Abstract
The structure of CO2 hydration complex I is not compatible with the view on how this complex is embedded and functions in the cyanobacterial carbon-concentrating mechanism.
Dear Editor,
Recently, Schuller et al. (2020) published the first structure of a cyanobacterial CO2-hydrating photosynthetic complex I (PCI), which plays a fundamental role in oxygenic photosynthesis. This type of photosynthesis is by far the most important process for organic carbon acquisition on Earth. Oxygenic photosynthesis evolved in cyanobacteria about 2.7 billion years ago. Photosynthetic CO2 fixation is primarily done by the enzyme Rubisco, producing two molecules of 3-phosphoglycerate. Those are then reduced and converted into various organic carbon compounds via the Calvin-Benson-Bassham cycle that predated oxygenic photosynthesis (Hohmann-Marriott and Blankenship, 2011). The rising level of molecular oxygen introduced two main problems to Rubisco activity.
First, oxygen competes with CO2 in the cleavage of ribulose 1,5-bisphosphate (RuBP) and thus slows the carboxylase reaction. The binding of oxygen in the oxygenase reaction forms the toxic product 2-phosphoglycolate, which needs to be salvaged via photorespiratory metabolism (which probably coevolved with oxygenic photosynthesis in ancient cyanobacteria; Eisenhut et al., 2008). Second, the tremendous success of oxygenic phototrophs, particularly after the appearance of eukaryotic algae and plants, resulted in a strong decline of atmospheric CO2, slowing Rubisco activity due to its low affinity to CO2 and poor specificity (Tcherkez et al., 2006). Cyanobacteria, like many other photosynthetic organisms, solved these problems, at least partly, with the evolution of inorganic carbon- or CO2-concentrating mechanisms (CCMs; Raven et al., 2012).
By concentrating CO2 in close proximity to Rubisco, the CCM raises the apparent photosynthetic affinity for extracellular CO2 well above that of the enzyme. Hence, in addition to saturating the carboxylase activity, the CCM significantly reduces the oxygenase reaction. Among the various CCMs, the cyanobacterial type is considered the best understood (Raven et al., 2012). Here, light energy is used to fuel various uptake systems for CO2 and bicarbonate, leading to the accumulation of high concentrations of the latter in the cytoplasm. Bicarbonate enters bacterial microcompartments, the carboxysomes, where carbonic anhydrase (CA) converts it to CO2, thereby raising its concentration in close proximity to Rubisco mostly located in these bodies (Fig. 1). This enables efficient CO2 fixation into RuBP and a marked decline in the oxygenase activity of the enzyme (Kaplan and Reinhold, 1999; Rae et al., 2013; Burnap et al., 2015). Three different bicarbonate transport complexes were identified at the cytoplasmic membrane of model cyanobacteria (Fig. 1): the ABC-type bicarbonate transporter BCT1 and two bicarbonate/sodium antiporters, SbtA and BicA (Omata et al., 1999; Shibata et al., 2002; Price et al., 2004). In addition, specialized NDH1 complexes residing in the thylakoid membrane have been shown to act as low-affinity (NDH14) or high-affinity (NDH13; Fig. 1) CO2-uptake systems (Shibata et al., 2001). They convert cytoplasm-located CO2 to bicarbonate, thereby forming an inward concentration gradient for CO2 diffusion into the cells and also minimizing the leak of CO2 formed either by carboxysomal CA activity or dehydration of the cytoplasmic bicarbonate pool. These specific NDH1 complexes recruit the subunits CupA and CupS that form a cytoplasm-exposed structure visible in single-particle analyses (Shibata et al., 2001; Folea et al., 2008). It has been proposed that this part of the specialized NDH1 complexes is particularly responsible for the CO2 hydration; however, the mechanism remained elusive. Because cyanobacterial NDH1 complexes receive electrons from ferredoxin (unlike the mitochondrial NDH complex I, where NADH serves as an electron donor), it was recently renamed as PCI (Laughlin et al., 2019; Schuller et al., 2019).
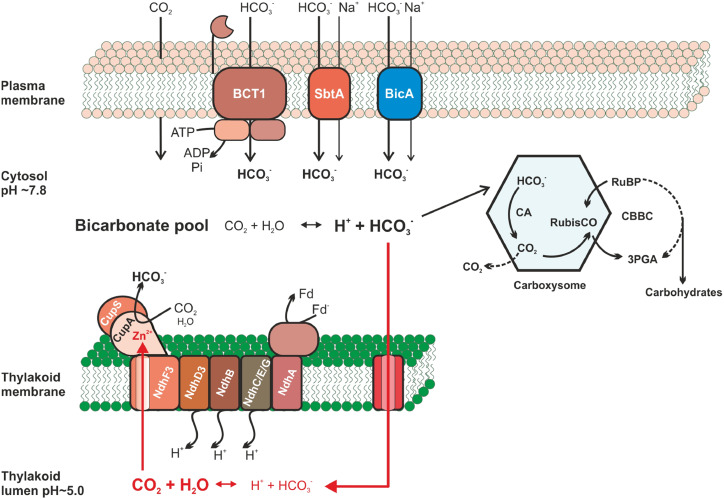
Figure 1.
Proposed model of the cyanobacterial CCM, including the structure-predicted bicarbonate/CO2 cycling in the thylakoid lumen. The cyanobacterial CCM (classical cycle in black arrows; structure-predicted bicarbonate/CO2 cycling in the thylakoid lumen in red arrows) utilizes three bicarbonate transporters: BCT1, SbtA, and BicA located in plasma membrane. The thylakoid-embedded CO2 hydration system (special CO2-hydrating PCI or NDH13 comprising the cyanobacteria-specific small subunits NdhD3, NdhF3 and CupA/B) converts cytoplasmic (classical view) or luminal (new prediction) CO2 to bicarbonate. The action of these uptake systems generates a large cytoplasmic bicarbonate pool, which penetrates into carboxysomes. There, bicarbonate is dehydrated back to CO2 by CA in proximity to Rubisco and bound to RuBP, leading to 3-phosphoglycerate (3PGA) that enters carbohydrate synthesis through the Calvin-Benson-Bassham cycle (CBBC). According to the newly resolved structure of the special CO2-hydrating PCI, the CA side is buried inside the CupA subunit (depicted as red Zn2+), which is connected to the luminal side of the thylakoids via a putative CO2 channel (Schuller et al., 2020). The most probable source for CO2 inside the lumen is a leakage of bicarbonate into the luminal space, where it becomes converted into CO2 due to the acidic pH.
Tremendous progress has been made in our understanding of the cyanobacterial CCM with the emergence of structures of carboxysomal proteins and the BicA transporter, mainly by cryoelectron microscopy (Kerfeld et al., 2018; Wang et al., 2019). The most recent hallmark in this development represents the structural analysis of the high-affinity CO2-hydrating PCI (formerly known as NDH13) from the cyanobacterium Thermosynechococcus elongatus (Schuller et al., 2020). The authors isolated a tagged version of this low CO2-induced complex and resolved its structure by cryoelectron microscopy at 3.2 Å resolution. The obtained structure allowed assignment of a Zn2+ atom bound to two helices of CupA near the NdhF3 subunit that is stabilized by CupS. The Zn2+ is a reactive metal center in most types of CAs (DiMario et al., 2018). Although the coordination of Zn2+ in the CO2-hydrating PCI differs from canonical CAs, the authors provided strong theoretical evidence based upon computational simulations that it is able to catalyze the conversion of CO2 into bicarbonate (Schuller et al., 2020), a thermodynamically unfavorable reaction due to the high cytoplasmic bicarbonate level. Hence, the structure analysis and the coupled theoretical calculations verified that the CupA/S-formed cytoplasm-exposed structure at NdhF3 represents the expected CA part of this PCI. It is proposed that the CO2 hydration process is closely coupled to a redox-driven mechanism including electron transport from ferredoxin toward plastoquinone and three proton extrusion steps from the cytoplasmic into the luminal side across the thylakoid membrane, which energizes the CO2 conversion (Schuller et al., 2020). The proton extrusion mechanism further explains how the predicted alkaline pocket is formed (Kaplan and Reinhold, 1999), which drives the CO2 hydration by the Zn2+-containing active site (Schuller et al., 2020). Contrary to previous models, the CA site appears inside the protein and not at its cytoplasm-exposed surface, where it was tentatively predicted to be, supported by the CA-like EcaB protein (Sun et al., 2019).
Summarizing, the proposed structure nicely explains the biochemical and molecular mechanisms of CO2 hydration by PCI as part of the large bicarbonate accumulation inside the cyanobacterial cell. However, the presented structure is not easily compatible with the traditional view of how this complex is embedded and functions in the cyanobacterial CCM. Most pronounced and surprising is the proposed occurrence of a putative CO2 channel in the structure. The structure revealed a nonpolar tunnel that is surrounded by hydrophobic and bulky amino acid residues, which connects the reactive Zn2+ inside CupA with the luminal side of the thylakoids. Molecular dynamic simulations made it possible to conclude that this channel might be the path by which CO2 reaches the CA site (Schuller et al., 2020). The existence of this channel in the structure implies that CO2 mostly reaches the CA site from the thylakoid lumen instead of from the cytoplasmic space, which is contrary to the classical view of CO2 appearance in the cytoplasm but not in the thylakoid lumen. Furthermore, the CA site with the bound Zn2+ is densely packed and surrounded by charged amino acid residues, which seem to shield it from cytoplasmic CO2 (Schuller et al., 2020). These findings are puzzling, since they are not compatible with the current CCM model for cyanobacteria (Rae et al., 2013; Burnap et al., 2015). This model assumes that the substrate CO2 used by PCI arises only in the cytoplasm from three different sources (Fig. 1): (1) CO2 uptake from the medium, the diffusion of which is accelerated due to aquaporin gating and rapid conversion of dissolved CO2 into bicarbonate in the cytoplasm; (2) leakage of nonfixed CO2 from the carboxysome into the cytoplasm; and (3) pH-dependent physicochemical conversion of the cytoplasmic bicarbonate into CO2. Hence, according to the existing CCM model, the cytoplasmic CO2 pool should have direct access to the CA site inside CupA of this special PCI, which is obviously not the case in the newly proposed structural model (Schuller et al., 2020).
Can we reconcile the structural with the physiological interpretations? If the predicted CO2 channel is working as postulated and CO2 is mostly reaching the CA site from the luminal side, then we must assume that the cytoplasm-accumulated bicarbonate is the most likely source of CO2 inside the lumen. The negatively charged bicarbonate ion could be driven into the lumen by its electrochemical gradient, using some pores or transporters (Fig. 1), and rapidly converted into CO2 due to the acidic lumen pH, which is built up by the photosynthetic electron transport. This is a vicious inorganic carbon cycle, breaking and forming bicarbonate and on the way dissipating the CCM. Furthermore, as the magnitude of the required CO2/HCO3− flux across the thylakoid is very high, it would be expected to dissipate the light-driven electrochemical proton gradient essential for ATP formation. If this scenario is correct, the cyanobacterial CCM includes a bicarbonate/CO2 cycle at the thylakoid membrane. This is reminiscent of the function of algal CCMs, where CO2 is released inside specific thylakoids traversing the Rubisco-containing structure, the pyrenoid (Matsuda et al., 2017). However, in cyanobacteria, Rubisco is located in the carboxysomes, which are not directly attached to the thylakoids.
According to the new proposed structure (Schuller et al., 2020), CO2 access is predicted to occur mainly from the luminal side of thylakoids, which would severely diminish the photosynthetic efficiency of cyanobacteria. How to solve this puzzling finding? First, the accessibility of the CupA-localized CA site to cytoplasmic CO2 is still possible, which is in agreement with the traditional view of the function of the CO2-hydrating PCI within the cyanobacterial CCM. Moreover, the putative CO2 channel still needs experimental validation. However, if the channel is really functional, this finding may suggest that the CO2-hydrating PCI might have additional/alternative functions. For example, can it also function to dissipate excess light energy or to dissipate the internal inorganic carbon pool upon darkening? In this case, the conversion of cytoplasmic CO2 may be of secondary importance. Clearly, more function-related studies with isolated CO2-hydrating PCI preparations are needed; these should include site-specific variants affecting the CO2 channel and the accessibility of the CA site.
Acknowledgments
We thank Dr. Eva-Maria Brouwer (University of Rostock, Plant Physiology) for the preparation of Figure 1.
Footnotes
Articles can be viewed without a subscription.
References
- Burnap RL, Hagemann M, Kaplan A(2015) Regulation of CO2 concentrating mechanism in cyanobacteria. Life (Basel) 5: 348–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario RJ, Machingura MC, Waldrop GL, Moroney JV(2018) The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci 268: 11–17 [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M(2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci USA 105: 17199–17204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folea IM, Zhang P, Nowaczyk MM, Ogawa T, Aro EM, Boekema EJ(2008) Single particle analysis of thylakoid proteins from Thermosynechococcus elongatus and Synechocystis 6803: Localization of the CupA subunit of NDH-1. FEBS Lett 582: 249–254 [DOI] [PubMed] [Google Scholar]
- Hohmann-Marriott MF, Blankenship RE(2011) Evolution of photosynthesis. Annu Rev Plant Biol 62: 515–548 [DOI] [PubMed] [Google Scholar]
- Kaplan A, Reinhold L(1999) The CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50: 539–570 [DOI] [PubMed] [Google Scholar]
- Kerfeld CA, Aussignargues C, Zarzycki J, Cai F, Sutter M(2018) Bacterial microcompartments. Nat Rev Microbiol 16: 277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin TG, Bayne AN, Trempe JF, Savage DF, Davies KM(2019) Structure of the complex I-like molecule NDH of oxygenic photosynthesis. Nature 566: 411–414 [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Hopkinson BM, Nakajima K, Dupont CL, Tsuji Y(2017) Mechanisms of carbon dioxide acquisition and CO2 sensing in marine diatoms: A gateway to carbon metabolism. Philos Trans R Soc Lond B Biol Sci 372: 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Price GD, Badger MR, Okamura M, Gohta S, Ogawa T(1999) Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA 96: 13571–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L(2004) Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA 101: 18228–18233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae BD, Long BM, Badger MR, Price GD(2013) Functions, compositions, and evolution of the two types of carboxysomes: Polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol Mol Biol Rev 77: 357–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Giordano M, Beardall J, Maberly SC(2012) Algal evolution in relation to atmospheric CO2: Carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos Trans R Soc Lond B Biol Sci 367: 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller JM, Birrell JA, Tanaka H, Konuma T, Wulfhorst H, Cox N, Schuller SK, Thiemann J, Lubitz W, Sétif P, et al. (2019) Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 363: 257–260 [DOI] [PubMed] [Google Scholar]
- Schuller JM, Saura P, Thiemann J, Schuller SK, Gamiz-Hernandez AP, Kurisu G, Nowaczyk MM, Kaila VRI(2020) Redox-coupled proton pumping drives carbon concentration in the photosynthetic complex I. Nat Commun 11: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, Kaplan A, Ogawa T(2002) Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: Function and phylogenetic analysis. J Biol Chem 277: 18658–18664 [DOI] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Kaneko T, Fukuzawa H, Tabata S, Kaplan A, Ogawa T(2001) Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: Genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci USA 98: 11789–11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Han X, Xu M, Kaplan A, Espie GS, Mi H(2019) A thylakoid-located carbonic anhydrase regulates CO2 uptake in the cyanobacterium Synechocystis sp. PCC 6803. New Phytol 222: 206–217 [DOI] [PubMed] [Google Scholar]
- Tcherkez GGB, Farquhar GD, Andrews TJ(2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA 103: 7246–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sun B, Zhang X, Huang X, Zhang M, Guo H, Chen X, Huang F, Chen T, Mi H, et al. (2019) Structural mechanism of the active bicarbonate transporter from cyanobacteria. Nat Plants 5: 1184–1193 [DOI] [PubMed] [Google Scholar]