Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease (COVID-19), spreading from Wuhan to worldwide has been emerged since December 2019. Although scientists and researchers have been racing to develop specific therapeutic agents or vaccines against SARS-CoV-2 since the identification of the agent, either a drug or a vaccine has not been approved to treat or to prevent COVID-19 up to date. On the base of historical experiences, Convalescent Plasma (CP), a passive antibody therapy, has been evaluated as a hopeful and potential therapeutic option since the beginning of the COVID-19 outbreak. Immune plasma had been used previously for the treatment of H1N1 influenza virus, SARS-CoV-1 and MERS-CoV epidemics successfully. In this scope competent authorities are responsible to set up certain principles and criteria for the collection and clinical use of COVID-19 Convalescent Plasma (CCP). This document has been prepared to aid both for the convalescent plasma suppliers and the clinicians. The first part encompasses the supply of CCP and the second part lead the clinical use of CCP for the treatment of patients with severe COVID-19 infection.
Turkish Ministry of Health developed a guide on collection and clinical use of CCP and created a web-based monitoring system to follow-up the patients treated with convalescent plasma in universal. This follow-up process is thought to be crucial for the creation and development of current and future treatment modalities. This guide would be a pathfinder for clinicians and/or institutions those eager to conduct CCP treatment more effectively.
Keywords: COVID-19, Convalescent plasma, Plasma donation, Pandemic
1. Introduction
Coronaviruses are a large family of viruses that cause a common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV) [1].
The novel Coronavirus (2019-nCoV) was isolated from a cluster of patients who developed respiratory tract symptoms (fever, cough, shortness of breath) in Wuhan Province in late December and named as a new coronavirus COVID-19 [2]. On 30 January 2020, World Health Organization (WHO) has declared “Public Health Emergency of International Concern” as the 2019-nCoV outbreak continued to be spreaded outside of China and a pandemic on 11 March 2020 to be posed a threat to public health worldwide [3,4]. Although the fatality rate of 2019-nCoV was not as high as MERS-CoV it has kept on rather a high rate as the emergence of the outbreak. On the other hand it was likely to be related to all aspects of the pathogenesis of COVID-19 infection that has not been clarified as well as the fact that antiviral treatment for the agent has not been developed yet. Current treatment approaches include supportive therapy, such as prevention and treating seconder infections and complications. In this context, passive immunization has been became the main topic for the treatment based on historical experiences. “Passive Immune Transfer” is defined as the use of immune plasma for critically ill hospitalized patients for therapeutic purposes. Immune plasma had been used previously for the treatment of 2009–2012 H1N1 influenza virus, 2003 SARS-CoV-1 and 2012 MERS-CoV epidemics successfully [5,6]. Passive immune antibodies may neutralize viruses or reduce target organ damages. In this scope convalescent plasma or immunoglobulin has been decided to be collected from the patients who had been recovered from COVID-19 to prevent or to treat COVID-19 infection.
On 28 February 2020, WHO Blood Regulators Network published a position paper on the Use of Convalescent Plasma and Serum or Immune Globulin Concentrates. According to this paper; an organized program, to collect convalescent plasma or serum from recovered patients, could provide a potentially valuable alternative therapy before the availability of effective vaccines and/or antiviral therapies while data on effectiveness and safety of its use are obtained through orderly scientific studies was recommended. WHO has emphasized that those local regulatory authorities should organize administrative and legal arrangements for the collection of plasma or serum [7].
Food and Drug Administration (FDA) has worked out on a guidance to provide recommendations to health care providers and investigators on COVID-19 Convalescent Plasma (CCP) during the public health emergency. In this guide, it is recalled that immune plasma has already been studied in other respiratory infections in the past twenty years and stated that the use of CCP might be effective against the infection based on those experiences [8].
Since COVID-19 pandemic has been emerged, a number of the scientific studies on vaccine development, the use of existing pharmaceutical agents, or the development of new agents to prevent or to treat infection as well as alternative therapy methods like immune plasma are increasing in day by day. A limited number of studies have been reported the benefits of immune plasma therapy in COVID-19 patients [[9], [10], [11]].
Considering the scientific viewpoints, CCP is an option to treat patients with COVID-19 infection as far as to be collected and stored according to the universal standards. This guide was intended for an additional regulation to support the collection, preparation and clinical use of CCP containing the anti-SARS CoV-2 antibodies for the treatment of COVID-19 patients in Turkey.
1.1. Supply of COVID-19 convalescent plasma
1.1.1. Selection criteria of candidates for CCP donation
In this section, selection criteria of voluntary CCP apheresis donors, from which the plasma will be collected to be used in the treatment of COVID-19 were defined. Candidates of CCP should meet the standard eligibility requirements in accordance with the National Guide on Preparation, Use and Quality Assurance of Blood and Blood Components and European Directorate for the Quality of Medicines (EDQM) instructions with the exceptional criteria of “deferral period of 28 days for previous viral infection”. CCP donors should meet the additional eligibility requirements for the collection of CCP by plasmapheresis defined below, as well as the eligibility requirements for the whole blood donation in accordance with the National Legislation [12,13].
-
a.For the individuals of CCP donor candidates who has been infected with COVID-19 virus infection and have been recovered afterwards [14,15];
-
i.The diagnosis of COVID-19 infection should be based on the results of laboratory tests (positive PCR test from the nasopharyngeal swab sample or serological test for SARS-CoV-2 antibodies), AND
-
ii.At least 14 days passed after the complete clinical recovery (cough, fever, shortness of breath, weakness, etc.), AND
-
iii.At least 2 negative PCR test results (one of the tests must have been conducted within the last 48 h) from the nasopharyngeal swab samples. Test negativity is not required in case of 28 days have been passed since the clinical recovery,
-
iv.The record of these individuals related to the disease period should be complete, traceable and documented.
-
i.
-
b.Individuals who have had COVID-19 infection to be an CCP donor is based on voluntary. In order to become a volunteer CCP donor, candidates should meet the requirements for “Whole Blood Donation”;
-
i.In addition to the forms related with whole blood donation, “CCP Informed Consent Form”, and “Questionnaire Form of CCP Voluntary Donation” should be conducted to the candidates along with a short physical examination,
-
ii.Microbiological screening tests [(HBsAg, anti-HCV, anti-HIV 1-2 and anti-syphilis serologic tests and HBV-DNA, HCV-RNA, HIV 1,2-RNA Nucleic Acid Amplification Screening Tests (NAT)] should be negative.
-
i.
-
c.
Convalescent plasma donors should preferably be selected from male, and female who have never been pregnant (birth / abortion / curettage), and individuals who have not received any blood transfusion. Women who have been pregnant and individuals who have had blood transfusion should be screened for HLA antibodies and should be negative,
-
d.
The antibody screening (indirect-coombs) test result of CCP donors should be negative,
-
e.
Convalescent plasma could be donated no more than 3 times in a month, at least once every 7–10 days, based on the date of the first donation that is accepted as the started date. Within a month at most 1800 mL of plasma can be collected from a donor.
1.1.2. Procedures to be applied to COVID-19 immun plasma donors and the product
-
a.If technical facilities are available [14,15];
-
i.It is necessary to select plasma, those with anti-SARS-CoV-2 titers with a neutralizing antibody level 1:80 or above, or those with antibody level above this threshold level after the threshold level defined in the literature by ELISA test corresponding to this threshold level, (retention sample should be stored from the CCP donation for to test antibody titers at a later time when it is available),
-
ii.In order to maximize transfusion safety, it is recommended to subject the received plasma to “Pathogen Inactivation”.
-
i.
-
b.
Plasma should be collected via apheresis from 200 to 600 mL ignoring the amount of anticoagulant solution. In case of plasma is collected more than 200 mL the plasma should be divided to 200 mL volum and be labeled individulally including unique idenfification number(s),
-
c.
Collected plasma component should be labeled spesific (COVID-19 Immune Plasma) by using ISBT 128 Coding System. Blood centers except than Turkish Red Crescent (TRC) those are collecting CCP should provide the provision of TRC before the collection of CCP,
-
d.
In terms of traceability witness sample should be stored in accordance with the National Regulation,
-
e.
CCP should be irradiated if the collected plasma would be used without freezing within 6 h after collection,
-
f.
CCP should be stored for one year of expiration date at −18 °C or cooler circumstances.
1.2. Clinical use of COVID-19 convalescent plasma
1.2.1. Patients selection criteria for CCP
Currently, there is no controlled study available to clearly define the patient group on which the use of CCP is effective. Only case series with a small number of patients have been published to date. In these reports, CCP has been added to the existing treatments in patients who were unresponsive to the existing protocol [[9], [10], [11]].
Duan et al. documented clinical success with the application of CCP to 10 severe COVID-19 patients in 16.5 days (median, range: 11–20 days) after the symptoms started. All patients had extensive pulmonary infiltrations and the severity criteria were defined with respiratory distress as; respiratory rate ≥30 /min, oxygen saturation < 93 % in resting state, and partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa). When compared to ten COVID-19 patients who did not receive CCP in the same center, CCP-group had significantly higher rates of survival and recovery with no appreciable adverse effect [9]. Zang et al. started CCP between 16–19 days to four critically ill patients two of which were treated with V—V ECMO. All four patients (including a pregnant woman) recovered from SARS-CoV-2 infection [10]. Similarly, Shen et al. reported a case series of five critically ill COVID-19 patients. Clinical statuses of all patients were improved after administration of CCP between 10–22 days after admission [11].
Any serious adverse effects has not been reported in a limited number of observations... However, Fleming and Raabe have emphasized, in their letter to Journal of Medical Virology, that potential adverse events such as allergies, acute lung injury and antibody-dependent enhancement should not be overlooked [16].
Convalescent plasma has usually been administered to severely/critically ill patients in the case series reported so far. However, it is not recommended to apply this treatment after the initiation of cytokine storm. In a guidance issued by FDA, CCP is recommended to be administered between 7–14 days in COVID-19 patients that comply with the specified criteria [8].
The criteria of this guidance for CCP treatment have been determined based on the current literature with the contributions of Turkish scientists Inittialy, CCP is recommended to the confirmed (PCR positive) or probable (clinical/radiological evidence compatible with COVID-19, but PCR test result not yet available) cases with COVID-19 infection who are above 18 years old within the first 14 days of the disease and 7–10 days after the symptoms started. It is not recommended beyond 14 days of the disease and the initiation of cytokine storm CCP should not be used in IgA-deficient patients. In case, IgA levels should be tested before the administration of CCP.
The efficiency of CCP treatment would be improved with correct indication and timing. Thus CCP treatment should be added to the existing treatment protocol and CCP should be used in critically ill patients who comply with at least one of the following criteria:
-
-
Persistent fever (7 days)
-
-
Lung infiltration > 50 % within 24–48 hours
-
-
Respiratory frequency ≥ 30/min
-
-
PaO2 / FiO2 < 300 mm Hg
-
-
Oxygen saturation <90 % despite nasal oxygen support ≥5 L/min
-
-
Partial oxygen pressure <70 mm Hg despite nasal oxygen support ≥5 L/min
-
-
Need for mechanical ventilation
-
-
Minimum 2 points increase in SOFA score
-
-
Need for vasopressor
-
-
Patients expected to have fast clinical worsening and those with poor prognostic parameters (severe lymphopenia; high CRP; high ESR, ferritine, LDH or d-dimer)
1.2.2. Request for COVID-19 convalescent plasma
The decision for the treatment of a COVID-19 patient with CCP should be approved by a committee consisting of an infectious disease specialist, a pulmonologist and a critical care specialist.
Informed consent and approval of therapy related to CCP is obtained from the patient or his/her first-degree relatives. If therapy is deemed urgent, the above-mentioned committee may decide to apply CCP despite the failure to meet this condition. CCP therapy should be prescribed with COVID-19 Immune Plasma Request Form (Table 1 ) that should include a brief clinical history of the patient.
Table 1.
COVID-19 CONVALESCENT PLASMA REQUEST FORM.
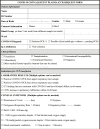 | |
 |
The recommended minimum dose for one patient is one unit (200 mL per unit) of CCP. Second unit can be administered 48 h following the completion of the transfusion of the first unit of convalescent plasma and can be administered up to a maximum of 3 units (600 mL). The decision for the total dose is taken by the physician in charge and based on the clinical and laboratory findings of the patient. Scientific rationale of this application is to avoid volume overload in these patients who are instable in terms of cardiopulmonary functions [16].
The ABO blood group of the patient should be compatible with the CCP (AB is a common donor for plasma transfusion). Rh group may be omitted.
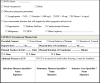
A web base monitoring system is created by Ministry of Health in scope of Table 2 to monitor patients those are treated with CCP.
Table 2.
COVID-19 CONVALESCENT PLASMA TREATMENT FOLLOW-UP FORM.
 | |
 |
2. Discussion
Different treatment approaches, including convalescent plasma, are currently being in use based on prior experiences with respiratory viruses since there is no specific therapeutic agent or vaccine for COVID-19 infection. In scope of the COVID-19 outbreak spread to Turkey, convalescent plasma therapy has been included into the treatment protocols of COVID-19 infection in the country as well as in many other countries (17 - 19). In this context, a “Guide on Supply and Clinical Use of COVID-19 Immune (Convalescent) Plasma” has been prepared and published in Turkey in order to standardize the collection, preparation, and use of CCP and to monitor all the processes including the follow-up of the patients those are treated with CCP.
The content of this guideline has been separated in to two sections. The first part encompasses the supply of CCP and the second part provides the clinical use of CCP for the treatment of patients with severe COVID-19 infection.
Various national and international guides were referred to establish donor selection criteria for the eligibility of CCP donation and preparation convalescent plasma [8,14,17]. Candidates that comply with the CCP donor selection criteria were sorted from the electronic health management system of the Turkish Ministry of Health, which was updated for additional questionnaire data for CCP donation. All recovered patients who were considered to meet the criteria for donation were selected and reached via phone at the end of the clinical recovery period, which has been indicated in the guidelines, to ask if they were willing to donate convalescent plasma. The list of the identified eligible volunteer candidates to donate plasma was shared with Turkish Red Crescent to be contacted for donation. An appointment was scheduled for the candidates who were eager to donate plasma. In addition, via social media and websites, to raise wider patients in those recovered from COVID-19 infection were encouraged to donate convalescent plasma. The main goal was to raise wider awareness to reach all donor candidates that are eligible for the convalescent plasma donation.
Although neutralizing antibody test was indicated as one of the criteria for donor selection, this test was not conducted due to an unavailability in our country. In order to perform neutralizing antibody test after achieving the capacity, blood sample was stored from each plasma donor for the further investigation since it is considered to be critically important in demonstrating the effectiveness of transfused convalescent plasma. With the onset of the outbreak, although plasma inactivation is not routinely performed in our country, pathogen inactivation system is established at Regional Blood Centers in Istanbul and Ankara for CCP, where the outbreak was more intensive.
The second part of the guideline was prepared for the patients who will receive convalescent plasma therapy. In scope of this section, indications for the clinical use of CCP were defined and the the request form of the CCP was created. In order to follow-up the patients treated with CCP in universal “Convalescent Plasma Treatment and Follow-up Care Report Form” was also created. These forms are filled by clinicians for each patient and submitted to the Turkish Ministry of Health via web-based information system to enable to evaluate the effectiveness of convalescent plasma therapy by the authority. This follow-up process is thought to be crucial for the creation and development of current and future treatment modalities.
Convalescent plasma seems to retain its importance as an alternative treatment method for patients with COVID-19 infection until a specific effective anti-viral agent or vaccine is found. In conclusion, this guideline may be an important reference guide for the collection, preparation and use of CCP, especially with the clinical follow-up section.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Declaration of Competing Interest
None.
References
- 1.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R., Features . StatPearls Publishing; Treasure Island (FL): 2020. Evaluation and Treatment Coronavirus (COVID-19) StatPearls [Internet] [PubMed] [Google Scholar]
- 2.She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med. 2020;9(February 20 (1)):19. doi: 10.1186/s40169-020-00271-z. https://doi.org/0.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) World Health Organization (WHO); 2005. Statement on the second meeting of the International Health Regulations.https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (Accessed 15 April 2020) [Google Scholar]
- 4.WHO . World Health Organization (WHO); 2020. Director-General’s opening remarks at the media briefing on COVID-19-11.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 March (Accessed 15 April 2020) [Google Scholar]
- 5.WHO Blood Regulators Network . 2017. Position Paper on Use of Convalescent Plasma, Serum of Immune Globuline Concentrates as an Element in Response to an Emerging Virus.https://www.who.int/bloodproducts/brn/2017_BRN_PositionPaper_ConvalescentPlasma.pdf?ua=1 (Accessed 15 April 2020) [Google Scholar]
- 6.WHO Blood Regulators Network . 2014. Position Paper on Collection and Use of Convalescent Plasma or Serum as an Element in Middle East Respiratory Syndrome Coronavirus Response.https://www.who.int/blood-products/brn/BRN_PositionPaperConvPlasmaMERSCoV_March2014.pdf?ua=1 (Accessed 15 April 2020) [Google Scholar]
- 7.WHO Blood Regulators Network (BRN) 2020. Interim position Paper on blood regulatory reponse to the evolving out-break of the 2019 novel coronavirus SARS-CoV-2.https://zdravstvo.gov.hr/UserDocsImages//2020%20Transplantacija%20i%20biomedicina//BRN%20Position%20COVID-19%202020-.pdf (Accessed 15 April) [Google Scholar]
- 8.FDA . 2020. Investigational COVID-19 Convalescent Plasma Emergency INDs.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds (Accessed 15 April) [Google Scholar]
- 9.Duan K., Liu B., Li C. et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS, 117; 17:9490-9496. https://doi.org/10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed]
- 10.Zhang B., Liu S., Tan T. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. (article in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients withCOVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Guide on Preperation . 2020. Use and Quality Assurance of Blood and Blood Components.https://www.kanver.org/Upload/Dosya/ulusal_kan_rehberi.pdf (Accessed 15 April) [Google Scholar]
- 13.Council of Europe European Committee on Blood Transfusion (CD-P-TS). 19th Edition of the Guide to the Preparation, Use and Quality Assurance of Blood Components. https://www.edqm.eu/en/blood-guide (Accessed 15 April 2020)
- 14.Epstein J., Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang. 2020;(April 22) doi: 10.1111/vox.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2020. COVID-19 Convalescent Plasma Collection: Donor Eligibility, Processing, Labeling, and Distribution. (AABB protochol) http://www.aabb.org/advocacy/regulatorygovernment/ Documents/COVID-19-Convalescent-Plasma-Collection.pdf (Accessed 15 April) [Google Scholar]
- 16.Fleming A.B., Raabe V. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody-dependent enhancement. J Clin Virol. 2020;28(April (127)) doi: 10.1016/j.jcv.2020.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-19 Blood supply document library . 2020. Documents on securing blood supply during COVID-19, ISBT.https://isbtweb.org/covid-19resources/covid-19-blood-supply-document-library/ (Accessed 15 April) [Google Scholar]


