DA, Björkman KM, Quay PD, Church MJ, Karl DM(2016) Application of membrane inlet mass spectrometry to measure aquatic gross primary production by the 18O in vitro method. Limnol Oceanogr Methods 14: 610–622 [Google Scholar]
- Field CB, Behrenfeld MJ, Randerson JT, Falkowski P(1998) Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 281: 237–240 [DOI] [PubMed] [Google Scholar]
- Flori S, Jouneau P-H, Bailleul B, Gallet B, Estrozi LF, Moriscot C, Bastien O, Eicke S, Schober A, Bártulos CR, et al. (2017) Plastid thylakoid architecture optimizes photosynthesis in diatoms. Nat Commun 8: 15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A(2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13: 230–235 [DOI] [PubMed] [Google Scholar]
- Hoch G, Kok B(1963) A mass spectrometer inlet system for sampling gases dissolved in liquid phases. Arch Biochem Biophys 101: 160–170 [DOI] [PubMed] [Google Scholar]
- IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY [Google Scholar]
- Jokel M, Nagy V, Tóth SZ, Kosourov S, Allahverdiyeva Y(2019) Elimination of the flavodiiron electron sink facilitates long-term H2 photoproduction in green algae. Biotechnol Biofuels 12: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola RA, Lauritsen FR(2016) Membrane inlet mass spectrometry (MIMS) in historical perspective In Gross ML, and Caprioli RM, eds, The Encyclopedia of Mass Spectrometry. Elsevier, Boston, pp 143–148 [Google Scholar]
- Kong F, Burlacot A, Liang Y, Légeret B, Alseekh S, Brotman Y, Fernie AR, Krieger-Liszkay A, Beisson F, Peltier G, et al. (2018) Interorganelle communication: Peroxisomal MALATE DEHYDROGENASE2 connects lipid catabolism to photosynthesis through redox coupling in Chlamydomonas. Plant Cell 30: 1824–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosourov S, Jokel M, Aro E-M, Allahverdiyeva Y(2018) A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii. Energy Environ Sci 11: 1431–1436 [Google Scholar]
- Kotiaho T, Lauritsen FR(2002) Membrane inlet mass spectrometry In Comprehensive Analytical Chemistry, Vol Vol 37 Elsevier, New York, pp 531–557 [Google Scholar]
- Mackinder LCM.(2018) The Chlamydomonas CO2-concentrating mechanism and its potential for engineering photosynthesis in plants. New Phytol 217: 54–61 [DOI] [PubMed] [Google Scholar]
- Mackinder LCM, Chen C, Leib RD, Patena W, Blum SR, Rodman M, Ramundo S, Adams CM, Jonikas MC(2017) A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 171: 133–147.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milrad Y, Schweitzer S, Feldman Y, Yacoby I(2018) Green algal hydrogenase activity is outcompeted by carbon fixation before inactivation by oxygen takes place. Plant Physiol 177: 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfelder JR.(2011) Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu Rev Mar Sci 3: 291–315 [DOI] [PubMed] [Google Scholar]
- Santana-Sanchez A, Solymosi D, Mustila H, Bersanini L, Aro E-M, Allahverdiyeva Y(2019) Flavodiiron proteins 1-to-4 function in versatile combinations in O2 photoreduction in cyanobacteria. eLife 8: e45766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevela D, Schröder WP, Messinger J(2018) Liquid-phase measurements of photosynthetic oxygen evolution In Covshoff S, ed, Photosynthesis: Methods and Protocols. Springer, New York, pp 197–211 [DOI] [PubMed] [Google Scholar]
- Sorigué D, Légeret B, Cuiné S, Blangy S, Moulin S, Billon E, Richaud P, Brugière S, Couté Y, Nurizzo D, et al. (2017) An algal photoenzyme converts fatty acids to hydrocarbons. Science 357: 903–907 [DOI] [PubMed] [Google Scholar]
- Stephens E, Ross IL, Mussgnug JH, Wagner LD, Borowitzka MA, Posten C, Kruse O, Hankamer B(2010) Future prospects of microalgal biofuel production systems. Trends Plant Sci 15: 554–564 [DOI] [PubMed] [Google Scholar]
- Sültemeyer D, Klughammer B, Badger MR, Dean Price G(1998) Fast induction of high-affinity HCO3− transport in cyanobacteria. Plant Physiol 116: 183–192 [Google Scholar]
- Sültemeyer D, Rinast K-A(1996) The CO2 permeability of the plasma membrane of Chlamydomonas reinhardtii: Mass-spectrometric 18O-exchange measurements from 13C18O2 in suspensions of carbonic anhydrase-loaded plasma-membrane vesicles. Planta 200: 358–368 [Google Scholar]
- Tansik AL, Fitt WK, Hopkinson BM(2015) External carbonic anhydrase in three Caribbean corals: Quantification of activity and role in CO2 uptake. Coral Reefs 34: 703–713 [Google Scholar]
- Tolleter D, Chochois V, Poiré R, Price GD, Badger MR(2017) Measuring CO2 and HCO3− permeabilities of isolated chloroplasts using a MIMS-18O approach. J Exp Bot 68: 3915–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T, Auroy P, Adriano JM, Beyly A, et al. (2011) Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23: 2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]