Abstract
Introduction
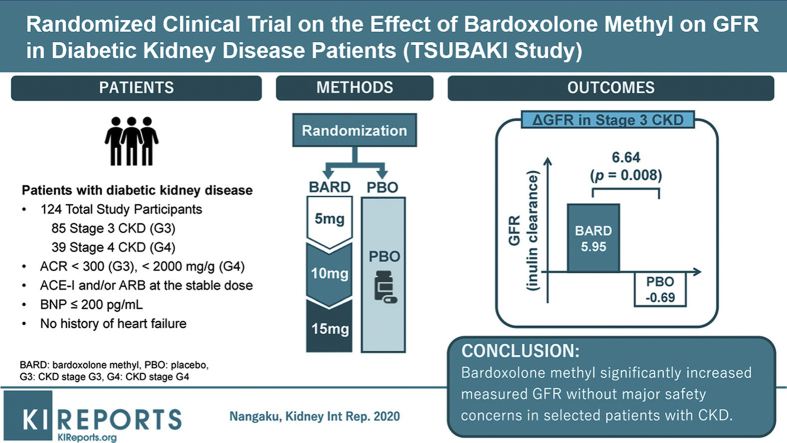
Bardoxolone methyl significantly increases estimated glomerular filtration rate (eGFR) in patients with chronic kidney disease (CKD). However, the phase 3 study, Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events (BEACON), was terminated prematurely because bardoxolone methyl increased the risk for early-onset fluid overload in patients with identifiable risk factors for heart failure (elevated baseline B-type natriuretic peptide levels >200 pg/ml and prior history of hospitalization for heart failure). The Phase 2 Study of Bardoxolone Methyl in Patients with Chronic Kidney Disease and Type 2 Diabetes (TSUBAKI) study aimed to determine if patients without risk factors can mitigate the risk for fluid overload and whether changes in eGFR with bardoxolone methyl reflect true increases in GFR.
Methods
This phase 2, randomized, multicenter, double-blind, placebo-controlled study enrolled patients with type 2 diabetes and stage 3–4 CKD. Patients were randomized 1:1 to bardoxolone methyl (n = 41) or placebo (n = 41) (cohort G3), or 2:1 to bardoxolone methyl (n = 24) or placebo (n = 14) (cohort G4), administered orally once daily for 16 weeks using a dose-titration scheme. The primary efficacy endpoint was change from baseline in GFR measured by inulin clearance at week 16 in the cohort G3.
Results
A total of 40 patients were evaluated for the prespecified primary efficacy analysis. Mean change (95% confidence interval [CI]) from baseline in GFR was 5.95 (2.29 to 9.60) and −0.69 (−3.83 to 2.45) ml/min per 1.73 m2 for patients randomized to bardoxolone methyl and placebo, respectively, with a significant intergroup difference of 6.64 ml/min per 1.73 m2 (P = 0.008). Increases in the albumin/creatinine ratio were observed in the bardoxolone methyl group vs the placebo group. The most common adverse events (≥15% in either group) were viral upper respiratory tract infection, increased alanine aminotransferase, increased aspartate aminotransferase, increased γ-glutamyltransferase, and constipation. Peripheral edema was reported by 4 patients receiving bardoxolone methyl and by 1 patient receiving placebo; all events were mild and self-limiting. No patient died or experienced heart failure. The study discontinuation rate was higher in the bardoxolone methyl group (cohort G3, n = 8; cohort G4, n = 7) than the placebo group (cohort G3, n = 1; cohort G4, n = 0).
Conclusion
Bardoxolone methyl significantly increased measured GFR, and further investigation is ongoing to evaluate whether it provides clinical benefit without major safety concerns in selected patients with CKD.
Keywords: bardoxolone methyl, chronic kidney disease, diabetic kidney disease, glomerular filtration rate, heart failure, inulin clearance
Graphical abstract
The number of patients with CKD, particularly diabetic kidney disease (DKD), is increasing globally, and prevention of disease progression is an important concern.1,2 Inflammation and oxidative stress contribute to the pathogenesis of CKD.3 Bardoxolone methyl activates a master regulator of redox homeostasis, the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (Nrf2) signal transduction pathway, and may target the inflammatory pathways that contribute to kidney function decline in CKD.4, 5, 6, 7 In multiple clinical studies that enrolled more than 2600 patients with CKD, bardoxolone methyl increased the eGFR in a significant and sustained manner.8, 9, 10 The largest of these studies was the phase 3 trial, Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events (BEACON), which assessed the efficacy and safety of bardoxolone methyl in patients with stage 4 CKD. BEACON was prematurely terminated in 2012 due to an increased risk for heart failure (HF) events with bardoxolone methyl treatment. Post hoc analyses of BEACON showed that the increase in HF events was most likely caused by fluid overload, which occurred in the first 4 weeks after randomization.11 An additional analysis identified elevated baseline B-type natriuretic peptide (BNP) levels >200 pg/ml and history of hospitalization for HF as risk factors for HF; for patients without these baseline characteristics, the risk for HF among bardoxolone methyl−treated and placebo-treated patients was similar (2%).12 Accordingly, a phase 2 study was conducted to determine whether prospective enrollment of patients without these clinical characteristics could mitigate the risk for fluid overload with bardoxolone methyl in patients with DKD. In addition, the study was designed to determine whether the observed increases in eGFR with bardoxolone methyl reflected a true increase in GFR.
Methods
Study Design and Participants
TSUBAKI (The Phase 2 Study of Bardoxolone Methyl in Patients with Chronic Kidney Disease and Type 2 Diabetes, ClinicalTrials.gov: NCT02316821) was a randomized, multicenter, double-blind, placebo-controlled trial conducted at 36 hospitals in Japan. The trial enrolled patients 20 to 79 years of age with type 2 diabetes and stage 3 CKD (eGFR ≥30 to <60 ml/min per 1.73 m2) and albumin to creatinine ratio (ACR) <300 mg/g (cohort G3). After a protocol amendment, a subsequent cohort included patients with type 2 diabetes and stage 4 CKD (eGFR ≥15 to <30 ml/min per 1.73 m2) and ACR <2000 mg/g (cohort G4). Concomitant administration of angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers was required. Patients with baseline BNP >200 pg/ml or significant cardiovascular histories were excluded from the study. Additional inclusion/exclusion criteria are presented in Supplementary Table S1. The study protocol and its amendments were approved by the institutional review board at each study site. Written informed consent was obtained from all patients.
Procedures
Eligible patients were randomized 1:1 (cohort G3) or 2:1 (cohort G4) to receive bardoxolone methyl or placebo, with stratification by ACR (cohorts G3 and G4) and CKD stage (cohort G3 only). Patients, investigators, site medical staff, and the sponsor were masked to the treatment assignment and to parameters that could potentially be affected by bardoxolone methyl treatment (Supplementary Table S1). Patients received bardoxolone methyl or placebo orally once daily for 16 weeks. The starting dose was 5 mg/d, followed by dose escalation, as tolerated, to 10 mg/d at week 4 and 15 mg/d at week 8. Patients were assessed weekly at the study site during the treatment period.
The primary efficacy endpoint parameter, GFR (inulin clearance, Cin), was measured twice at baseline and week 16 of treatment. To curtail variations in Cin measurements, patients were hospitalized 1 to 2 days prior to control for diet, water intake, and physical conditions. Patients fasted for at least 6 hours before INULEAD INJECTION (inulin solution for injection; Fujiyakuhin Co., Ltd., Saitama, Japan) was i.v. infused for the first 30 minutes at a rate of 300 ml/h, followed by 100 ml/h for 90 minutes.13 This continuous infusion method was performed under adequate water intake (other beverages were prohibited); patients drank 500 ml of water 30 minutes before inulin infusion, and 60 ml of water was given at 30, 60, and 90 minutes after the start of infusion. Patients were asked to void completely 30 minutes after inulin infusion and then underwent blood collection every 30 minutes (45, 75, and 105 minutes after inulin infusion) and urine collection every 30 minutes (60, 90, and 120 minutes after inulin infusion). GFR was calculated as the mean of 3 Cin measurements. Cin was calculated as follows13:
Body surface area of each patient was calculated from the body height at baseline and the body weight at GFR measurement using the Dubois equation; 24-hour urine samples were collected during hospitalization. The inulin assay was performed using an enzymatic method at the central laboratory (SRL Inc., Tokyo, Japan).
Outcomes
The primary efficacy endpoint was the change from baseline in GFR at week 16 in patients randomized to bardoxolone methyl relative to placebo. A prespecified interim analysis was performed by an independent data monitoring committee when 50% of the target sample size was reached. Because GFR measurements are time consuming and burdensome, once the primary endpoint was met, enrollment continued but no additional GFR measurements were collected. The secondary endpoint was the change from baseline in eGFR at week 16. The eGFR was calculated using an equation best tailored to the Japanese population13: eGFR (ml/min per 1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 (× 0.739 in female patients). The primary and secondary efficacy endpoints were analyzed using a per-protocol set (PPS) of cohort G3. The reason that PPS was used was to evaluate more precisely the efficacy with which bardoxolone methyl would increase GFR. In addition, 24-hour urine parameters (creatinine clearance, urine creatinine, urine volume, and urine sodium) were assessed as exploratory endpoints in PPS of cohort G3. Exploratory endpoints evaluated in the full analysis set of cohorts G3 and G4 included changes in eGFR and ACR over time during the study period.
Statistical Analysis
A target sample size of 72 patients (36 patients in each treatment group) provided the trial with approximately 80% statistical power to detect a 7 ml/min per 1.73 m2 difference in change from baseline in GFR between the bardoxolone methyl and placebo groups, which was expected based on eGFR values observed in a previous study (H. Takama, personal communication, 2, October, 2014). The calculations assumed an SD of 10 ml/min per 1.73 m2 and used a z test (incorporating data from 1 interim analysis) with a significance level of .025 for a 1-sided test. The 1-sided test was chosen to demonstrate bardoxolone methyl increases GFR compared to placebo to be considered effective, because previous studies have shown that bardoxolone methyl increases eGFR from baseline.
We analyzed the primary efficacy endpoint using an analysis of covariance model with baseline GFR and log-transformed baseline ACR as covariates. The PPS excluded untreated patients and those with unavailable GFR data at week 16. The adjusted least-squares mean (LSM) and its 95% CI were calculated for each group. The t statistics of the difference between treatment groups were calculated with respect to LSM, and a group sequential test was performed. To correct for a type 1 error, the critical value obtained from a normal distribution was corrected for t distribution using the Lan and DeMets α-spending function (Pocock type), which allows flexible timing of interim analysis.14
Exploratory analyses of other kidney function parameters (eGFR and ACR) were performed using a mixed-effects model with repeated measures, with factors (fixed effects) for treatment, visit, treatment-by-visit interaction, and relevant baseline value. All other continuous measures were analyzed using an analysis of covariance model with treatment as a fixed effect and relevant baseline value as a covariate. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
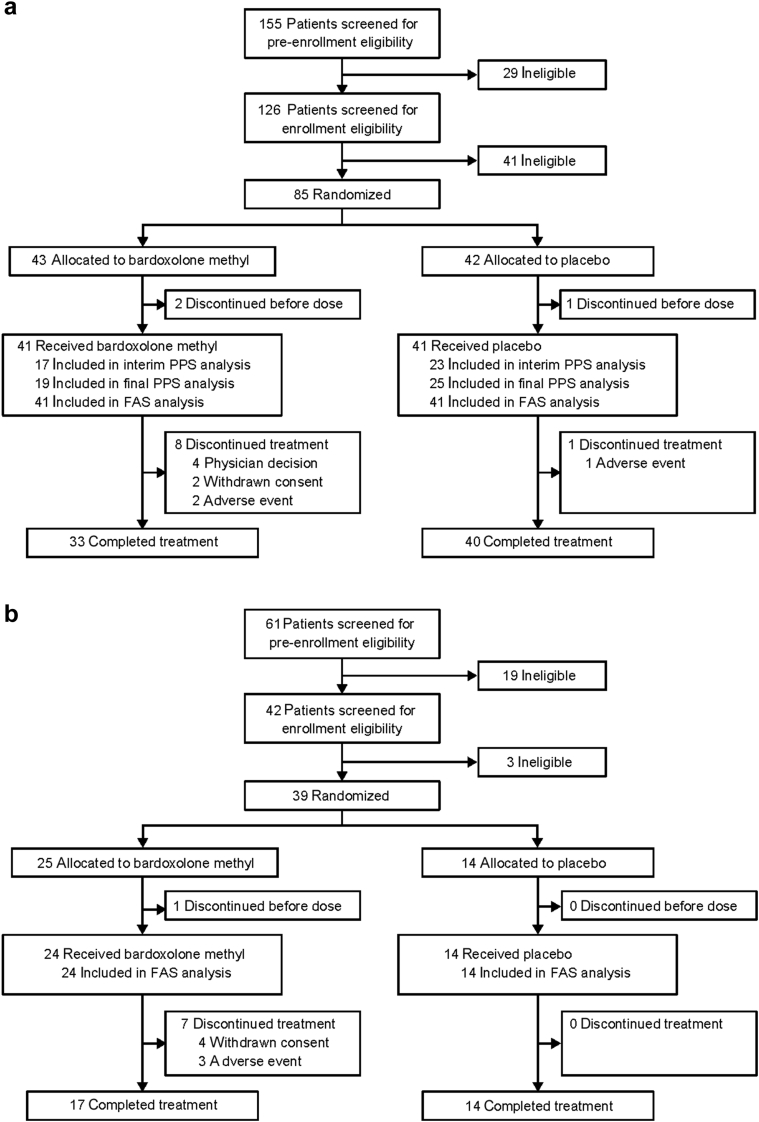
Between 15 December 2015 and 28 July 2016, a total of 155 patients provided written informed consent, 85 of whom met the eligibility criteria and were randomized 1:1 to either bardoxolone methyl or placebo in cohort G3 (Figure 1a). At the time of the interim analysis of the primary efficacy endpoint, 40 patients (bardoxolone methyl, n = 17; placebo, n = 23) had GFR data through week 16 and were included in the interim PPS. The final PPS population comprised all patients whose GFR was measured prior to the date that the primary endpoint was met and included 44 patients (bardoxolone methyl, n = 19; placebo, n = 25). The full analysis set for cohort G3 included a total of 82 patients (bardoxolone methyl and placebo, n = 41 each). Overall, 3 patients discontinued the study before treatment initiation, 9 patients (bardoxolone methyl, n = 8; placebo, n = 1) discontinued treatment prior to the end of the study, and 73 patients completed the study.
Figure 1.
Patient disposition in (a) cohort G3 and (b) cohort G4. FAS, full (safety) analysis set; PPS, per-protocol set.
After a prespecified interim analysis showed that the trial had met its primary efficacy endpoint, the protocol was amended to include cohort G4. Between 25 August 2016 and 26 February 2017, a total of 61 patients provided written informed consent, 39 of whom met the eligibility criteria and were randomized 2:1 to receive bardoxolone methyl or placebo (Figure 1b) in cohort G4. A total of 38 patients received bardoxolone methyl (n = 24) or placebo (n = 14) treatment and were included in the full analysis set for cohort G4. One patient discontinued the study before treatment initiation, and 7 patients, all in the bardoxolone methyl group, discontinued treatment prior to week 16. Specific reasons for discontinuation in both cohorts are summarized in Supplementary Table S2.
In both cohorts, baseline characteristics of patients were generally comparable between the bardoxolone methyl and placebo groups (Table 1). The baseline characteristics of patients in the interim and final PPS populations (Supplementary Tables S3 and S4, respectively) were also similar to those of the rest of the cohort G3.
Table 1.
Baseline characteristics
| Cohort G3 |
Cohort G4 |
|||
|---|---|---|---|---|
| Bardoxolone methyl |
Placebo |
Bardoxolone methyl |
Placebo |
|
| (n = 41) | (n = 41) | (n = 24) | (n = 14) | |
| Sex, n (%) | ||||
| Female | 15 (36.6) | 11 (26.8) | 7 (29.2) | 5 (35.7) |
| Male | 26 (63.4) | 30 (73.2) | 17 (70.8) | 9 (64.3) |
| Age, yr, mean (SD) | 67.9 (6.6) | 70.9 (6.8) | 68.0 (8.9) | 66.0 (6.8) |
| Weight, kg, mean (SD) | 66.71 (10.76) | 66.57 (11.05) | 64.65 (8.87) | 77.94 (19.03) |
| BMI, kg/m2, mean (SD) | 25.77 (3.69) | 25.17 (3.08) | 24.45 (2.01) | 28.44 (5.59) |
| eGFR, ml/min per 1.73 m2, mean (SD) | 46.82 (7.87) | 46.79 (6.85) | 25.55 (3.68) | 23.37 (3.16) |
| Serum creatinine, mg/dl, mean (SD) | 1.135 (0.235) | 1.148 (0.226) | 2.001 (0.356) | 2.151 (0.448) |
| ACR, mg/g, mean (SD) | 75.78 (104.47) | 51.55 (80.51) | 1082.70 (774.22) | 765.64 (669.58) |
| BNP, pg/ml, mean (SD) | 25.60 (28.32) | 30.44 (27.86) | 52.52 (40.56) | 39.55 (27.79) |
| Blood pressure, mm Hg, mean (SD) | ||||
| Systolic | 128.0 (17.3) | 125.1 (17.7) | 136.0 (12.6) | 134.8 (14.8) |
| Diastolic | 73.9 (10.6) | 70.2 (10.6) | 74.0 (8.9) | 71.3 (9.1) |
| HbA1c, %, mean (SD) | 6.99 (0.85) | 7.11 (0.73) | 6.78 (0.88) | 7.47 (1.15) |
ACR, albumin/creatinine ratio; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin.
Primary Endpoint
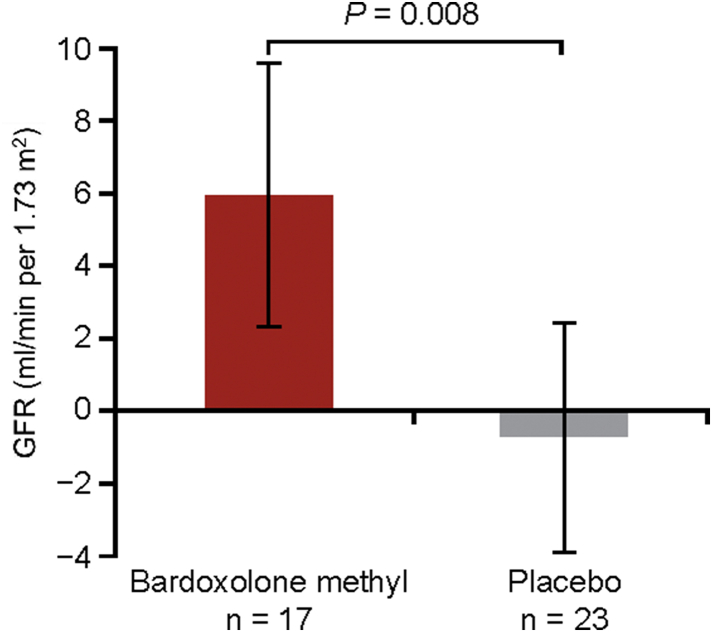
After 16 weeks of treatment, the LSM change (95% CI) from baseline in GFR for patients randomized to bardoxolone methyl was 5.95 (2.29 to 9.60) ml/min per 1.73 m2. In contrast, patients randomized to placebo had a mean decline in GFR of −0.69 (−3.83 to 2.45), resulting in a significant intergroup difference of 6.64 ml/min per 1.73 m2 (P = 0.008) (Figure 2).
Figure 2.
Change from baseline in glomerular filtration rate (GFR) assessed by inulin clearance (interim per-protocol set). Data are least-squares mean (LSM) (95% confidence interval). P value indicates the difference between the LSM using the analysis of covariance model with treatment as a factor, and baseline values of GFR and log-transformed albumin/creatinine ratio as covariates,
Secondary Endpoint
Consistent with the measured GFR results, patients randomized to bardoxolone methyl had significant increases from baseline in eGFR relative to patients randomized to placebo. The LSM change (95% CI) from baseline in eGFR at week 16 was 12.30 (10.10 to 14.49) and 0.22 (−1.69 to 2.13) ml/min to 1.73 m2 in the bardoxolone methyl and placebo groups, respectively (P < 0.0001).
Exploratory Endpoints
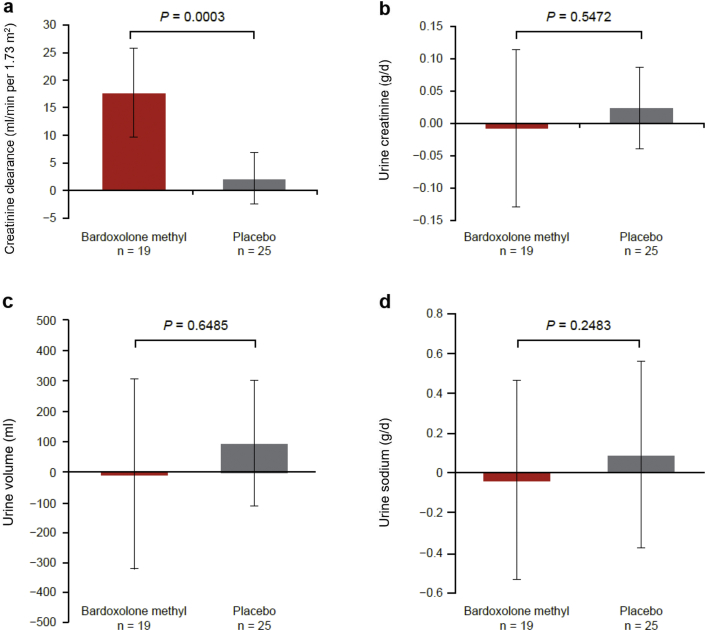
Results of the 24-hour urine collection parameters at week 16 are presented in Figure 3a to d. Bardoxolone methyl treatment significantly increased creatinine clearance relative to placebo (P = 0.0003) (Figure 3a). Urinary creatinine excretion, urine volume, and urinary sodium excretion were not significantly different between the 2 groups (Figure 3b−d).
Figure 3.
Twenty-four-hour urine collection parameters (final per-protocol set) at week 16 in cohort G3: (a) creatinine clearance (CCr), (b) urine creatinine (Cr), (c) urine volume, and (d) urine sodium. Data for (a–d) are mean (95% confidence interval). P value is based on non−multiplicity-adjusted P value by t test and post hoc analyses.
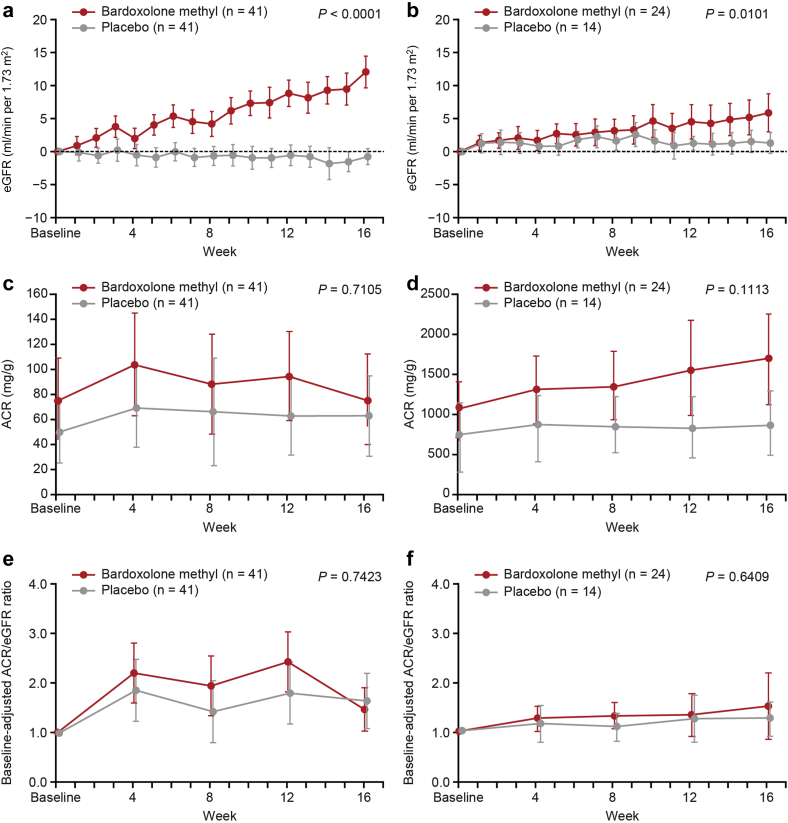
Changes in eGFR over time for cohort G3 or cohort G4 are presented in Figure 4a and b, respectively. In both cohorts, bardoxolone methyl treatment significantly increased eGFR relative to placebo. The magnitude of increases in eGFR with bardoxolone methyl treatment appeared to be greater in cohort G3, but the relative changes in eGFR, presented as percentages, were comparable between the 2 cohorts (Supplementary Figure S1).
Figure 4.
Estimated glomerular filtration rate (eGFR) (full analysis set [FAS]) in (a) cohort G3 and (b) cohort G4; urinary albumin/creatinine ratio (ACR) (FAS) in (c) cohort G3 and (d) cohort G4; and baseline-adjusted ACR/eGFR in (e) cohort G3 and (f) cohort G4. Data are mean (95% confidence interval). P value is based on non−multiplicity-adjusted P value by t test and post hoc analyses. P value indicates the difference between the treatment groups at 16 weeks using the mixed-effects model with repeated measures with treatment and time as factors, treatment-by-visit interaction, and relevant baseline value as covariate.
The LSM change (95% CI) from baseline in eGFR (ml/min per 1.73 m2) 4 weeks after treatment withdrawal (week 20) in the bardoxolone methyl versus placebo group was 3.65 (2.15 to 5.15) versus −0.76 (−2.23 to 0.70) for cohort G3 and 1.26 (−1.45 to 3.97) versus −0.04 (−2.49 to 2.41) for cohort G4.
Changes in the ACR over time in both cohorts, including individual-level data, are presented in Figure 4c and d, and Supplementary Figures S2 and S3. Increases in ACR were observed with bardoxolone methyl treatment relative to placebo. However, when indexed to eGFR values at each time point, bardoxolone methyl did not increase ACR/eGFR ratios (Figure 4e and f).
Safety
Peripheral edema was reported as an adverse event (AE) in 4 patients treated with bardoxolone methyl and in 1 patient treated with placebo. All reports of peripheral edema were mild in severity and resolved during the study. The most commonly reported AEs (≥15% in either group) were viral upper respiratory tract infection, increased alanine aminotransferase, increased aspartate aminotransferase increased γ-glutamyltransferase, and constipation (Table 2), which were mild in severity and occurred more frequently in patients treated with bardoxolone methyl than in those treated with placebo. The reports of increases in aminotransferase were not accompanied by increases in total bilirubin, and no patient met Hy’s law criteria.15
Table 2.
Most common adverse events (≥15% in either group) and serious adverse events
| Cohort G3 |
Cohort G4 |
|||
|---|---|---|---|---|
| Bardoxolone methyl |
Placebo |
Bardoxolone methyl |
Placebo |
|
| (n = 41) | (n = 41) | (n = 24) | (n = 14) | |
| Any adverse event, n (%) | ||||
| Viral upper respiratory tract infection | 8 (19.5) | 6 (14.6) | 12 (50.0) | 5 (35.7) |
| Alanine aminotransferase increased | 16 (39.0) | 1 (2.4) | 6 (25.0) | 0 (0) |
| Aspartate aminotransferase increased | 10 (24.4) | 1 (2.4) | 6 (25.0) | 0 (0) |
| γ-Glutamyltransferase increased | 10 (24.4) | 0 (0) | 2 (8.3) | 0 (0) |
| Constipation | 8 (19.5) | 4 (9.8) | 1 (4.2) | 0 (0) |
| Serious adverse event, n (%) | ||||
| Myocardial infarction | 1 (2.4) | 0 (0) | 0 (0) | 0 (0) |
| Tibia fracture | 1 (2.4) | 0 (0) | 0 (0) | 0 (0) |
| Spinal osteoarthritis | 1 (2.4) | 0 (0) | 0 (0) | 0 (0) |
| Pyelonephritis, acute | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Chronic kidney disease | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Diabetic gangrene | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Influenza | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Pneumonia pneumococcal | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
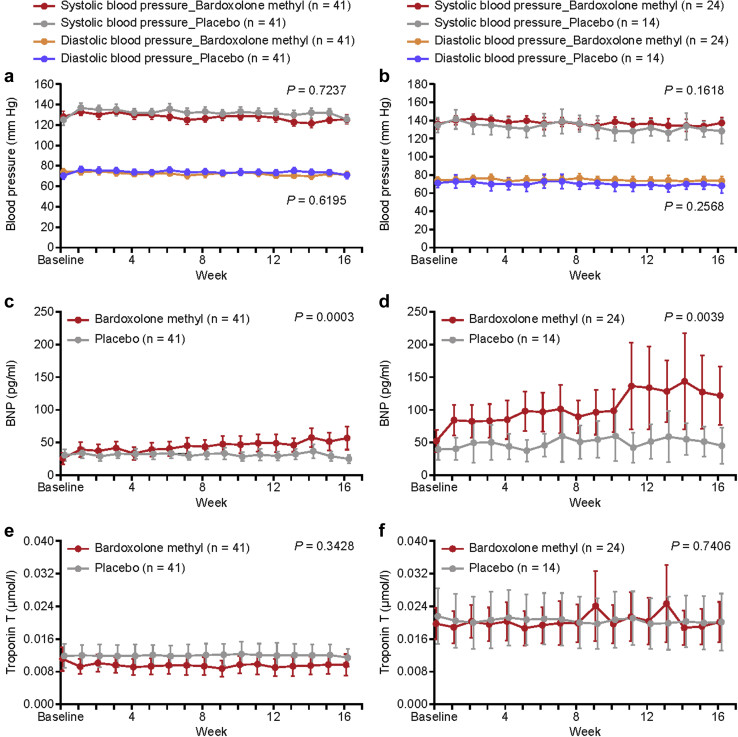
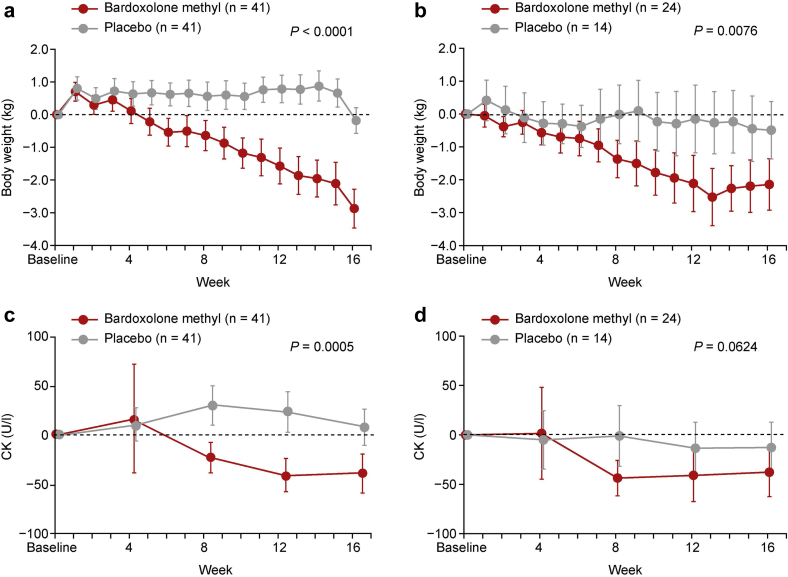
No significant increases in systolic or diastolic blood pressure were observed with bardoxolone methyl treatment during the study (Figure 5a and b). Slight, but statistically significant, increases in BNP were observed with bardoxolone methyl treatment relative to placebo (Figure 5c and d; Supplementary Figures S4 and S5). An observable increase in BNP levels was reported as an AE in 2 patients in the bardoxolone methyl group versus 1 in the placebo group. The increase in BNP levels was not associated with treatment withdrawal (data not shown). Troponin T levels were unchanged from baseline and relative to placebo with bardoxolone methyl treatment (Figure 5e and f). There were no clinically significant changes in electrocardiography, echocardiography, or chest X-ray findings; no changes were noted in left ventricular fractional shortening or cardiothoracic ratio (Supplementary Figures S6 and S7). A significant decrease in body weight was observed with bardoxolone methyl treatment, which was not associated with creatine kinase (CK) elevations (Figure 6a−d). Other AEs commonly observed in prior studies with the drug,8, 9, 10 such as mild muscle spasms (bardoxolone methyl, 6 vs. placebo, 1), weight loss (bardoxolone methyl, 1 vs. placebo, 0), increased amount of protein in the urine (bardoxolone methyl, 4 vs. placebo, 0), and increase in ACR (bardoxolone methyl, 4 vs. placebo, 0), were observed in more patients treated with bardoxolone methyl than in those given placebo (Supplementary Table S5). No deaths or hospitalizations due to HF were reported in the study.
Figure 5.
Systolic and diastolic blood pressure (safety analysis set) in (a) cohort G3 and (b) cohort G4; B-type natriuretic peptide (BNP) levels (safety analysis set) in (c) cohort G3 and (d) cohort G4; and troponin T levels in (e) cohort G3 and (f) cohort G4. Data are mean (95% confidence interval). P value is based on non−multiplicity-adjusted P value by t test and post hoc analyses. P value indicates the difference between the treatment groups at 16 weeks using the mixed-effects model with repeated measures with treatment and time as factors, treatment-by-visit interaction, and relevant baseline value as covariate.
Figure 6.
Change in body weight (safety analysis set) in (a) cohort G3 and (b) cohort G4 and change in creatine kinase (CK) in (c) cohort G3 and (d) cohort G4. Data are mean (95% confidence interval). P value is based on non−multiplicity-adjusted P value by t test and post hoc analyses.
Discussion
The trial met its primary efficacy endpoint and, using the inulin clearance (Cin) method, demonstrated that treatment with bardoxolone methyl significantly increased measured GFR. Furthermore, consistent with prior studies, patients treated with bardoxolone methyl in TSUBAKI had significant increases in eGFR. Bardoxolone methyl treatment also significantly increased creatinine clearance, and these increases were not associated with changes in total 24-hour excretion of creatinine. Collectively, these data establish that the increases in eGFR with bardoxolone methyl are not due to changes in creatinine metabolism but reflect true increases in GFR.
Two potential mechanisms by which bardoxolone methyl may increase GFR include dynamic increases in glomerular filtration surface area16, 17, 18, 19 and chronic antifibrotic effects.20 Dynamic increases in GFR may be attributed to maintenance of intracellular redox homeostasis in mesangial cells16 and/or endothelial cells17, 18, 19; preclinical studies show that bardoxolone methyl reduces endothelial dysfunction and angiotensin II−induced mesangial cell contraction to increase the filtration coefficient and GFR. Interventions that cause pressure-mediated injury would be expected to accelerate the rate of decline in kidney function relative to placebo after 1 year or more of treatment.21 In contrast, patients treated with bardoxolone methyl experience increases in eGFR that are sustained for at least 1 year, and a sizeable fraction of that increase is retained 4 weeks after the drug has been withdrawn, long after active concentrations of the drug are evident.9 Therefore, the mechanism by which bardoxolone methyl increases GFR and eGFR appears to be distinct from that of agents associated with increased intraglomerular pressure. Thus, there is no evidence that the GFR changes produced by bardoxolone methyl are due to pressure-mediated hyperfiltration. Importantly, a recent study in mice has found that Nrf2 activation increases GFR without affecting the afferent/efferent arteriole ratio.22 Moreover, we found no evidence of an increased risk of kidney toxicity, as assessed by serious AEs involving the kidneys, end-stage kidney disease events, and proportion of patients with clinically meaningful loss of eGFR. Finally, partially sustained benefit after cessation of bardoxolone methyl in other studies with longer treatment durations may be indicative of the antifibrotic effects observed with bardoxolone methyl in preclinical studies.20 It should be noted, however, that long-term observation is required to clarify the true biological significance of increased GFR.
As part of a broader risk mitigation strategy to reduce the risk for HF with bardoxolone methyl treatment, patients with risk factors for fluid overload were excluded from TSUBAKI. In addition, a dose-titration scheme was used; patients received 5 mg/d of bardoxolone methyl (a quarter of the dose used in BEACON [20 mg/d]) during the first 4 weeks and were dose escalated to a maximum dose of 15 mg/d. Furthermore, body weight and blood pressure were monitored daily throughout the treatment period to assess for signs and symptoms of fluid overload. Notably, no hospitalizations due to HF or fluid overload were reported in the trial. Although a slight increase in BNP and a higher incidence of peripheral edema were observed with bardoxolone methyl, there were no increases in blood pressure, body weight, or troponin T levels and no changes in electrocardiography, echocardiography, or chest X-ray findings, supporting the absence of clinically significant fluid retention. The relationship between fluid retention and increases in BNP caused by bardoxolone methyl is not clear at this moment; however, BNP measurement during the treatment may help prediction of future HF risk. Moreover, unlike BEACON, there were no signs of overt volume changes, and 24-hour urine collections showed no changes in urinary volume or sodium excretion with bardoxolone methyl treatment. The absence of overt fluid overload or subclinical measures of fluid retention supports that TSUBAKI used an effective risk mitigation strategy to allow safe administration of bardoxolone methyl to patients with moderate-to-advanced CKD.
In concert with the increases in eGFR, treatment with bardoxolone methyl also increased ACR. The result is consistent with the finding of post hoc analyses of BEACON showing that changes in albuminuria are directly related to changes in eGFR.23 Besides, the increases in urinary albumin excretion with bardoxolone methyl treatment are not associated with signs of disease progression or kidney injury, but likely are the results of a pharmacological effect of increased GFR and decreased albumin reabsorption that are distinct from alterations to glomerular selectivity. This implication is supported by the fact that glomerular permeability of albumin was not changed by bardoxolone methyl analogue treatment in mice.22
Although the rate of study discontinuation was higher in the bardoxolone methyl group for both cohorts, only 5 discontinuations were associated with AEs. The most commonly reported AEs (≥15% in either group) were viral upper respiratory tract infection, increased alanine aminotransferase, increased aspartate aminotransferase, increased γ-glutamyltransferase, and constipation. Viral upper airway inflammation appeared to be seasonal. Increases in aminotransferases were transient and followed a pattern similar to those observed in other clinical studies with bardoxolone methyl. Importantly, aminotransferase increases were not associated with elevations in total bilirubin, and no patient met Hy’s law criteria. Genetic manipulation of Nrf2, the target of bardoxolone methyl, has been shown to regulate the induction of aminotransferase genes and the serum activity of alanine aminotransferase and aspartate aminotransferase.24 Similar to increases in alanine aminotransferase and aspartate aminotransferase, elevations in serum γ-glutamyltransferase, a protein involved in glutathione synthesis and also controlled by Nrf2, were also reported. The mechanism by which bardoxolone methyl elevates aminotransferases and γ-glutamyltransferase appears to relate to its pharmacologic actions and not intrinsic liver toxicity.
Also consistent with previous studies, bardoxolone methyl treatment reduced body weight in TSUBAKI. The absence of creatine kinase elevations and the lack of changes in urinary creatinine excretion support that the observed weight loss was not associated with muscle injury or muscle wasting. As observed in prior studies, weight loss was more pronounced in patients with higher body mass index at baseline in this study.25 In BEACON, reductions in body weight coincided with significant decreases in waist circumference, a surrogate measure of adiposity, as well as reductions in glycated hemoglobin.25 Thus, improvements in glycemic control and lipid metabolism observed in preclinical studies with bardoxolone methyl may explain the reductions in body weight observed in humans.26, 27, 28, 29, 30
Strengths of this trial include a randomized, placebo-controlled design and the measurement of GFR using the gold standard Cin method in an interventional study. In addition, through strict handling procedures, we were able to control for diet, water intake, and physical conditions to minimize variability. Limitations include the sample size; measuring Cin involved overnight hospitalizations and complex and invasive measurements that precluded assessment of Cin in a larger sample size and broader patient population. The magnitude of increases in GFR observed with bardoxolone methyl was less than the increases in eGFR, and likely reflects the technical challenges associated with Cin measurements that may have resulted in underestimating GFR because of incomplete voiding. Another limitation is that only a limited number of patients with cohort G4 were added to ensure that selected patients with stage G4 DKD could be safely treated with bardoxolone methyl. This was the first study to be conducted after the premature termination of the BEACON study in patients with stage G4 DKD; therefore, the eligibility criteria for the TSUBAKI study were limited to patients with stage G3 DKD at the beginning of the study. However, cohort G4 was added to assess the safety of the drug in patients with later-stage CKD in the discussion with the Japanese regulatory agency (Pharmaceuticals and Medical Devices Agency [PMDA]) after the primary efficacy endpoint was achieved in the interim analysis. In addition, because of the small numbers of patients in this study, additional information about safety is definitely needed to evaluate the overall risk−benefit relationship of the drug, especially in advanced CKD. Although the 16-week study duration and small sample size in TSUBAKI could not comprehensively profile the safety and efficacy of bardoxolone methyl, the ongoing phase 3 AYAME study will provide longer-term safety and efficacy data, and will evaluate how increases in GFR can affect kidney failure outcomes in patients with DKD.
Disclosure
MN reports grants and personal fees from Kyowa Kirin, Astellas, Daiichi-Sankyo, Mitsubishi-Tanabe, JT, Takeda, and Chugai, and personal fees from GSK, Boehringer-Ingelheim, and AstraZeneca, during the conduct of the study. TA reports personal fees from Kyowa Kirin during the conduct of the study and personal fees from Astellas, Bayer, GSK, JT, Kissei, Chugai, Ono, Fuso, Nipro, Torii, Otsuka, and Sanwa Chemical outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
This work was supported by Kyowa Kirin. The study sponsor was involved in the study design, data collection and management, statistical analysis, and the decision to submit the manuscript for publication. The sponsor also worked with the coauthors in interpreting data and provided medical writing and editorial assistance for the development of this manuscript. The corresponding author has full access to all data in this study and had the final responsibility for the decision to submit this manuscript for publication. We thank the participants, investigators, research staff at the study sites, and members of the independent data monitoring committee (Supplementary Table S6) for their involvement in and support of the study. We also thank Yoshinari Yasuda and Tomio Suzuki for their contributions to the study design.
Author Contributions
MN, HK, HT, TI, HH, and TA designed the study; HT and TI prepared the protocol and managed the study; MN, HH, and TA contributed to data interpretation; HK, HT, and TI wrote the first draft; all authors edited this draft; and all authors critically revised and approved the final version.
Footnotes
Table S1. TSUBAKI study inclusion/exclusion criteria and details of masking.
Table S2. Breakdown of reasons for discontinuation.
Table S3. Baseline characteristics (interim PPS).
Table S4. Baseline characteristics (final PPS).
Table S5. Adverse events of special interest.
Table S6. TSUBAKI study groups.
Figure S1. Relative changes in eGFR (FAS) in (A) cohort G3 and (B) cohort G4.
Figure S2. Urinary ACR (FAS) in (A) cohort G3 and (B) cohort G4. Data are median (± IQR). P value: difference between treatment groups at week 16 were calculated by the Wilcoxon rank sum test and post hoc analyses. ACR, albumin/creatinine ratio; IQR, interquartile range, FAS, full analysis set.
Figure S3. Change from baseline in ACR for individual patients (safety analysis set) in (A) placebo-treated cohort G3, (B) bardoxolone methyl-treated cohort G3, (C) placebo-treated cohort G4, and (D) bardoxolone methyl-treated cohort G4.
Figure S4. BNP levels (safety analysis set) in (A) cohort G3 and (B) cohort G4. Data are median (± IQR). P value: difference between treatment groups at week 16 were calculated by the Wilcoxon rank sum test and post hoc analyses. BNP, B-type natriuretic peptide; IQR, interquartile range.
Figure S5. Change from baseline in BNP for individual patients (safety analysis set) in (A) placebo-treated cohort G3, (B) bardoxolone methyl-treated cohort G3, (C) placebo-treated cohort G4, and (D) bardoxolone methyl-treated cohort G4.
Figure S6. Change from baseline at week 16 in FS (safety analysis set) in (A) cohort G3 and (B) cohort G4.
Figure S7. Change from baseline at week 16 in CTR (safety analysis set) in (A) cohort G3 and (B) cohort G4.
Supplementary Material
References
- 1.Coresh J., Turin T.C., Matsushita K. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai E., Horio M., Watanabe T. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz S., Pergola P.E., Zager R.A. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinkova-Kostova A.T., Liby K.T., Stephenson K.K. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates M.S., Tauchi M., Katsuoka F. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 6.Sporn M.B., Liby K.T., Yore M.M. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74:537–545. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pergola P.E., Krauth M., Huff J.W. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b-4 CKD. Am J Nephrol. 2011;33:469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 9.Pergola P.E., Raskin P., Toto R.D. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 10.de Zeeuw D., Akizawa T., Audhya P. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin M.P., Reisman S.A., Bakris G.L. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol. 2014;39:499–508. doi: 10.1159/000362906. [DOI] [PubMed] [Google Scholar]
- 12.Chin M.P., Wrolstad D., Bakris G.L. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Card Fail. 2014;20:953–958. doi: 10.1016/j.cardfail.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Lan K.K.G., DeMets D.L. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 15.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER) Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. July 2009. https://www.fda.gov/downloads/guidances/UCM174090.pdf Available at:
- 16.Ding Y., Stidham R.D., Bumeister R. The synthetic triterpenoid, RTA 405, increases the glomerular filtration rate and reduces angiotensin II-induced contraction of glomerular mesangial cells. Kidney Int. 2013;83:845–854. doi: 10.1038/ki.2012.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heiss E.H., Schachner D., Werner E.R. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem. 2009;284:31579–31586. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aminzadeh M.A., Reisman S.A., Vaziri N.D. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol. 2013;1:527–531. doi: 10.1016/j.redox.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aminzadeh M.A., Reisman S.A., Vaziri N.D. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014;44:570–578. doi: 10.3109/00498254.2013.852705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan S.M., Sharma A., Stefanovic N. Derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes. 2014;63:3091–3103. doi: 10.2337/db13-1743. [DOI] [PubMed] [Google Scholar]
- 21.Wright J., Bakris G., Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 22.Kidokoro K, Nagasu H, Satoh M, et al. Activation of the Keap1/Nrf2 pathway increases GFR by increasing glomerular effective filtration area without affecting the afferent/efferent arteriole ratio. Poster presented at: ASN Kidney Week 2019; November 5–10, 2019; Washington, DC.
- 23.Rossing P., Block G.A., Chin M.P. Effect of bardoxolone methyl on the urine albumin-to-creatinine ratio in patients with type 2 diabetes and stage 4 chronic kidney disease. Kidney Int. 2019;96:1030–1036. doi: 10.1016/j.kint.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Miller GA, Bumeister R, Laidlaw J, et al. Bardoxolone methyl transcriptionally regulates transaminase levels and increases glutathione levels. Poster presented at: ASN Kidney Week 2011; November 8–13, 2011; Philadelphia, PA.
- 25.Chertow G.M., Appel G.B., Block G.A. Effects of bardoxolone methyl on body weight, waist circumference and glycemic control in obese patients with type 2 diabetes mellitus and stage 4 chronic kidney disease. J Diabetes Complications. 2018;32:1113–1117. doi: 10.1016/j.jdiacomp.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Shin S., Wakabayashi J., Yates M.S. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha P.K., Reddy V.T., Konopleva M. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem. 2010;285:40581–40592. doi: 10.1074/jbc.M110.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uruno A., Furusawa Y., Yagishita Y. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013;33:2996–3010. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludtmann M.H., Angelova P.R., Zhang Y. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem J. 2014;457:415–424. doi: 10.1042/BJ20130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinh C.H., Szabo A., Yu Y. Bardoxolone methyl prevents mesenteric fat deposition and inflammation in high-fat diet mice. Sci World J. 2015;2015:549352. doi: 10.1155/2015/549352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.