Abstract
Anemia is a commonly occurring comorbidity among patients with heart failure with preserved ejection fraction (HFpEF) but limited data exists on the cardiovascular phenotype of anemia in HFpEF. We sought to characterize the clinical features, exercise capacity and outcomes in patients with HFpEF to elucidate the phenotype and pathophysiology of anemia in HFpEF. Post-hoc analyses of participants enrolled in the RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure) trial was performed. Anemia was defined as hemoglobin <13g/dL in men and <12 g/dL in women. Multivariate adjusted regression modeling was done to assess for differences in peak oxygen uptake. Adjusted hazard ratios were generated to assess difference in hospitalization events using a Cox proportional hazards model. Anemic HFpEF patients were more likely to be older, male, and have worse renal function (p<0.05 for all). NT-proBNP, troponin I, pro-collagen III N-terminal peptide, C-telopeptide for type I collagen, uric acid, cystatin-c and galectin-3 (p<0.05 for all) levels were higher in anemic HFpEF patients. In adjusted models, anemic HFpEF patients had worse exercise capacity (peak oxygen uptake: 11.3 vs 12.1 mL/kg/min; p=0.004). The hazard for cardiac or renal cause of hospitalization in those with anemia was 2.0 (95% CI: 0.9–4.3). Anemic HFpEF patients have worse exercise capacity and are more likely to be hospitalized. A better understanding of the physiologic phenotypes of HFpEF patients may allow for greater personalization of treatment and prognostication in HFpEF patients.
Keywords: Heart Failure, Anemia, Cardiopulmonary Exercise Test, Echocardiography, Cardiac Magnetic Resonance Imaging
Introduction
Anemia occurs in approximately half of all individuals with chronic heart failure,1 and lower hemoglobin levels are independently associated with worse exercise capacity, longer and more frequent hospitalizations, lower quality of life, and poor cardiovascular outcomes.2 The comorbidity burden of anemia is similar in patients of heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).2,3 There is a complex and multifactorial relationship between anemia and outcomes in HFpEF, with left ventricular (LV) dilation, hypertrophy, remodeling, and increased myocardial work playing a contributory role.2 The differences in the characteristics and the impact of anemia on outcomes in HFrEF have been previously described,4 and but data there is limited information on the impact of anemia in a well phenotyped and followed HFpEF population from clinical trials.2 The RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure) trial enrolled and comprehensive phenotyped clinical features, cardiac imaging parameters, biomarkers, exercise capacity, and quality of life of HFpEF patients.5 We sought to characterize the anemia-related differences in clinical features, cardiac structure and function by imaging, exercise capacity, and outcomes in patients with HFpEF. We hypothesized that anemia in HFpEF has a distinct phenotype and pathophysiology, and that anemia is associated with worse exercise capacity, and clinical outcomes in RELAX trial participants.
Methods
The RELAX trial was a multicenter, randomized, double-blind, placebo-controlled, 24-week trial conducted by the Heart Failure Clinical Research Network and funded by the National Heart, Lung, and Blood Institute (NHLBI).5 The trial was conducted between October 1, 2009, and February 1, 2012, in 216 (215 with data on anemia) stable HFpEF patients enrolled across 26 centers in the United States and Canada randomized to 24-weeks of sildenafil versus placebo. The data for current analyses were obtained from the NHLBI BioLINCC data repository. Anemia was defined as hemoglobin <13g/dL in men and <12 g/dL in women. The study was conducted in accordance with the principles in the Declaration of Helsinki. Written and informed consent was obtained from all participants in the parent trial and the trial was approved by protocol review and data safety monitoring committee and the institutional review board at the respective sites.6
Study design, inclusion and exclusion criteria, and results have been previously described.5,6. In brief, patients enrolled in the RELAX trial had a left ventricular ejection fraction (LVEF) ≥50% measured in the last 12 months and had objective evidence of heart failure. Additionally, the enrolled patients had elevated N-terminal pro-B-type natriuretic peptide levels (NT-proBNP) ≥400 pg/mL or B-type natriuretic peptide ≥200 pg/mL or elevated invasively measured filling pressures [pulmonary capillary wedge pressure (>20 mm Hg on rest or >25 mm Hg on exertion)], in addition to peak oxygen consumption (VO2) ≤60% of the age and sex-adjusted normative values while achieving an exercise respiratory exchange ratio ≥1.0.
Participants underwent baseline testing and investigation before randomization. In brief, the baseline evaluation included history and physical examination, quality of life assessment using the Minnesota Living with Heart Failure Questionnaire (MLWHFQ), laboratory examination for routine assessment and biomarkers, 6-minute walk testing, echocardiography, cardiac magnetic resonance imaging (CMR; if in sinus rhythm), and cardiopulmonary exercise testing.6
Assessment of laboratory parameters and biomarkers was done as per the previously described RELAX trial protocol.6 Lab parameters that included, hemoglobin, glomerular filtration rate, serum electrolytes, creatinine, and blood urea nitrogen, were assessed locally at the respective trial sites. Analyses of plasma biomarkers representing myocardial injury or inflammation [high-sensitivity Troponin-I (hs-TropI), and high-sensitivity C-reactive protein], fibrosis [Pro-Collagen III N-terminal peptide, C-telopeptide for type I collagen (CITP), galectin-3], renal function (creatinine, uric acid, cystatin-c) and neurohormonal activation (endothelin-1, NT-proBNP, aldosterone) was performed at the Heart Failure Clinical Research Network biomarker core lab (University of Vermont, Burlington, VT).
Doppler echocardiography and CMR imaging was performed as per the study protocol,6 and measurements were carried out at the Heart Failure Clinical Research Network core echocardiography lab (Mayo Clinic, Rochester, MN) and Heart Failure Clinical Research Network core CMR imaging lab (Duke University, Durham, NC). Echocardiography and CMR parameters included aortic thickness, aortic distensibility, cardiac index, deceleration time, E/A ratio, E/e’ ratio, left atrial (LA) volume, LA volume index, medial and lateral e’ velocity, LV end-diastolic volume, LV end-diastolic volume index, LV ejection fraction, LV end-systolic volume, LV end-systolic volume index, LV mass, LV mass index, pulmonary artery systolic pressure, and stroke volume. Grading of LV diastolic dysfunction was done as per the American Society of Echocardiography 2016 guidelines.7
The cardiopulmonary exercise testing was performed on a bicycle or a treadmill as per the predefined RELAX protocol, the details of which have been previously described.5,6 Blinded analysis of the measurements was done at the Heart Failure Clinical Research Network core cardiopulmonary exercise testing lab (Harvard University, Cambridge, MA). Exercise capacity parameters evaluated included chronotropic index, chronotropic incompetence, exercise duration, peak heart rate and blood pressure, peak oxygen consumption (VO2), percent predicted peak VO2, peak VO2 at ventilatory anaerobic threshold, and respiratory exchange ratio. The percent predicted peak VO2 was calculated using age and sex-specific normative values as previously described.5 Additionally, 6-minute walk distance (6MWD) and percent predicted 6MWD was measured to assess the sub-maximal exercise capacity. The percent predicted 6MWD was calculated using normative values adjusted for age, sex, weight, and height.
Baseline characteristics including cardiac imaging and exercise parameters of study individuals were compared, stratified by anemia using descriptive statistics. In brief, continuous data were summarized as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were summarized as number with percentages and compared using the Chi-Square test. Continuous variables such as NT-proBNP, hs-TropI, CITP, and galectin-3 levels were found to be non-normal (based on histogram, Q-Q plot, and Shapiro Wilk test) and were log-transformed for analyses.
Linear regression models were used to assess the relationship between anemia and baseline evaluation for echocardiography, CMR parameters, and exercise capacity. Furthermore, baseline parameters which were found to be significant in the aforementioned models were further adjusted for the factors known to affect imaging variables and exercise testing based on prior studies.8,9 In brief, we included age, sex, race, body mass index, systolic blood pressure, diabetes mellitus, resting heart rate, smoking, glomerular filtration rate (from modified MDRD [Modification of Diet in Renal Disease study] equation), history of atrial fibrillation, chronic obstructive pulmonary disease, NYHA (New York Heart Association) class, ischemic heart disease, valvular heart disease, and anti-hypertension medications for the adjustment.
The association between anemia and hospitalization was assessed using Cox proportional hazard model adjusted for age, glomerular filtration rate, and NYHA functional class based on prior studies.8 Potential effect modification by status of anemia was assessed using multiplicative interaction term (anemia*treatment) with respect to change (from randomization to end of study) in peak VO2, 6MWD, and MLWHFQ score.
We also performed ranking of relative strength of association between peak VO2 and various clinical and demographic factors by using likelihood ratio test as previously described.10 Multivariate linear regression models with and without respective covariates were used to calculate the Chi-Square values for each covariate. Additionally, we calculated the H2FPEF score, which has been recently developed to assess the diagnostic probability of HFpEF in clinical setting.11 The HFpEF probability was calculated using regression coefficients for the H2FPEF score parameters as previously described.11 For all analyses, a two-tailed p-value <0.05 was considered statistically significant. All statistical procedures and analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Two hundred and fifteen participants from the RELAX trial had hemoglobin data and were eligible for analyses. The prevalence of anemia in the entire cohort was 35%. Those with anemia were likely to be older (median age 71 vs. 67 years; p=0.07) and males (64.5% vs. 44.6%; p=0.005) (Table 1). At the time of enrollment, the symptom severity, comorbidities burden, and quality of life were similar in HFpEF patients with and without anemia (Table 1). Diabetes mellitus (51.3% vs. 38.1%; p=0.06) and valvular heart disease (82.9% vs. 66.2%; p=0.009) was more common in people with anemia. Those with anemia had worse glomerular filtration rate (48.4 vs. 62.2 mL/min/1.73 m2; p<0.001), blood urea nitrogen (31.7 vs. 21.0 mg/dL; p<0.001), and creatinine levels (1.5 vs. 1.0 mg/dL; p<0.001) (Table 1). Apart from a trend for higher statin use in anemics (72.4% vs. 59.7%; p=0.06), the medication use was similar in both groups (Table 1). H2FPEF score was alike in both the groups [6 (IQR: 5, 8) vs. 6 (IQR: 5, 8); p=0.78] with comparable high estimated probability of HFpEF for the population with anemia and without anemia (96.7% vs. 95.4%; p=0.16) (Table 1).
Table 1:
Baseline Characteristics of Heart Failure With Preserved Ejection Fraction Patients With and Without Anemia
| Variable | Anemia (n=76) | Without Anemia (n=139) | p-value |
|---|---|---|---|
| Age (years), median, (IQR) | 71 (63, 79) | 67 (61, 76) | 0.07 |
| Body mass index (kg/m2), median, (IQR) | 32.9 (27.8, 38.9) | 33.3 (28.9, 38.7) | 0.47 |
| Body Surface Area (m2), median, (IQR) | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) | 0.49 |
| Women | 27 (36%) | 77 (55%) | 0.005 |
| Heart Rate (beats/min), median, (IQR) | 66 (59,77) | 70 (62, 79) | 0.10 |
| White | 68 (90%) | 129 (92%) | 0.40 |
| Systolic Blood Pressure, mm Hg, median, (IQR) | 122 (112, 135) | 128 (115, 139) | 0.09 |
| Atrial fibrillation/flutter | 42 (55%) | 69 (50%) | 0.43 |
| Chronic Obstructive Pulmonary Disease | 12 (16%) | 29 (21%) | 0.37 |
| Diabetes Mellitus | 39 (51%) | 53 (38%) | 0.06 |
| Hyperlipidemia | 55 (72%) | 105 (76%) | 0.61 |
| Hypertension | 68 (89%) | 114 (82%) | 0.15 |
| Coronary Heart Disease | 31 (41%) | 53 (38%) | 0.70 |
| Malignancy | 1 (1%) | 7 (5%) | 0.17 |
| Myocardial Infarction | 7 (9%) | 18 (13%) | 0.42 |
| Peripheral Vascular Disease | 13 (17%) | 18 (13%) | 0.41 |
| Valvular Heart Disease | 63 (83%) | 92 (66%) | 0.009 |
| JVP ≥8 cm | 35 (48%) | 60 (44%) | 0.63 |
| MLWFQ Score, median, (IQR) | 42 (28, 58) | 44 (30, 66) | 0.34 |
| NYHA class II | 33 (43%) | 68 (49%) | 0.44 |
| NYHA class III | 43 (57%) | 71 (51%) | 0.44 |
| Orthopnea | 25 (34%) | 48 (35%) | 0.97 |
| Peripheral Edema ≥2 | 18 (24%) | 26 (19%) | 0.39 |
| Blood Urea Nitrogen, mg/dL, median, (IQR) | 31.7 (23.0, 42.3) | 21.0 (16.0, 27.0) | <0.001 |
| Creatinine level, mg/dL, median, (IQR) | 1.5 (1.2, 1.8) | 1.0 (0.9, 1.4) | <0.001 |
| GFR, mL/min/1.73 m2, median, (IQR) | 48.4 (33.8, 63.4) | 62.2 (49.0, 80.1) | <0.001 |
| Hemoglobin, g/dL, median, (IQR) | 11.5 (11.0, 12.2) | 13.5 (13.0, 14.5) | <0.001 |
| Sodium level, mEq/L, median, (IQR) | 140 (139, 142) | 140 (138, 141) | 0.24 |
| H2FPEF Score*, median, (IQR) | 6 (5, 8) | 6 (5, 8) | 0.78 |
| H2FPEF Score based HFpEF Probability, Median | 96.7 (91.0, 98.6) | 95.4 (84.0, 98.1) | 0.16 |
| Percentage, (IQR) | |||
| ACE inhibitors or ARB | 51 (67%) | 93 (67%) | 0.98 |
| Aldosterone Antagonist | 11 (15%) | 12 (9%) | 0.18 |
| Calcium Channel Blocker | 22 (29%) | 44 (32%) | 0.68 |
| Statin | 55 (72%) | 83 (60%) | 0.06 |
| β-Blockers | 56 (35%) | 108 (65%) | 0.51 |
Abbreviations: ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; GFR = glomerular filtration rate; IQR=interquartile range; JVP = jugular venous pressure; MLWFQ = Minnesota Living with Heart Failure Questionnaire; NYHA = New York Heart Association.
Note: Data are presented as median (25th–75th percentile) or as number (percentage).
H2FPEF score is a composite of age>60 years; use of ≥2 anti-hypertensive medications, obesity, presence of atrial fibrillation, E/e’ ratio >9, and pulmonary artery systolic pressure >35 mm Hg. The score is available in 126 participants (77 with anemia, 49 without anemia).
Anemic HFpEF patients showed higher levels of profibrotic biomarkers galectin-3 (16.1 vs. 13.1 ng/mL; p<0.001), CITP (9.0 vs. 5.4 μg/L; p<0.001), and pro-collagen III N-terminal peptide (8.1 vs. 7.5 μg/L; p=0.06)]. NT-proBNP levels were significantly higher in anemic patients (976.9 vs. 633.3 pg/mL; p=0.008), whereas other neurohormonal activation markers were similar when compared to patients without anemia (Table 2). Higher levels of high-sensitivity Troponin I (hs-TropI) were noted in anemic patients (12.1 vs. 7.8 ng/L; p<0.001). However, high-senstivity C-reactive protein levels did not differ significantly between the two groups (Table 2). Anemic HFpEF patients also had evidence of worse renal function compared to patients without anemia (Table 2).
Table 2.
Biomarkers Profile of Heart Failure with Preserved Ejection Fraction Patients with and without Anemia
| Plasma Biomarkers | Anemia | (n = 76) | Without Anemia | (n = 139) | p-value* |
|---|---|---|---|---|---|
| Aldosterone (pg/mL), median, (IQR) | 201.0 (115.8, 267.1) | 76 | 184.6 (122.3, 290.4) | 137 | 0.93 |
| cGMP levels (pmol/mL), median, (IQR) | 73.4 (56.5, 94.8) | 76 | 80.4 (59.7, 103.6) | 137 | 0.35 |
| CITP level (μg/L), median, (IQR) | 9.0 (5.9, 14.9) | 76 | 5.4 (4.3, 7.5) | 137 | <0.001 |
| C-reactive protein (mg/dL), median, (IQR) | 4.3 (1.9, 8.3) | 76 | 3.5 (1.7, 7.9) | 137 | 0.27 |
| Creatinine level (mg/dL), median, (IQR) | 1.3 (1.1, 1.7) | 75 | 1.0 (0.8, 1.2) | 136 | <0.001 |
| Cystatin-C level (mg/L), median, (IQR) | 1.7 (1.3, 2.3) | 69 | 1.2 (1.0, 1.5) | 128 | <0.001 |
| Endothelin-I (pg/mL), median, (IQR) | 2.4 (2.0, 3.4) | 76 | 2.3 (1.9, 3.2) | 137 | 0.19 |
| Galectin-3 level (ng/mL), median, (IQR) | 16.1 (12.1, 21.9) | 74 | 13.1 (10.9, 15.8) | 133 | <0.001 |
| NT-proBNP(pg/mL), median, (IQR) | 976.9 (401.7, 2395.0) | 75 | 633.3 (203.7, 1388.0) | 137 | 0.008 |
| Procollagcn III NTP level (μg/L), median, (IQR) | 8.1 (6.6, 10.8) | 76 | 7.5 (5.6, 9.3) | 137 | 0.06 |
| Troponin I level (ng/L), median, (IQR) | 12.1 (6.5, 37.2) | 75 | 7.8 (4.2, 14.2) | 136 | <0.001 |
| Uric acid level (mg/dL), median, (IQR) | 8.0 (6.3, 9.7) | 75 | 6.7 (5.7, 8.1) | 136 | <0.001 |
Abbreviations: NT-proBNP = N-terminal pro B-type natriuretic peptide; PA = pulmonary artery; CITP=C-telopeptide for type I collagen; procollagen III NTP = procollagen type III N-terminal peptide; IQR-interquartile range.
Note: Data are presented as median (25th–75th percentile).
p-values generated after log transformation of the variables
On echocardiography, anemic HFpEF patients had a similar ejection fraction compared to those without anemia. Anemic patients had a greater LA volume (LA volume index: 54.5 vs. 42.1 mL/m2; p=0.02), LV volume (LV end-diastolic volume index: 57.7 vs. 49.9 mL/m2; p=0.05), relatively higher LV filling pressure (E/e’ ratio: 17.5 vs. 15.0; p=0.06), and right ventricular systolic pressure (pulmonary artery systolic pressure: 45.5 vs. 38.9 mmHg; p=0.07) (Table 3). Grading of LV diastolic dysfunction was similar in the patients with and without anemia (Supplementary Table). In the subgroup of patients who underwent CMR imaging, the ejection fraction was similar between the two populations, while anemic HFpEF patients had a higher stroke volume (80.1 vs. 67.9 mL; p=0.001) and indexed LV end-diastolic volume (65.1 vs. 60.0 mL/m2; p<0.001) (Table 3). The indexed LV mass was also greater in anemics (LV mass index: 70.3 vs. 60.0 gm/m2; p=0.005) compared to their counterparts without anemia. While other parameters were not statistically significant in multivariable-adjusted models (p>0.05 for all), stroke volume measured on CMR remained significant by the status of anemia (p=0.03).
Table 3:
Cardiac Imaging Profile of Heart Failure With Preserved Ejection Fraction Patients with and without Anemia
| Anemia | (n=76) | Without Anemia | (n=139) | p-value | |
|---|---|---|---|---|---|
| Echocardiographic Parameters | |||||
| Cardiac Index (L/min per meters2), median, (IQR) | 2.5 (2.1, 3.0) | 64 | 2.5 (2.0, 3.0) | 121 | 0.60 |
| Deceleration time (m/s), median, (IQR) | 190 (159, 223) | 67 | 182 (153, 215) | 126 | 0.19 |
| E/A ratio, median, (IQR) | 1.5 (0.9, 2.6) | 46 | 1.4 (1.0, 2.0) | 96 | 0.76 |
| E/e’ ratio, median, (IQR) | 17.5 (13.3, 25.0) | 63 | 15.0 (11.3, 20.0) | 125 | 0.06 |
| LA volume index (mL/m2), median, (IQR) | 54.5 (38.8, 62.8) | 45 | 42.1 (33.6, 55.2) | 104 | 0.02 |
| LA volume (mL), median, (IQR) | 104.2 (80.3, 137.8) | 45 | 87.8 (72.5, 110.5) | 104 | 0.03 |
| Lateral e’ velocity (m/s), median, (IQR) | 0.09 (0.06, 0.11) | 64 | 0.08 (0.06, 0.10) | 127 | 0.86 |
| LV diastolic dimension (cm), median, (IQR) | 4.6 (4.2, 5.2) | 59 | 4.6 (4.2, 5.1) | 104 | 0.84 |
| LV dimension/BSA, (cm/m2), median, (IQR) | 2.3 (2.0, 2.5) | 59 | 2.2 (2.1, 2.4) | 104 | 0.61 |
| LV end-diastolic volume index (mL/m2), median, (IQR) | 57.7 (46.5, 69.6) | 34 | 49.9 (43.5, 61.9) | 70 | 0.05 |
| LV end-diastolic volume (mL), median, (IQR) | 116 (87, 159) | 34 | 103 (87, 138) | 70 | 0.17 |
| LVEF (%), median, (IQR) | 60 (55, 65) | 73 | 60 (55, 65) | 139 | 0.91 |
| Medial e’ velocity (m/s), median, (IQR) | 0.06 (0.05, 0.07) | 66 | 0.06 (0.05, 0.08) | 130 | 0.23 |
| PA Systolic Pressure (mmHg), median, (IQR) | 45.5 (36.4, 51.4) | 54 | 38.9 (32.0, 51.0) | 84 | 0.07 |
| Stroke Volume (mL), median, (IQR) | 81.9 (65.8, 93.5) | 64 | 76.3 (62.3, 87.9) | 121 | 0.29 |
| Magnetic Resonance Imaging Parameters | |||||
| Aortic distensibility (10−3/mm Hg), median, (IQR) | 1.02 (0.67, 1.76) | 31 | 1.19 (0.63, 1.64) | 54 | 0.93 |
| Aortic Thickness (mm), median, (IQR) | 1.3 (1.1, 1.5) | 34 | 1.3 (1.1, 1.4) | 55 | 0.89 |
| Cardiac Index (L/min per meters2), median, (IQR) | 2.5 (2.2, 2.8) | 38 | 2.2 (1.9, 2.6) | 76 | 0.01 |
| LV end-diastolic volume index, (mL/m2), median, (IQR) | 65.1 (53.2, 75.7) | 40 | 60.0 (50.4, 72.5) | 76 | <0.001 |
| LV end-diastolic volume (mL), median, (IQR) | 132.8 (109.0, 156.3) | 40 | 108.5 (91.6, 133.1) | 76 | <0.001 |
| LV mass index (gm/m2), median, (IQR) | 70.3 (58.0, 85.6) | 40 | 60.0 (50.4, 72.5) | 76 | 0.005 |
| LV mass (gm), median, (IQR) | 148.8 (125.8, 176.3) | 40 | 126.5 (99.0, 168.9) | 76 | 0.01 |
| LVEF (%), median, (IQR) | 65.9 (54.5, 69.6) | 40 | 65.8 (58.9, 70.9) | 76 | 0.58 |
| LV-end systolic volume index, (mL/m2), median, (IQR) | 20.7 (17.5, 31.1) | 40 | 18.4 (13.9, 23.8) | 76 | 0.01 |
| LV-end systolic volume (mL), median, (IQR) | 46.0 (35.6, 64.7) | 40 | 35.9 (27.1, 52.7) | 76 | 0.02 |
| Stroke Volume (mL), median, (IQR) | 80.1 (70.5, 94.2) | 40 | 67.9 (59.2, 85.5) | 76 | 0.001 |
Abbreviations: BMI = body mass index; BSA = body surface area; E/A ratio = ratio of early diastolic to late diastolic mitral inflow Doppler velocity; e’ = tissue Doppler velocity in early diastole at mitral annulus; E/e’ = ratio of early diastolic mitral inflow velocity to tissue Doppler velocity in early diastole at mitral annulus; IQR-interquartile range; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction.
Note: Data are presented as median (25th–75th percentile).
In comparison to HFpEF patients without anemia, anemic HFpEF patients had a shorter exercise duration (9.2 vs. 9.9 minutes; p=0.03), lower absolute peak VO2 (11.3 vs 12.1 mL/kg/min; p=0.003), percent of predicted peak VO2 (38.6% vs 42.6%; p=0.001), and had a higher respiratory effort (respiratory exchange ratio: 1.11 vs. 1.07; p=0.007) during cardiopulmonary exercise testing (Table 4). Poorer sub-maximal exercise performance was observed in anemics as demonstrated by a lower 6MWD (303.5 vs. 317.0 m; p=0.03), percent predicted 6MWD (65.0% vs 72.1%; p=0.01), and peak VO2 at ventilatory anaerobic threshold (673.0 vs 730.5 mL/min; p=0.02) (Table 4). In multivariate-adjusted models, there was significant difference in peak VO2 (p=0.004) (Figure 1), percent of predicted peak VO2 (p=0.02), respiratory exchange ratio (p=0.04), and peak VO2 at ventilatory anaerobic threshold (p=0.02). The ranking of relative strength of association between peak VO2 and various clinical and demographic factors in multivariate adjusted models was also done for patients with and without anemia (Figure 2).
Table 4.
Exercise Capacity of Heart Failure with Preserved Ejection Fraction Patients with and without Anemia
| Exercise Parameter | Anemia | (n=76) | Without Anemia | (n=139) | p-value |
|---|---|---|---|---|---|
| 6-minute walk distance (m), median, (IQR) | 303.5 (193.5, 355.5) | 76 | 317.0 (241.0, 396.0) | 139 | 0.03 |
| Chronotropic incompetence (%) | 80% | 75 | 76% | 137 | 0.50 |
| Chronotropic index, median, (IQR) | 0.42 (0.25,0.62) | 75 | 0.52 (0.31,0.68) | 137 | 0.08 |
| Exercise Duration (min), median, (IQR) | 9.2 (7.3, 10.9) | 68 | 9.9 (7.8, 11.6) | 135 | 0.03 |
| Peak heart rate (beats/min), median, (IQR) | 104 (86, 122) | 75 | 111 (93, 128) | 137 | 0.02 |
| Peak systolic blood pressure (mmHg), median, (IQR) | 158 (132, 170) | 74 | 149 (130, 170) | 134 | 0.36 |
| Peak VO2 (ml/kg/min), median, (IQR) | 11.3 (9.6, 13.2) | 75 | 12.1 (10.7, 15.5) | 139 | 0.003 |
| Peak VO2 at VAT (ml/min) | 673.0 (544.0, 817.0) | 74 | 730.5 (550.0, 919.0) | 138 | 0.02 |
| Predicted 6-minute walk distance (% achieved), median, (IQR) | 65.0 (45.9, 77.2) | 76 | 72.1 (54.3, 84.7) | 139 | 0.01 |
| Predicted peak VO2 (% achieved), median, (IQR) | 38.6 (32.9, 44.6) | 75 | 42.6 (36.5, 51.3) | 139 | 0.001 |
| Respiratory exchange ratio, median, (IQR) | 1.11 (1.04, 1.19) | 75 | 1.07 (1.02, 1.15) | 139 | 0.007 |
Abbreviations: VO2=oxygen consumption; VAT=ventilatory anaerobic threshold; m=meters Chronotropic index = [peak HR — rest HR] / [(220-age) — rest HR].
Note: Data are presented as median (25th–75th percentile) or %.
Figure 1. Exercise Capacity in Heart Failure With Preserved Ejection Fraction Patients With and Without Anemia.
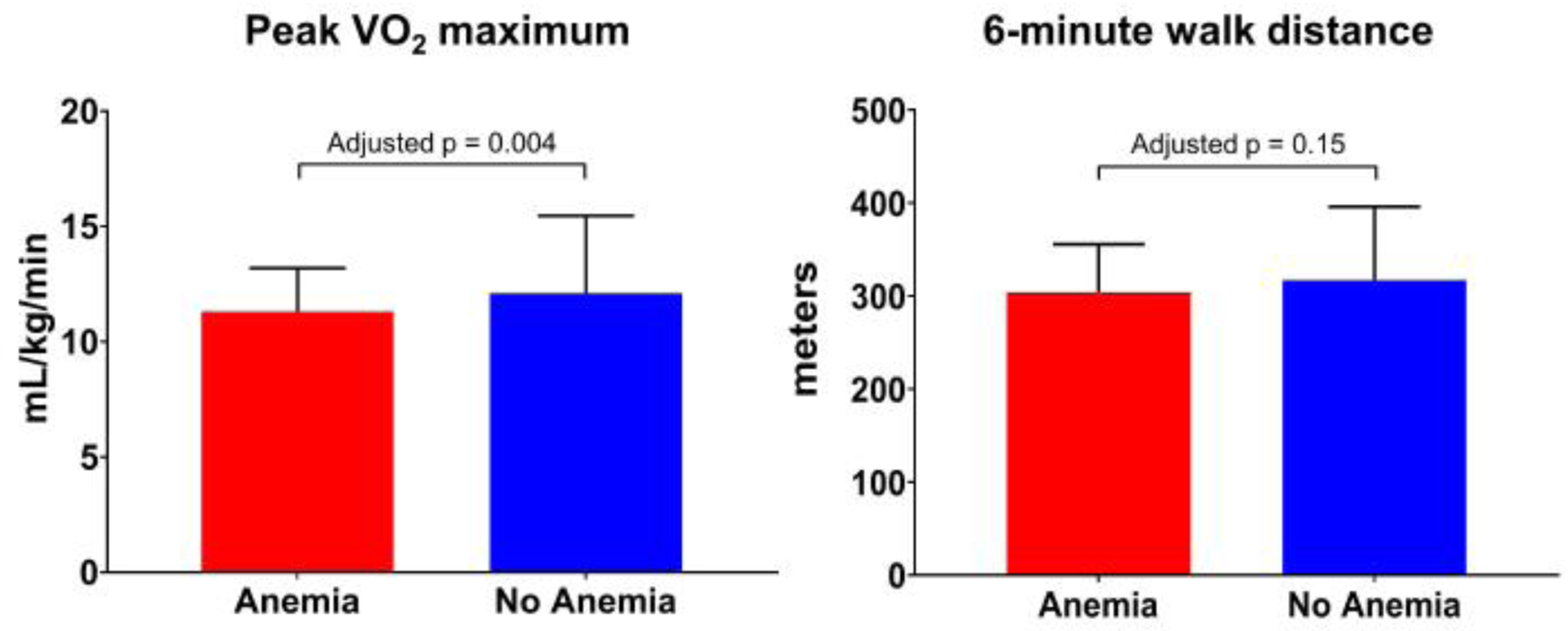
The red bars represent the values in patients with anemia. The blue bars represent the values in patients without anemia. Data are shown as median and IQR.
VO2: oxygen consumption.
Figure 2. Ranking of Strength of Association Between Peak VO2 and Clinical and Demographic Factors.
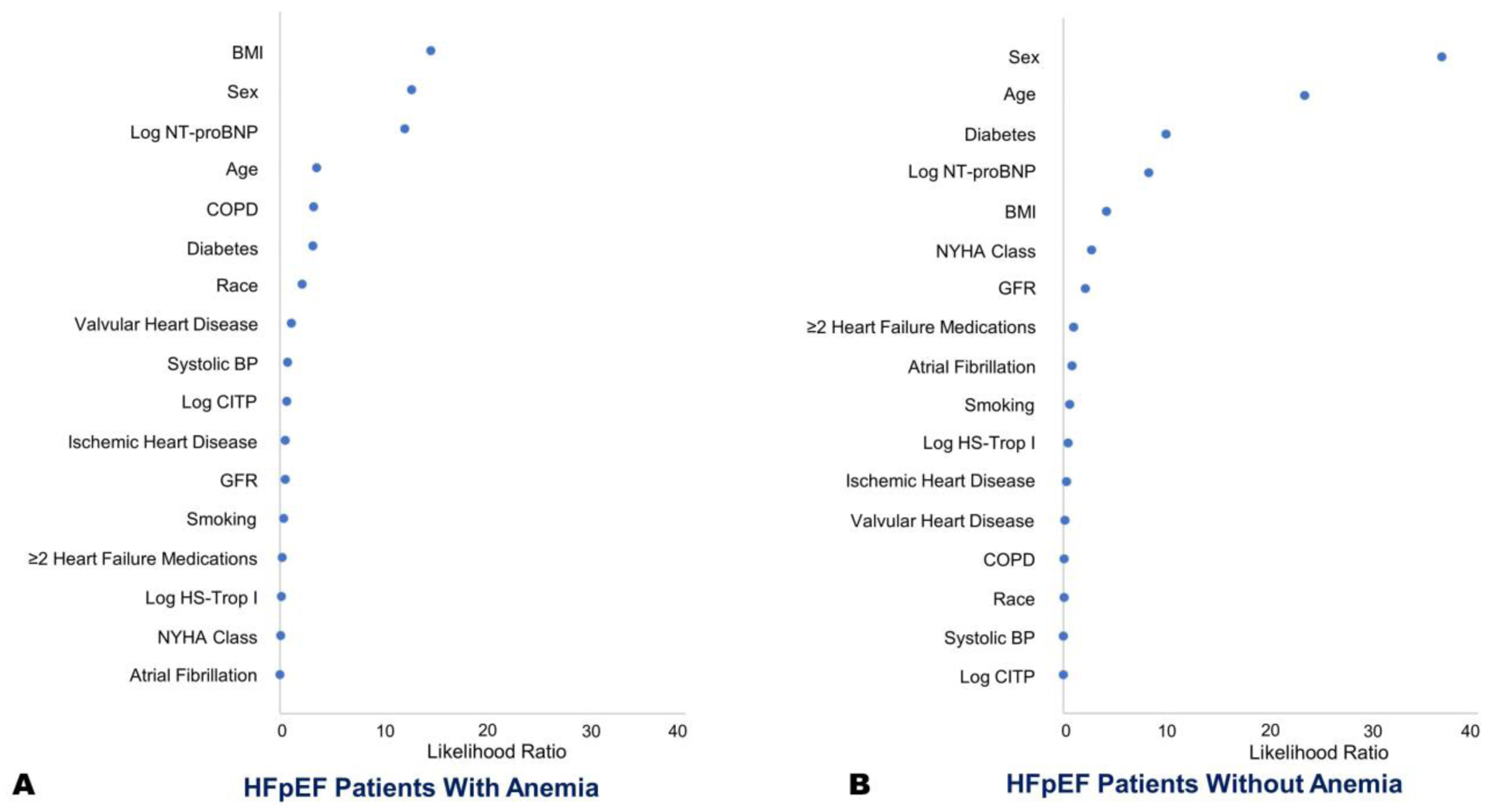
The panel shows likelihood ratios for various factors contributing to peak VO2 in patients with (Panel A) and without anemia (Panel B). Multivariate linear regression models with and without respective covariates were used to calculate the Chi-Square values for each covariate.
VO2: oxygen consumption.
Anemic HFpEF patients at the time of enrollment had a greater prevalence of at least one hospitalization in the preceding 12 months (47.4% vs. 30.2%; adjusted p=0.04). Anemic patients were more likely to be hospitalized (at least once) for cardiac or renal causes in the period after enrollment (19.7% vs. 9.4%: adjusted p=0.06) (Figure 3). After adjustment, the hazard for hospitalization due cardiac or renal causes at the end of 24-weeks was 2.0 (95% CI: 0.9–4.3). There was no significant interaction between treatment arm (sildenafil vs. placebo), and anemia for change in peak VO2, 6MWD, MLWHFQ score, and number of participants with one or more hospitalizations, respectively (p>0.10 for all).
Figure 3. Hospitalizations in Heart Failure With Preserved Ejection Fraction Patients With and Without Anemia.
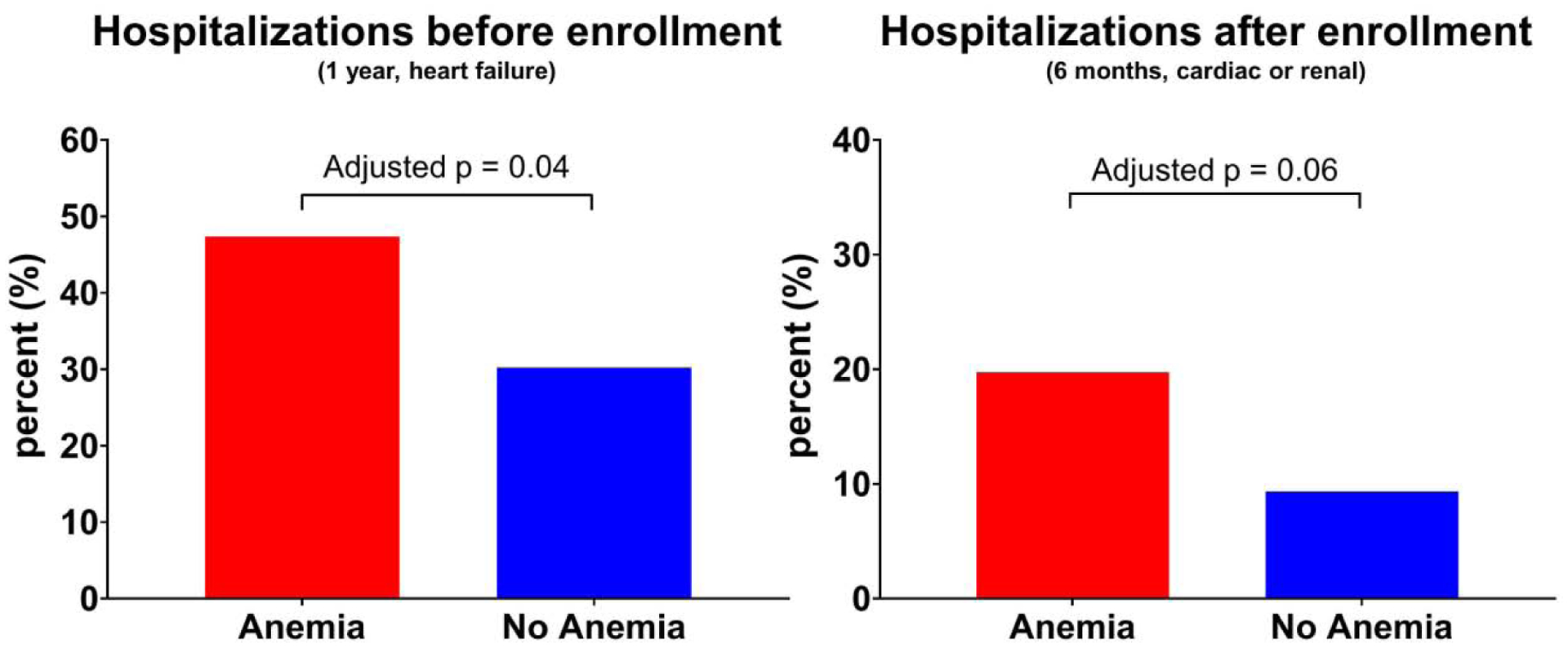
The red bars represent the values in patients with anemia. The blue bars represent the values in patients without anemia. Hospitalization prior to trial enrollment was defined as at least one heart failure hospitalization during the 12-month period before enrollment in the RELAX trial. Hospitalization after enrollment was defined as the percentage of patients with ≥1 hospitalization for cardiac or renal causes during the 6-month trial period. Data are shown as median and IQR.
Discussion
Our study provides a detailed characterization of the clinical, physiologic, and imaging phenotypic differences between HFpEF patients with and without anemia. Nearly 35% of HFpEF patients had anemia and these patients were clinically indistinguishable from those without anemia having a similar symptom severity, co-morbidity burden, and quality of life. However, there was greater evidence of fibrosis, oxidative stress, and myocardial injury as demonstrated by elevated galectin-3, Pro-Collagen III N-terminal peptide, NT-proBNP, and hs-TropI levels. Anemic patients had a worse functional capacity in comparison to HFpEF patients without anemia, as demonstrated by a significantly lower peak VO2 and 6MWD. Hospitalization rates before the study and during the follow-up period were higher in anemic patients.
Anemia has a multifactorial role in the pathophysiology of heart failure and has been previously reported in HFpEF patients with increasing age, diabetes mellitus, and renal dysfunction.12 A similar preponderance of comorbidities in anemic HFpEF patients was seen in the RELAX trial. Previous investigations in predominantly HFrEF populations suggest that chronic inflammation, inadequate erythropoietin production, bone marrow defects, marrow unresponsiveness, and iron deficiency contribute to the development of anemia.1,13, 14 Given the importance of iron in mitochondrial function, oxidative injury, and collagen synthesis,15 one can speculate its role in the underlying etiopathogenesis of HFpEF. We have studied the anemic phenotype of HFpEF independent of iron deficiency status, as we were limited by unavailability of data for iron deficiency markers. O’Meara et al.2 previously described the differences in anemic phenotype in HFpEF individuals from the CHARM trial, and observed worse renal function, differences in comorbidity burden, more hospitalization, and greater cardiovascular and all-cause mortality. We observed a similar finding in our cohort with poor renal function and more hospitalization among anemic HFpEF patients. Low rates of adverse outcomes in RELAX trial participants limited our ability to analyze the impact of anemia on mortality outcomes.
Anemic HFpEF patients had higher levels of biomarkers suggestive of worsening LV wall stress (NT-proBNP), increased oxidative stress (uric acid), ongoing myocardial injury (hs-TropI) and fibrosis (CITP, galectin-3, Procollagen III N-terminal peptide). Correlates of NT-proBNP are well recognized16 and its levels are used for risk stratification,16 prognostication, and grading of heart failure severity. NT-proBNP is stimulated by hypoxemia in healthy subjects which may possibly explain the observed difference in levels between the two groups.17 Biomarker profiles of anemic HFpEF patients as described in our study provide insight not only to disease severity and progression but also help to illustrate the pathophysiology in HFpEF.18
Anemia may be compensated for by an increase in stroke volume, a mechanism which may already be impaired in heart failure, resulting in poor oxygen delivery and impairment of functional capacity.19 In line with this physiological principle, we observed a higher stroke volume and reduced peak oxygen consumption and exercise capacity, in anemic HFpEF patients. Furthermore, the natural history of HFpEF is not well understood as literature mostly describes the disease progression after an index event.20 Exercise capacity is central to the understanding of the disease and outcomes. Reduced exercise capacity in HFpEF correlates with elevated filling pressures21 and usually precedes the development of overt HFpEF and acute decompensated heart failure. Decreased exercise capacity is an indicator of poor cardiovascular outcomes in patients with HFrEF, and with a modest increase in exercise capacityis associated with an improvement in cardiovascular outcomes.22 We found that anemic HFpEF patients had reduced exercise capacity and a higher risk for hospitalization. Physiologically, a reduced arteriovenous oxygen gradient due to reduced oxygen delivery or reduced oxygen extraction in exercising muscle is a significant determinant for reduced peak VO2.23 We observed a worse peak VO2 in the anemic HFpEF cohort, mirroring physiological role hemoglobin plays in oxygen delivery. Though it may be difficult to differentiate HFpEF patients with and without anemia based on clinical symptoms and comorbidity burden, literature suggests that exercise testing is valuable in HFpEF patients for risk stratification and prognostication.24
Burns et al.25 elaborated upon the association of anemia in HFpEF with markers of diastolic dysfunction, such as E/A ratio, E/e’ ratio, and pulmonary artery systolic pressure. With respect to echocardiographic markers, we observed a larger indexed LA volume, a trend for higher pulmonary artery systolic pressure, and E/e’ ratio in anemic HFpEF patients without any significant difference in E/A ratio, possibly due to inadequate study power. Additionally, on CMR imaging we demonstrated a higher indexed LV mass and a significantly higher LV end-diastolic volume in HFpEF patients with anemia. Increased LV mass has been showed to be an independent risk marker for adverse cardiac events with higher LV mass at baseline associated with incident HF.26 CMR imaging provides better spatial resolution and may elucidate more information for patients with HFpEF as the imaging modality gains utility in clinical practice.
HFpEF is now increasingly being recognized as a systemic disorder27 that involves multiple other organs including lungs skeletal muscle, adipose tissue, and kidneys with limited therapeutic options.28 HFpEF is characterized by multiple comorbidities existing alongside the cardiac dysfunction, and anemia appears to be part of the complex etiopathogenesis puzzle of HFpEF (Figure 4). The distinct biomarker profile we observed lays groundwork into exploring the role anemia and iron deficiency play in the pathophysiology of HFpEF and its impact on the disease morbidity. A better understanding of the pathophysiology may help us in addressing the pathological derangements by focusing on the development of novel therapeutic measures and by using pre-existing therapies like cardiopulmonary rehabilitation and iron supplementation. Understanding these differences in cardiopulmonary exercise testing in anemic HFpEF patients will allow for greater personalization of treatment and prognostication. Increasing awareness for the recognition of the anemic HFpEF phenotype as a high-risk group is important, particularly to stem the symptomatic deterioration and hospitalization. Multiple trials have tried to address the importance of anemia in heart failure29 by treating the frequently preceding and underlying iron deficiency.30,31 The CONFIRM-HF30 and FAIR-HF31 trial evaluated intravenous iron treatment in HFrEF and observed significant improvements in functional status and reduced risk for hospitalizations following treatment. The results of the ongoing FAIR-HFpEF study (NCT03074591) examining the role of intravenous iron therapy in improving symptoms in HFpEF patients are eagerly awaited.
Figure 4.
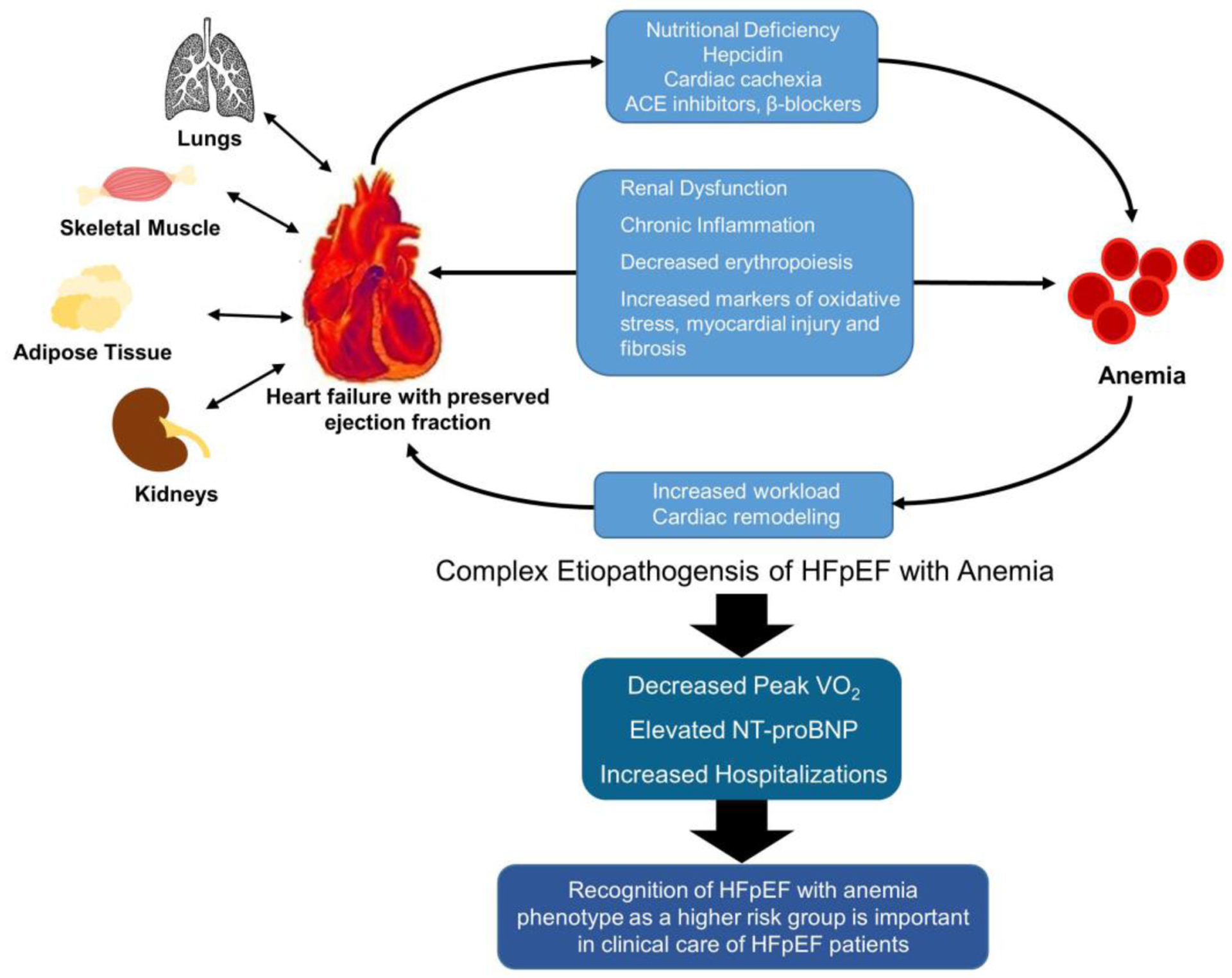
Insights into Cardiovascular Phenotype of HFpEF With Anemia
There are several limitations in our study while interpreting the results. There is a notable lack of other hematological parameters available to assess the anemia status and derive information on the iron deficiency. Serial hemoglobin assessments were also not available for the participants. RELAX trial outlined detailed phenotype of HFpEF patients, but due to limited number of patients and a relatively younger population, we may have been unable to detect smaller yet significant variations between the HFpEF phenotype with and without anemia. The limited sample size also restricted our ability to detect interactions and adjust for confounders in our statistical models.
In a multicenter, meticulously phenotyped cohort of HFpEF patients, we observed that differences in cardiac structure and function with evidence of underlying oxidative stress, fibrosis, myocardial injury, and poor renal function, may contribute to the impaired exercise capacity, and increased hospitalizations in anemic HFpEF patients. Instead of a singular derangement of myocardial function, a combination of multi-systemic factors may be responsible for the worse outcomes in HFpEF patients with anemia.
Supplementary Material
Acknowledgements
We would like to thank the RELAX trial investigators for making the study data available for public use through the NHLBI Biologic Specimen and Data Repository.
Source of Funding: This work was supported by National Institutes of Health Mentored Patient-Oriented Research Award [5K23HL146887-02] to Dr. Pankaj Arora.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors had any conflicts of interest or financial disclosures to declare.
References
- 1.Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575–582 e573. [DOI] [PubMed] [Google Scholar]
- 2.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, Young JB, Solomon SD, Granger CB, Ostergren J, Olofsson B, Michelson EL, Pocock S, Yusuf S, Swedberg K, Pfeffer MA, Committees C, Investigators. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 2006;113:986–994. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, Wong K, Rigby A, Goode K, Clark AL. Prevalence and Outcomes of Anemia and Hematinic Deficiencies in Patients With Chronic Heart Failure. JAMA Cardiol 2016;1:539–547. [DOI] [PubMed] [Google Scholar]
- 4.Komajda M, Anker SD, Charlesworth A, Okonko D, Metra M, Di Lenarda A, Remme W, Moullet C, Swedberg K, Cleland JG, Poole-Wilson PA. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: results from COMET. Eur Heart J 2006;27:1440–1446. [DOI] [PubMed] [Google Scholar]
- 5.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E, Heart Failure Clinical Research N. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail 2012;5:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent Liege B, Cleveland O, Novara I, Rochester M, Bucharest R, St Louis M. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 8.Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014;64:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauricio R, Patel KV, Agusala V, Singh K, Lewis A, Ayers C, Grodin JL, Berry JD, Pandey A. Sex differences in cardiac function, biomarkers and exercise performance in heart failure with preserved ejection fraction: findings from the RELAX trial. Eur J Heart Fail 2019. [DOI] [PubMed] [Google Scholar]
- 10.York MK, Gupta DK, Reynolds CF, Farber-Eger E, Wells QS, Bachmann KN, Xu M, Harrell FE Jr., Wang TJ. B-Type Natriuretic Peptide Levels and Mortality in Patients With and Without Heart Failure. J Am Coll Cardiol 2018;71:2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Tong W, Jain A, Francis GS, Harris CM, Young JB. Evaluation and long-term prognosis of new-onset, transient, and persistent anemia in ambulatory patients with chronic heart failure. J Am Coll Cardiol 2008;51:569–576. [DOI] [PubMed] [Google Scholar]
- 13.Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Open Heart 2019;6:e001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011;58:1241–1251. [DOI] [PubMed] [Google Scholar]
- 15.Dong F, Zhang X, Culver B, Chew HG Jr., Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond) 2005;109:277–286. [DOI] [PubMed] [Google Scholar]
- 16.Patel N, Gutierrez OM, Arora G, Howard G, Howard VJ, Judd SE, Prabhu SD, Levitan EB, Cushman M, Arora P. Race-based demographic, anthropometric and clinical correlates of N-terminal-pro B-type natriuretic peptide. Int J Cardiol 2019;286:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Due-Andersen R, Pedersen-Bjergaard U, Hoi-Hansen T, Olsen NV, Kistorp C, Faber J, Boomsma F, Thorsteinsson B. NT-pro-BNP during hypoglycemia and hypoxemia in normal subjects: impact of renin-angiotensin system activity. J Appl Physiol (1985) 2008;104:1080–1085. [DOI] [PubMed] [Google Scholar]
- 18.Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-La Rocca HP, investigators T-C. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 2015;17:1006–1014. [DOI] [PubMed] [Google Scholar]
- 19.Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, Steinbeck L, Kube J, Bekfani T, Scherbakov N, Valentova M, Sandek A, Doehner W, Springer J, Anker SD, von Haehling S. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co-morbidities Aggravating Heart Failure. Int J Cardiol 2016;205:6–12. [DOI] [PubMed] [Google Scholar]
- 20.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ Jr., Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH, New York Heart Failure C. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004;43:1432–1438. [DOI] [PubMed] [Google Scholar]
- 21.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou MC, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L, Kraus WE, Investigators H-A. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 2012;5:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadruz W Jr., West E, Sengelov M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H, Shah AM. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burns JA, Sanchez C, Beussink L, Daruwalla V, Freed BH, Selvaraj S, Shah SJ. Lack of Association Between Anemia and Intrinsic Left Ventricular Diastolic Function or Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction. Am J Cardiol 2018;122:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer MS, Teruya S, Chakraborty B, Helmke S, Mancini D. Treating anemia in older adults with heart failure with a preserved ejection fraction with epoetin alfa: single-blind randomized clinical trial of safety and efficacy. Circ Heart Fail 2013;6:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, Investigators C-H. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P, Investigators F-HT. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


