Dear Editor,
COVID-19 ARDS presentation often associates hypoxemia without appropriate dyspnoea, relatively preserved pulmonary compliance. Gattinoni et al. [1], [2] recently suggested that hypoxemia is mainly due to a large intrapulmonary shunt linked to loss of hypoxic vasoconstriction (HV). This has led some authors to suggest that using drugs to strengthen HV and/or inhibitors of endogenous vasodilator pathways could be helpful [3]. This study aimed to evaluate the effect on oxygenation of almitrine bismesylate (AB), inhaled nitric oxide (NO), or both in COVID-19 ARDS.
Twenty consecutive patients with COVID-19 pneumonia, fulfilling the Berlin criteria of ARDS with PaO2/FiO2 ratio < 120, and who were given NO inhalation and AB infusion tests, alone or together, were retrospectively investigated. All patients were deeply sedated and paralysed. Ventilator settings were set to volume-controlled mode, with a fixed tidal volume (Vt) of 6–8 ml.kg−1 of predicted body weight. Respiratory rate (RR) and a fraction of inspired oxygen (FiO2) was adjusted to maintain an end-tidal carbon dioxide tension and oxygen saturation within a range of 35–45 mmHg and 92–96%, respectively. PEEP was set according to the attending physician after a recruitment manoeuvre, and a decremental PEEP titration, inspiration/expiration (I/E) ratio was set, until trapping, according to the attending physician. Vt, RR, I/E ratio, and PEEP remained unchanged throughout the entire test period. NO was administered sequentially, at a dose of 10 to 20 ppm during inspiration within the ventilator inspiratory limb just after the humidifier via a delivery system (NO-A, EKU Elektronik, Germany). AB was infused at a loading dose of 0.5 mg.Kg−1 over 30 minutes with an electric syringe pump. The entire NO and AB tests, alone or together, were performed in prone position.
The choice between NO or AB or both was left to the discretion of the clinician, according to the practice of our ICU: we first use NO in cases of refractory hypoxemia despite PEEP titration, recruitment manoeuvre and prone position. AB is used alone or in combination with NO in case of NO ineffectiveness, and in the absence of pulmonary arterial hypertension. In the particular case of the COVID-19 epidemic, due to the large number of patients, not all patients were able to benefit from first-line NO, which explains why AB was used more often than NO.
Arterial Blood Gas analysis were performed just before NO or AB introduction, at one hour after inhaled NO beginning and immediately at the end of AB infusion.
In accordance with previous studies [4], patients with ≥ 20% improvement of PaO2/FiO2 ratio were defined as responders.
As it is a retrospective study, Sainte-Anne military teaching hospital's ethics committee gave its approval. Data are shown in median value with range (quantitative ones) and in number with percentage and 95% confident interval (95% CI) (qualitative ones)
The number of test was distributed as follows: NO alone 10, AB alone 13, combining both 7. The tests were performed in median on the 8th day (range: 5–13) after invasive ventilation, no patients had pulmonary superinfection. The median age was 73 years (range: 45–76).
Only one AB test was performed in a patient with norepinephrine (dose of 0.12 μg.Kg−1.min−1). The median PaO2/FiO2 was 106 (range: 69.1–120) with a median PEEP of 16 cmH20 (range: 10–20), median respiratory system compliance of 33.3 ml.cmH2O−1 (range: 17.5–40), and a median driving pressure of 14 cmH2O (range: 11–16). The median PaCO2 was 51.8 mmHg (range: 42.9–67), with a median PaCO2-EtCO2 difference of 11.3 (range: 1.9–27).
1. Effect of NO inhalation alone
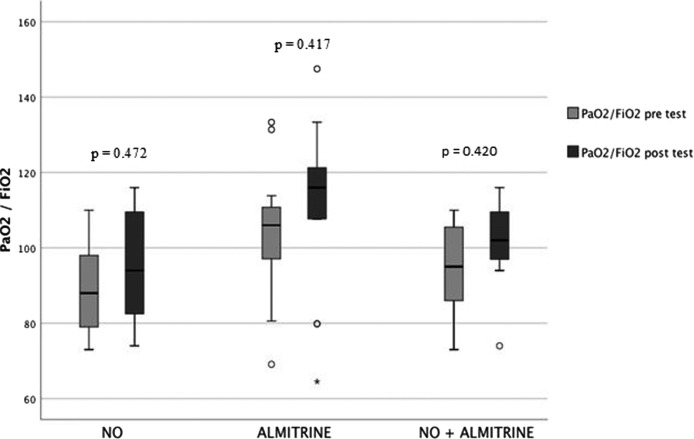
The median increase of PaO2/FiO2 ratio was 2.2% (95% CI: 1.3–12) (from 88 (range: 73–110) to 94 (range: 74–116)). No NO test resulted in an increase ≥ 20%. There was no significant difference between patients who tested at 10 ppm and those tested at 20 ppm.
2. Effect of almitrine bismesylate infusion alone
The median increase of PaO2/FiO2 ratio was 1.9% (95% CI: −4.8–11) [from 101.2 (range: 69.1–120) to 108 (range: 64.5–147)]. Only one patient was responder.
3. Effect of combined inhaled NO and almitrine bismesylate infusion
The median increase of PaO2/FiO2 ratio was 5% (95% CI: 1.4–7.8) [from 95 (range: 73–110) to 102 (range: 74–116)]. No patient was responder.
Used of inhaled NO and AB alone or together did not increase significantly PaO2/FiO2 ratio (Fig. 1 ).
Fig. 1.
effect on oxygenation of inhaled nitric oxide, almitrine bismesylate and inhaled nitric oxide + almitrine bismesylate.
In this small sample, the use of NO or AB, or both did not improve oxygenation in moderate to severe COVID-19 ARDS. This suggests that the loss of HV may not be the main reason for deep hypoxemia, as indicated by Gattinoni et al. [1].
4. Some limitations should be acknowledged
It is possible that the use of AB cannot sufficiently restore the loss of HV to improve oxygenation, and that the ineffectiveness of NO is due to a low inequality of the ventilated areas.
The majority of tests were performed after the first week of evolution under invasive ventilation. Indeed, patients worsened after the 5th day with a drop in the respiratory system compliances and an increase of hypoxemia (in spite of protective mechanical ventilation and in the absence of pulmonary superinfection). This explains why the use of NO and/or AB was performed during the second week of evolution. This evolution also seems unusual to us, without really being able to give a precise explanation (inflammatory evolution?).
In conclusion, in this small sample, pulmonary blood flow pharmacological manipulation did not improve ventilation/perfusion mismatching and therefore did not correct severe hypoxemia in moderate to severe COVID-19. These results remain to be confirmed by a study on a larger cohort of patients.
Author's Contributions
Michael Cardinale and Pierre Esnault designed the study. Michael Cardinale wrote the paper, and is its guarantor. Michael Cardinale, Pierre J. Cungi, Pierre Esnault and Jean Cotte, managed the data collection. All of the authors have contributed to the writing of the manuscript and approved the final version.
Disclosure of interest
The authors declare that they have no competing interests. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The publication has not been published elsewhere.
This paper has not been presented at a conference.
Ethical Approval
Approval for the study was obtained from the Sainte-Anne Military Teaching Hospital ethics committee, N°: 0011873-2020-08.
Consent for publication
All patients whose data were used for this study have given their consent.
References
- 1.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [rccm.202003-0817LE.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;382:727–734. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer S.L., Sharp W.W., Weir E.K. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome (ARDS) and high altitude pulmonary edema (HAPE): therapeutic implications. Circulation. 2020;124:1727. doi: 10.1161/CIRCULATIONAHA.120.047915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillart T., Bazin J.E., Cosserant B., Guelon D., Aigouy L., Mansoor O. Combined nitric oxide inhalation, prone positioning and almitrine infusion improve oxygenation in severe ARDS. Can J Anaesth. 1998;45:402–409. doi: 10.1007/BF03012574. [DOI] [PubMed] [Google Scholar]