Membrane scission is a fundamental reaction of vesicular transport. In clathrin-mediated endocytosis and similar coated vesicle transport pathways, vesicles bud from a parent membrane towards the cytosol. The nascent vesicle is initially connected to the parent membrane by a narrow membrane neck. Dynamin forms spirals that encircle these necks, hydrolyze GTP, and sever the necks. Dynamin is critical for endocytosis, as highlighted by its crucial role in the fast recycling of the plasma membrane at synapses [1]. Its importance in biology has made it the best studied of any membrane scission factor. More than a decade ago, it was discovered that dynamin hydrolyzes GTP to power the constriction of membrane tubes. However, the degree of constriction that was observed did not narrow the inner diameter of the tube enough to cross the energy barrier to scission. This and other questions spawned more than a decade of vigorous debate and experimentation.
In a recent paper in Cell, Morlot et al.[2] developed an apparatus in which the physical parameters of lipid tubes can be manipulated while the binding of dynamin and membrane scission are monitored in real time. This system provides a level of experimental control and quantitative insight more typically seen in experimental physics than in molecular and cell biology. The results show that constriction results from a GTP-powered torque, which contracts the dynamin coat by reducing the number of subunits per helical turn and more closely packing the subunits [3]. While constriction itself may not be enough for scission, the constriction leads to a sharp bend in the membrane at the edge of the constriction zone. The additional energy involved in this membrane bending is shown to be sufficient for scission at the edge of the constriction zone (Figure 1).
Figure 1. Dynamin, membrane bending and membrane scission.
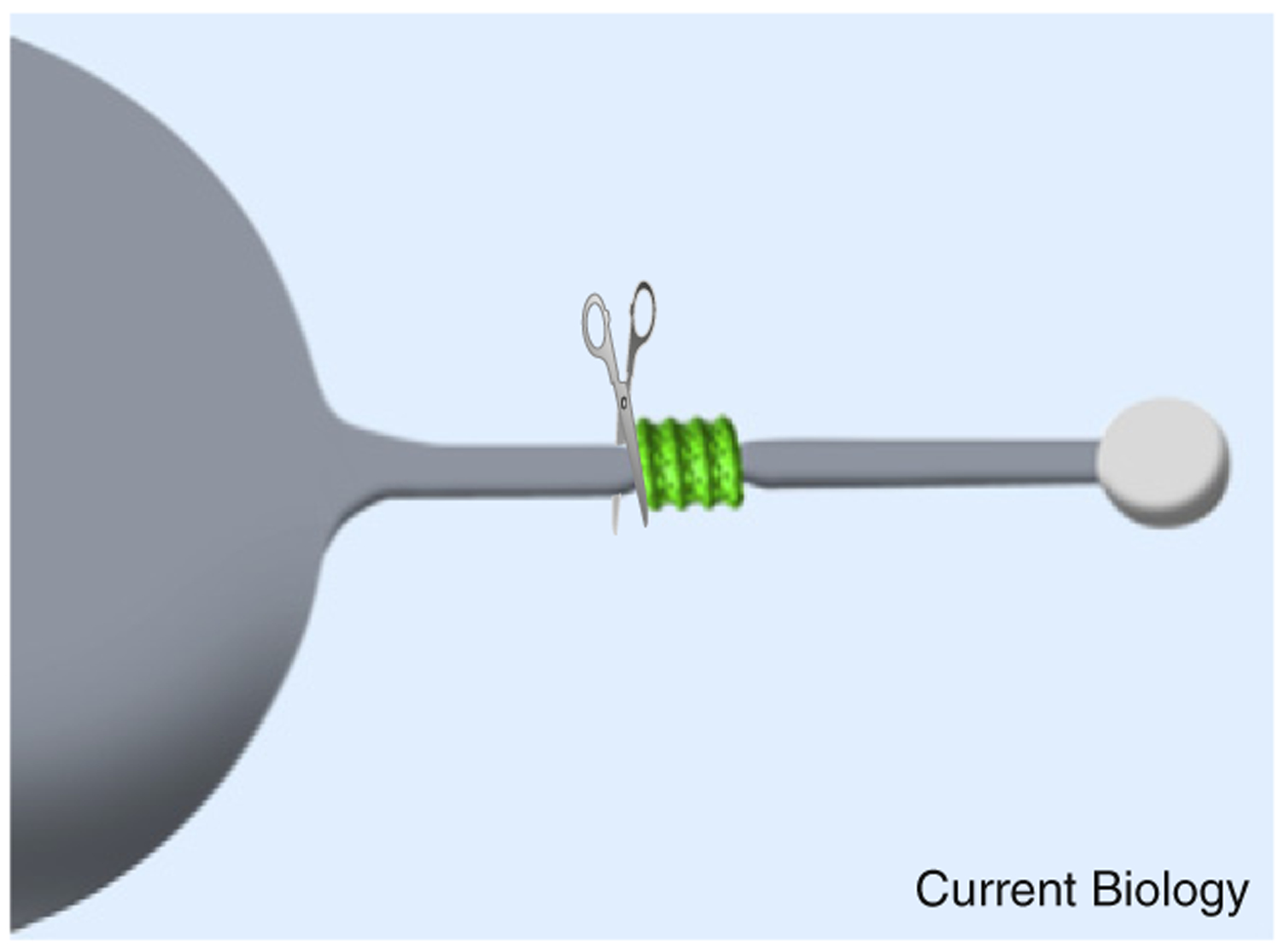
Constriction in a lipid tube (grey) pulled from a giant unilamellar vesicle as induced at the edge of the dynamin coat (green). The sharp bend in the membrane at the edge of the coat is important in promoting scission, which also occurs at the edge of the coat (denoted by scissors).
In the 1990s, purified dynamin was shown to self-assemble into spirals [4] and tubulate liposomes. The resulting tubes constrict, twist and fragment upon GTP addition [5,6]. This suggested that dynamin behaves as a mechanochemical catalyst of membrane fission. During the following decade and up to the present, there have been a burst of X-ray, NMR and cryo-EM structures of dynamin family members. These structures have included functional fragments and membrane-bound assemblies [7]. Key structures showed how the stalk domain drives self-assembly and how a three-bundle helix, connecting the GTPase domain to the stalk domain, undergoes a large swing upon GTP hydrolysis [8]. This power stroke most likely leads, somehow, to membrane fission. The biophysical mechanism of fission was not resolved by these structural studies, however. Considerable effort has gone into determining what membrane and biophysical properties are required for fission using a variety of real-time assays.
In the newly published work, Morlot et al. [2] show how GTP hydrolysis leads to fission at the edge of the dynamin assembly and show that dynamin stays bound to the lipid following fission [2]. Two other reports had suggested that the primary role of GTP hydrolysis was to disassociate dynamin from the membrane prior to fission [9,10]. It is clear from the elegant images in the study by Morlot et al. [2] that dynamin remains bound to the lipid tubes following fission. Future correlative light and electron microscopy studies may further resolve dynamin’s structural state during the fission process.
One of the key insights provided by the new study by Morlot et al. [2] is into the relationship between constriction and scission. An important consequence of constriction is to generate a sharp membrane bend at the edge of the dynamin coat. Membrane bending is energetically expensive, and the additional energy involved in the bend appears to be sufficient to cross the barrier to scission. By measuring the torque produced by dynamin, it was possible to calculate the work done by the dynamin coat upon GTP hydrolysis. The work was found to be great enough to bend the membrane to the extent needed to drive scission. Another key insight is that the membrane must be held under tension to be severed. An imperfect analogy to the tensionless situation would be an attempt to cut a limp noodle with a knife. To be more precise, without tension, the energy built up at the sharp membrane bend can be dissipated into the soft surrounding membrane. The membrane tension experiments were particularly critical to this study, in that they were replicated in a qualitative way for dynamin-mediated endocytosis in cells. The authors show that membrane tension is critical for membrane fission in the cellular setting.
Why has it taken fifteen years to go from the early observation of GTP and dynamin-powered scission to a biophysical mechanism? In some respects, dynamin has been experimentally and conceptually more tractable than other membrane scission machines. It is a single soluble protein, in contrast to the tightly membrane-associated multiprotein ESCRT III complex that severs vesicles budding away from the cytosol. Nevertheless, it is not easy to reach a high level of temporal, spatial, and energetic control simultaneously in systems where proteins are interacting with deformable membranes. The recent history of experimentation on dynamin-mediated scission in vitro has been one of successively closer approximations to an ideal experimental set-up.
Big advances in molecular biology normally derive either from better samples or from better instruments. The breakthrough in this case exemplifies instrumentation-driven progress. The authors adapted an ingenious methodology originally developed to study the curvature-dependent sorting of lipid in membrane tubules [11]. A device was built in which a lipid tube can be pulled out of a giant unilamellar vesicle (GUV) using optical tweezers. The membrane tension and the radius of the tube are controlled by a micropipette attached to the GUV. Both the membrane and dynamin are fluorescently labeled for visualization in real time, and the reaction is triggered by addition of GTP. Membrane scission rates can be analyzed as a function of membrane tension, GTP concentration, and lipid bending modulus in this system. In a particularly elegant twist, magnetic beads were attached to the dynamin coat, and the reaction was carried out in a magnetic field. This allowed the authors to measure the maximum torque generated by dynamin. This is important because the work done in membrane deformation is the integral of the torque exerted with respect to the rotation undergone by the dynamin coat. This apparatus allowed the authors to conduct a series of experiments that could be compared quantitatively to the predictions of membrane elasticity theory. The quantitative agreement that the authors achieve between theory and experiment is what brings dynamin into the world of physics, with its corresponding advance in insight and confidence in the model.
It is to be hoped that this study will put to rest some of the big questions that have dogged the dynamin field, including the roles and relative timing of GTP hydrolysis, tube constriction, torque, membrane tension, scission, and dissociation. Many questions remain to be answered at the structural level regarding how dynamin accomplishes all of this. These structural details could be quite important in destabilizing the membrane at the molecular scale, for example, as proposed for the amphipathic helices of BAR domain proteins [12].
With respect to other membrane scission machines such the ESCRT complexes [13], it will be important to determine whether sharp bending at the edge of a constriction is a general feature of such reactions. On the other hand, the GTP-propelled torque of dynamin is likely to be a specialized feature of the dynamin system. The precise role of nucleotide hydrolysis in powering scission reactions in other scission pathways, such as the ESCRT system, remains a contested question. Progress in the dynamin field, exemplified by the Morlot et al. paper [2], should be encouraging for the scientific optimists who believe that there is no paradox or controversy in molecular and cell biology that won’t eventually succumb to sufficiently clever technology.
References
- 1.Ferguson SM, and De Camilli P (2012). Dynamin, a membrane-remodeling GTPase. Nat. Rev. Mol. Cell Biol 13, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morlot S, Galli V, Klein M, Chiaruttini N, Manzi J, Humbert F, Dinis L, Lenz M, Cappello G, and Roux A (2012). Membrane shape at the edge of the dynamin helix sets location and duration of the fission reaction. Cell 151, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YJ, Zhang PJ, Egelman EH, and Hinshaw JE (2004). The stalk region of dynamin drives the constriction of dynamin tubes. Nat. Struct. Mol. Biol 11, 574–575. [DOI] [PubMed] [Google Scholar]
- 4.Hinshaw JE, and Schmid SL (1995). Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature 374, 190–192. [DOI] [PubMed] [Google Scholar]
- 5.Sweitzer SM, and Hinshaw JE (1998). Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93, 1021–1029. [DOI] [PubMed] [Google Scholar]
- 6.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, and De Camilli P (1998). Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131–141. [DOI] [PubMed] [Google Scholar]
- 7.Faelber K, Held M, Gao S, Posor Y, Haucke V, Noe F, and Daumke O (2012). Structural insights into dynamin-mediated membrane fission. Structure 20, 1621–1628. [DOI] [PubMed] [Google Scholar]
- 8.Chappie JS, Mears JA, Fang SM, Leonard M, Schmid SL, Milligan RA, Hinshaw JE, and Dyda F (2011). A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell 147, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, and Frolov VA (2008). GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell 135, 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pucadyil TJ, and Schimd SL (2008). Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135, 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost J, Goud B, and Bassereau P (2009). Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc. Natl. Acad. Sci. USA 106, 5622–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucrot E, Pick A, Camdere G, Liska N, Evergren E, McMahon HT, and Kozlov MM (2012). Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell 149, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley JH, and Hanson PI (2010). Membrane budding and scission by the ESCRT complexes: It’s all in the neck. Nat. Rev. Mol. Cell Biol 11, 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]


