Abstract
Background
Sore throat is a common condition caused by viruses or bacteria, and is a leading cause of antibiotic prescription in primary care. The most common bacterial species is group A streptococcus (’strep throat’). Between 50% to 70% of pharyngitis cases are treated with antibiotics, despite the majority of cases being viral in origin. One strategy to reduce antibiotics is to use rapid tests for group A streptococcus to guide antibiotic prescriptions. Rapid tests can be used alone or in combination with a clinical scoring system.
Objectives
To assess the efficacy and safety of strategies based on rapid tests to guide antibiotic prescriptions for sore throat in primary care settings.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Web of Science, and LILACS, as well as the trial registries ClinicalTrials.gov and the WHO ICTRP on 5 June 2019.
Selection criteria
We included randomised controlled trials (RCTs) comparing rapid tests with management based on clinical grounds to guide the prescription of antibiotics for people with a sore throat in ambulatory care settings. We included trials that randomised individuals, as well as cluster‐RCTs in which individual practitioners (or practices) or emergency departments were randomised.
Data collection and analysis
Two review authors independently extracted data on the primary outcomes (number of participants provided with an antibiotic prescription; number of participants with an antibiotic dispensed) and secondary outcomes (duration of sore throat symptoms; duration of other symptoms; quality of life measures; number of participants with a complication attributed to the index infection; number of participants in need of re‐consultation by the end of follow‐up; number of participants in need of hospital admission by the end of follow‐up; number of satisfied participants; number of participants with an adverse event attributed to the rapid test). We assessed the risk of bias of all included trials and used GRADE to assess the certainty of the evidence. We performed meta‐analyses and sensitivity analyses when feasible.
Main results
We included five trials (2891 children and adult participants in total; 2545 participants after adjusting for clustering). Management in the intervention group was as follows: in three trials rapid tests were used in combination with a clinical scoring system; in one trial, some physicians were asked to use rapid tests alone, while others were asked to use rapid tests in combination with a clinical scoring system; in one trial, rapid tests were used alone.
Based on data from five trials (2545 participants), a large reduction in prescribed antibiotics was found in the rapid test group (481/1197) versus management based on clinical grounds (865/1348), for a summary risk difference (RD) of −25%, 95% confidence interval (CI) −31% to −18%; I2 = 62%; moderate‐certainty evidence. Estimates of effect on antibiotic prescription rates were stable in various sensitivity analyses.
Based on data from two trials (900 people) originating from the same overarching study, the evidence suggests that rapid tests may not reduce dispensed antibiotic treatments: rapid test group (156/445) versus management based on clinical grounds (197/455); summary RD −7%, 95% CI −17% to 2%; I2 = 53%; low‐certainty evidence.
Four trials (2075 participants) reported data on the number of participants with a complication attributed to the index infection; the summary odds ratio (OR) was 0.85, 95% CI 0.03 to 26.65; P = 0.93; I2 = 62%; very low‐certainty evidence, which means that people in the rapid testing group were less likely to develop complications of the index infection, but the evidence is very uncertain.
Two trials (1161 participants) reported on the number of participants in need of re‐consultation by the end of follow‐up; the summary OR was 1.12, 95% CI 0.57 to 2.21; P = 0.74; I2 = 59%; low‐certainty evidence, which means that participants in the rapid testing group were more likely to be in need of re‐consultation by the end of the study follow‐up, but the evidence is uncertain.
Lack of data impeded assessment of other secondary outcomes (including safety outcomes) and of sources of heterogeneity.
Authors' conclusions
Rapid testing to guide antibiotic treatment for sore throat in primary care probably reduces antibiotic prescription rates by 25% (absolute risk difference), but may have little or no impact on antibiotic dispensing. More studies are needed to assess the efficacy and safety of rapid test‐guided antibiotic prescribing, notably to evaluate patient‐centred outcomes and variability across subgroups (e.g. adults versus children).
Plain language summary
Use of rapid point‐of‐care testing for strep throat to guide doctors prescribing antibiotics for sore throat in primary care settings
Review question
Can rapid point‐of‐care tests help reduce antibiotic use in people with acute sore throat in primary care?
Background
Sore throat is one of the most common reasons for primary care visits. It can be caused by viruses or bacteria. The bacterial species most frequently identified in cases of sore throat is group A streptococcus (’strep throat’). Antibiotics are commonly prescribed for people with a sore throat, even though the majority of sore throats are caused by viruses, in which case antibiotics are ineffective and unnecessary. The concern is that antibiotics may cause side effects and contribute to antibiotic resistance, causing difficult‐to‐treat infections. It is particularly challenging for physicians to distinguish between sore throats of viral and bacterial origin by observation alone (clinically distinguish), even for experienced physicians. Throat swab cultures may take up to 48 hours to grow. This has led to the development of rapid tests. Several rapid tests are currently available to identify sore throat cases caused by group A streptococcus and can be used by doctors during primary care consultations for sore throat. These rapid tests could help reduce antibiotic prescriptions by withholding antibiotics in people with a negative test result. We assessed the available evidence from randomised controlled trials (a type of study in which participants are assigned to one of two or more treatment groups using a random method) to evaluate the effectiveness and safety of using rapid tests in primary care.
Study characteristics
We searched for randomised controlled trials published in any language up to June 2019. We identified five randomised controlled trials with a total of 2545 participants with sore throat in primary care settings.
Key results
Participants in the rapid test group were less likely to be prescribed antibiotics than participants managed based on clinical grounds (481/1197 versus 865/1348). A 25% reduction (i.e., a decrease of 25 percentage points) in antibiotic prescription rates is likely to be achieved by using rapid testing in people with sore throat in primary care. However, there may be little or no reduction between groups in dispensed antibiotic treatments. Antibiotic prescriptions refer to medicines prescribed by healthcare providers. Antibiotic dispensing refers to medicines accessed in pharmacies. In some cases, patients may not present to the pharmacy to get their prescription filled. Four trials reported data on the number of participants with a complication attributed to the initial infection (e.g., tonsil abscess): complications were rare (0 to 3 per trial), and there may be little or no difference between people managed on clinical grounds alone and those managed with rapid testing but the evidence is very uncertain.
Certainty of the evidence
We ranked the certainty of the evidence as moderate for the number of participants provided with an antibiotic prescription, low for the number of participants with an antibiotic dispensed, and very low for the number of participants with a complication attributed to the episode of sore throat (e.g., abscess of the tonsils), respectively.
Conclusion
Compared with usual decision‐making based on clinical examination alone, implementing rapid tests can reduce antibiotic prescription rates, but may have little or no impact on antibiotic dispensing. More studies are needed to assess other outcomes that are important to patients, including safety.
Summary of findings
Summary of findings 1. Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined) to guide antibiotic prescriptions for sore throat.
| Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined) to guide antibiotic prescriptions for sore throat | |||||
| Patient or population: patients with sore throat Setting: primary care settings Intervention: management based on rapid tests Comparison: management based on clinical grounds | |||||
| Outcome | Illustrative comparative risks* (95% CI) | Effect size (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Clinical grounds (with and without scoring system combined) | Rapid test (with and without scoring system combined) | ||||
| Number of participants provided with an antibiotic prescription | 636 per 1000 | 394 per 1000 (343 to 445) | RD −25% (−31% to −18%) | 2545 (5 trials) | ⊕⊕⊕⊝ Moderatea |
| Number of participants with an antibiotic dispensed | 419 per 1000 | 344 per 1000 (277 to 432) | RD ‐7% (−17% to +2%) | 900 (2 trials) | ⊕⊕⊝⊝ Lowb,c |
| Number of participants with a complication attributed to the index infection | 2 per 1000 | 2 per 1000 (0 to 52) | OR 0.85 (0.03 to 26.65) | 2075 (4 trials) | ⊕⊝⊝⊝ Very lowd,e,f |
| Number of participants in need of re‐consultation by the end of follow‐up | 85 per 1000 | 94 per 1000 (51 to 174) | OR 1.12 (0.57 to 2.21) | 1161 (2 trials) | ⊕⊕⊝⊝ Lowg,h |
| No trial reported data on the other predefined outcomes. | |||||
| *Assumed risk in the control group (clinical grounds): mean control group risk across studies. CI: confidence interval; OR: odds ratio; RD: risk difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded due to high heterogeneity (I2 = 62%). bDowngraded due to high heterogeneity (I2 = 53%). cDowngraded due to study limitations: two trials embedded within the same overarching study. dDowngraded due to high heterogeneity (I2 = 62%). eDowngraded due to study limitations: two trials embedded within the same overarching study. fDowngraded due to imprecision. gDowngraded due to high heterogeneity (I2 = 59%). hDowngraded due to study limitations: two trials embedded within the same overarching study.
Background
Description of the condition
Sore throat accounts for an estimated 1.0% of ambulatory care visits to physicians in the USA (including visits to physician offices, walk‐in clinics, family medicine centres, and hospital outpatient and emergency departments) (CDC 2012), for a total of 7 million visits by adults (Hong 2011), and about the same number of visits by children each year (Linder 2005). Group A streptococcus (GAS) is found in about 35% of cases of childhood sore throat, Shaikh 2010, and 5% to 25% of adulthood cases (Ebell 2000; Wessels 2011). Non‐GAS cases are considered viral.
Antibiotic treatment may be indicated for GAS infection to prevent suppurative (e.g. retropharyngeal abscess and quinsy) and non‐suppurative complications (e.g., acute rheumatic fever and rheumatic heart disease) and to reduce the duration of symptoms and the spread of the infection (Spinks 2013). In industrialised settings where poststreptococcal diseases have become uncommon, the public health goal is shifting from preventing complications to minimising the inappropriate use of antibiotic agents in order to contain antimicrobial resistance.
About 50% to 70% of visits by patients with sore throat to ambulatory care result in antibiotic agents being prescribed (Fleming‐Dutra 2016; Hong 2011; Linder 2005; Pouwels 2018). The association between antibiotic use and antibiotic resistance in bacteria has been clearly demonstrated (Goossens 2005), and reducing antibiotic resistance has become a public health priority internationally (Carlet 2012; Laxminarayan 2013). Antibiotics also carry a risk of side effects for patients, including adverse digestive effects such as diarrhoea and Clostridioides difficile infection, and allergic reactions such as skin rash and anaphylaxis (a whole‐body and life‐threatening allergic reaction).
Description of the intervention
Because the signs and symptoms of GAS and viral sore throat overlap, some guidelines recommend that the diagnosis of GAS infection be confirmed by a throat culture or rapid test, both based on a throat swab, before using antibiotics (AAP 2015; Shulman 2012). Throat culture on a blood agar plate is the reference standard for the diagnosis of GAS in patients with sore throat. The major advantage of throat culture is its detection of GAS from swabs with a very low number of bacteria, thus conferring a high sensitivity. Its limitations are the need for special laboratory equipment and trained personnel and the 48‐hour delay in obtaining results.
Rapid tests detect a cell wall carbohydrate, a GAS‐specific antigen, by an immunologic reaction (Gerber 2004). They provide an indication for the clinician about the presence or absence of GAS in the throat sample within 5 to 10 minutes and can be performed directly on throat swabs at the point‐of‐care. They produce binary results (positive or negative) based on a colour change induced by a specific immunologic reaction with a sensing material (e.g. a test strip). Most rapid tests do not require any specific equipment. Their sensitivity is on average around 85% for a specificity of 95% (Cohen 2016; Lean 2014). In a recent cost‐effectiveness study in primary care in the UK, a cost of GBP 3.25 (around USD 4.00) per rapid test was applied (Little 2014). Rapid tests are easy to perform, but specific training is needed (a one‐hour training session is usually sufficient before implementation). A limitation of rapid tests is their inability to detect other bacteria that can cause sore throat (e.g. groups C and G beta‐haemolytic streptococci). The decision to prescribe antibiotics is guided by the result of the rapid test: patients with a positive test result receive antibiotics, whereas those with a negative result are managed without antibiotics.
Rapid tests can be used alone or in combination with a clinical scoring system. Various scoring systems, such as Centor's, McIsaac's, and the feverPAIN scores, have been proposed. They combine signs and symptoms to help clinicians define groups of patients according to the clinical likelihood of GAS infection (Ebell 2000; Shaikh 2012). These scoring systems can be used alone to guide the decision to prescribe antibiotics. They can also be used to select patients in whom a rapid test should be performed (Figure 1) (Cohen 2015). Clinicians often make decisions based on clinical observation: in the USA, GAS testing (rapid test or throat culture) may be performed in only one‐third of adults with a sore throat (Hong 2011), and in only half of cases in children (Linder 2005).
1.

Type of interventions: Rapid tests can be used alone or in combination with a clinical scoring system.
How the intervention might work
Rapid tests can be performed at the point‐of‐care to assist decision‐making regarding the need for an antibiotic in patients with sore throat. Such a test‐treatment strategy may prevent against unnecessary use of antibiotics compared to standard care.
Potential limitations of rapid tests compared to clinical management include the time and expertise needed to collect the throat sample and perform the test, and patient dissatisfaction. In terms of misclassification, patients with false‐negative results may be exposed to complications of untreated GAS infection, and patients with false‐positive results may receive unnecessary antibiotics.
Why it is important to do this review
It is critical to implement strategies that allow limiting antibiotic prescriptions in ambulatory care settings to curtail the emergence of antibiotic resistance. Rapid tests could be effective in achieving this goal. There is no systematic review of the literature investigating this question.
Objectives
To assess the efficacy and safety of strategies based on rapid tests to guide antibiotic prescriptions for sore throat in primary care settings.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing rapid tests with management based on clinical grounds, to guide the prescription of antibiotic treatment in people with sore throat in primary care settings. In the intervention arm, rapid tests could have been used non‐selectively in all participants or in a selected group of participants based on a scoring system (Figure 1); initiation of antibiotic treatment in the intervention arm must have been based on a positive rapid test result. We defined management based on clinical grounds as unstructured clinical examination, use of a scoring system without a rapid test, or treatment as usual. We included trials that randomised individuals as well as cluster‐RCTs in which individual practitioners (or practices) or emergency departments were randomised.
Types of participants
We included ambulatory care participants of all ages with a chief complaint of acute sore throat, or a clinical diagnosis of pharyngitis or tonsillitis, regardless of sex, severity, or duration of symptoms. In this review, ambulatory care settings included physician offices, walk‐in clinics, family medical centres, and hospital outpatient and emergency departments.
Types of interventions
Use of a rapid test, alone or in combination with a scoring system, with antibiotics being prescribed in case of a positive result. The comparator was management based on clinical grounds (with or without a scoring system).
Types of outcome measures
We focused on efficacy (antibiotic use) and patient‐relevant outcomes, including safety. We did not extract microbiological outcomes, such as microbiological cure.
Primary outcomes
Number of participants provided with an antibiotic prescription.
Number of participants with an antibiotic dispensed.
Secondary outcomes
Duration of sore throat symptoms.
Duration of other symptoms (e.g. fever).
Quality of life measures.
Number of participants with a complication attributed to the index infection (e.g. quinsy, acute rheumatic fever).
Number of participants in need of re‐consultation by the end of follow‐up.
Number of participants in need of hospital admission by the end of follow‐up.
Number of satisfied participants.
Number of participants with an adverse event attributed to the rapid test (i.e. discomfort, dissatisfaction, vomiting).
Search methods for identification of studies
Electronic searches
We developed the search strategy in consultation with a medical librarian and the Information Specialist for the Cochrane Acute Respiratory Infections Group. We searched the following bibliographic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 5, May), which includes the Cochrane Acute Respiratory Infections Group Specialised Register, in the Cochrane Library (searched 5 June 2019);
MEDLINE Ovid (1946 to 5 June 2019);
Embase Elsevier (1947 to 5 June 2019);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature, 1982 to 5 June 2019);
LILACS (Latin American and Caribbean Health Science Information database, 1982 to 5 June 2019); and
Web of Science (1900 to 5 June 2019).
The search strategies are shown in Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5, and Appendix 6. We did not use any language or publication restrictions.
Searching other resources
We searched the following trial registries for additional clinical trials:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 5 June 2019); and
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) (searched 5 June 2019).
We handsearched reference lists of included articles and any relevant review articles identified by the search.
Data collection and analysis
Selection of studies
Two review authors (JYP, NH) independently assessed studies for inclusion, first by screening titles and abstracts, and then by evaluating the full texts of relevant articles. One review author (JFC) acted as arbiter in case of discrepancies. We recorded and reported reasons for excluding studies. We summarised the selection process in a PRISMA flowchart (Figure 2) (Moher 2009).
2.
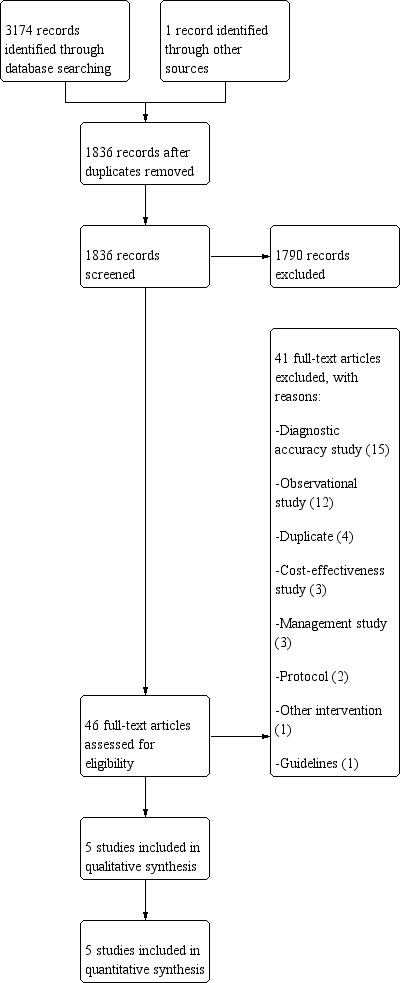
Study flow diagram.
Data extraction and management
Two review authors (JYP, NH) independently extracted a standard set of data from each included study using a prespecified data extraction form (Table 2); one review author (JFC) acted as arbiter in case of discrepancies. We extracted the following data:
1. Data extracted from each study.
| Study ID | First author and year of publication |
| RCT registration number | |
| Type of study | Journal article or conference abstract |
| Methods | Clinical setting (office‐based, walk‐in clinics, hospital outpatient clinics, emergency department, family medicine centres, mixed, other) |
| Single‐ or multicentre study | |
| Country of study | |
| Unit of allocation (clusters, individual participants) | |
| Inclusion criteria | |
| Exclusion criteria | |
| Follow‐up (follow‐up method, duration, outcome(s) assessed) | |
| Intervention(s) | Management in intervention group(s) (rapid test alone or in combination with clinical examination/scoring system) |
| Type of rapid test system used (EIA, OIA, or latex agglutination) | |
| Commercial name and brand of the rapid test | |
| Control(s) | Management in control group(s) (management based on clinical grounds, with or without a scoring system) |
| Participants | Number of clusters (n) |
| Number of participants (n) | |
Participant characteristics:
| |
| Outcomes | Primary outcome(s):
|
Secondary outcome(s):
| |
| Funding | Source(s) of funding (whether any of the authors are affiliated with the manufacturer of the rapid test, the study was directly funded by the manufacturer, authors reported conflicts of interests related to the manufacturer or other funding sources) |
| Notes | Anything else of relevance |
EIA: enzyme immunoassay OIA: optical immunoassay RCT: randomised controlled trial
intervention characteristics: type of intervention (rapid test used alone or in combination with a scoring system), type of rapid test system used (latex agglutination, enzyme immunoassay, optical immunoassay), commercial name and brand of the rapid test used;
outcome data: number of participants, number of participants provided with an antibiotic prescription, number of antibiotic prescriptions dispensed, duration of symptoms, quality of life measures, number of participants with a complication attributed to the index GAS infection (quinsy, acute rheumatic fever), number of participants in need of re‐consultation by the end of follow‐up, number of participants in need of hospital admission by the end of follow‐up, number of satisfied participants, number of participants with an adverse event attributed to the rapid test;
population characteristics: mean age, clinical severity (McIsaac/Centor score); and
study characteristics: year of publication, funding.
One review author (NH) transferred data into Review Manager 5 (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (JYP, NH) independently assessed the risk of bias of included RCTs using the Cochrane 'Risk of bias' tool (Higgins 2011). We scored studies as high, low, or unclear risk of bias in the following domains: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. One review author (JFC) acted as arbiter in case of discrepancies.
Measures of treatment effect
We expected antibiotic prescriptions to be reported dichotomously. We expressed the result from each RCT in tables, where we summarised the number of participants receiving and not receiving antibiotics in each arm. We reported the primary outcome as a risk difference (RD) to summarise the reduction in antibiotic prescription rate between the intervention and control arms. If the baseline risks varied substantially across studies, we would use risk ratios (RRs) instead of RDs. Binary secondary outcomes were reported as odds ratios (OR).
Unit of analysis issues
The unit of analysis was the individual participant. If we included cluster‐RCTs (e.g. where general practitioners were randomised), we adjusted the unit of analysis accordingly. We adjusted for clustering by calculating the effective sample size, based on the number of clusters and the intracluster correlation coefficient (Higgins 2011). If the number of clusters and the intracluster correlation coefficient were not published, we used external estimates obtained from similar studies.
Studies with multiple treatment groups
In case of RCTs with several intervention arms (e.g. rapid test alone and rapid test used in combination with a scoring system), we extracted data from each arm separately. Similarly, in the case of multiple control groups (e.g. unstructured clinical examination and use of a scoring system without a rapid test), we extracted data separately.
Dealing with missing data
We extracted data to permit an intention‐to‐treat analysis, that is participants were analysed according to the group to which they had been randomised. We reported any discrepancies between randomised and analysed participants. In a sensitivity analysis, we considered participants with missing outcome data as having received antibiotics in the meta‐analysis (i.e. intervention failures).
Assessment of heterogeneity
We assessed the statistical heterogeneity across included RCTs using the I2 statistic.
Assessment of reporting biases
We planned to use funnel plots to assess the presence of a small‐study effect.
Data synthesis
Because the between‐study variance was a priori expected to be substantial, we used random‐effects meta‐analysis models to summarise the data from the included RCTs, regardless of the estimate of the I2 statistic results. We planned that if the data allowed, we would perform the following separate meta‐analyses.
Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined).
Comparison 2: Rapid test alone versus clinical grounds (with scoring system).
Comparison 3: Rapid test alone versus clinical grounds (without scoring system).
Comparison 4: Rapid test used with a scoring system versus clinical grounds (with scoring system).
Comparison 5: Rapid test used with a scoring system versus clinical grounds (without scoring system).
We carried out each meta‐analysis for the two primary outcomes (number of participants provided with an antibiotic prescription and number of antibiotic prescriptions dispensed). We performed other analyses (secondary outcomes, subgroup analyses, and sensitivity analyses) only for Comparison 1.
Subgroup analysis and investigation of heterogeneity
We planned that if the data allowed, we would perform the following subgroup analyses:
children (≤ 18 years) versus adults (> 18 years);
type of rapid test (latex agglutination, enzyme immunoassay, or optical immunoassay); and
setting: office‐based versus hospital‐based.
Sensitivity analysis
We carried out sensitivity analyses to explore the robustness of the results when restricting to:
RCTs with low risk of bias (i.e. risk of bias judged as low in at least three domains) according to the 'Risk of bias' tool; and
RCTs where individual participants were randomised (versus cluster‐RCTs).
We also carried out a sensitivity analysis in which participants with missing outcome data were considered as having received antibiotics (i.e. intervention failures).
Summary of findings and assessment of the certainty of the evidence
For Comparison 1 (Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined)), we created a Table 1 for the following outcomes:
number of participants provided with an antibiotic prescription;
number of participants with an antibiotic dispensed;
number of participants with a complication attributed to the index infection; and
number of participants in need of re‐consultation by the end of follow‐up.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We justified all decisions to down‐ or upgrade the certainty of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of studies awaiting classification.
Results of the search
We performed the electronic searches on 5 June 2019. The searches identified 2217 titles (1836 non‐duplicates), of which 1790 articles were excluded based on title or abstract (Figure 2). After assessing the full text of 46 articles, we excluded 41 trials. We included five trials at this stage (Little 2013a; Little 2013b; Llor 2011a; Maltezou 2008; Worrall 2007a). We searched other resources, including trial registries, and identified one additional clinical trial that is currently awaiting classification (Wächtler 2018).
Included studies
The included RCTs were conducted in Canada (Worrall 2007a), Greece (Maltezou 2008), Spain (Llor 2011a), and the UK (Little 2013a; Little 2013b), between 2005, Worrall 2007a, and 2011, Little 2013a; Little 2013b.
Two included trials, Little 2013a; Little 2013b, were part of the same overarching study, the PRImary care Streptococcal Management (PRISM) study (Little 2014).
In Maltezou 2008, children were enrolled in three groups:
Group A: enrolment by private‐practice paediatricians with diagnosis by clinical picture only;
Group B: recruitment by private‐practice paediatricians with diagnosis by a rapid antigen detection test (RADT) and culture; and
Group C: enrolment by hospital‐affiliated paediatricians in the paediatric outpatient clinic with diagnosis by RADT and culture.
We contacted the trial authors for clarification, who informed us that only participants enrolled by private‐practice paediatricians were truly randomised, thus only participants from Groups A and B were included in the review.
Three trials included subgroups that were not eligible for inclusion (Little 2013a; Little 2013b; Maltezou 2008). In Little 2013a and Little 2013b, the “Delayed antibiotics” group (“Group 1”) was excluded because, by definition, all participants were expected to be prescribed antibiotics. In Maltezou 2008, Group C was excluded because participants were not truly randomised. After exclusion of these participants (n = 764), the review contained a total of 2891 participants.
Three RCTs included both adults and children (Little 2013a; Little 2013b; Llor 2011a); one included only children (Maltezou 2008); and one included only adults (Worrall 2007a).
The unit of randomisation was the participant in two RCTs (Little 2013a; Little 2013b), the physician in two RCTs (Maltezou 2008; Worrall 2007a), and the healthcare centre in one RCT (Llor 2011a). The number of clusters ranged from 17, Maltezou 2008, to 37, Worrall 2007a; average cluster size ranged from 14, Worrall 2007a, to 38, Maltezou 2008. We adjusted for clustering by calculating the effective sample size, using an intracluster correlation coefficient of 0.01 (Adams 2004); the total number of participants included in the quantitative synthesis after adjusting for clustering was 2545. Effective sample sizes were used in both numerators and denominators in every table, figure, and analysis. The design effect was of 1.26 in Llor 2011a, 1.37 in Maltezou 2008, and 1.13 in Worrall 2007a.
Management in the intervention group was based on the use of an RADT combined with a clinical scoring system, with RADT use initiated above a prespecified score in three RCTs (Little 2013a; Little 2013b; Maltezou 2008). In Worrall 2007a, some physicians were asked to use RADT on all participants, whilst others were asked to use RADT only above a certain clinical score. In Llor 2011a, RADT was used on all participants in the intervention group.
Management in the control group relied on a clinical scoring system in three RCTs (Little 2013a; Llor 2011a; Maltezou 2008). In Worrall 2007a, some physicians were asked to use a clinical scoring system, whilst others were asked to rely on usual clinical practice for decision‐making. Management in Little 2013b was not clearly described for either the intervention or the control group.
Clinical scoring systems used across studies were the Centor score, Llor 2011a; Maltezou 2008; Worrall 2007a, and FeverPAIN (Little 2013a; Little 2013b).
Excluded studies
Of the 46 trials assessed as full text, we excluded 41. Thirty‐three trials were not RCTs (diagnostic accuracy studies, n = 15; observational studies, n = 12; cost‐effectiveness studies, n = 3; management studies, n = 3). Four trials were duplicates; two references led to trial protocols; one trial evaluated another intervention; and one led to a clinical guideline.
Risk of bias in included studies
The overall risk of bias is presented graphically and summarised in Figure 3. For further information on the included studies, see Characteristics of included studies table.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation (selection bias)
In three trials, participants were randomised to treatment and control groups using appropriate random sequence generation methods. Two trials did not report random sequence generation; we assessed the risk of bias for these studies as unclear.
Allocation
In two trials, we judged the method used to randomise participants to the treatment and control groups as appropriate. Allocation concealment of individuals does not apply to cluster‐RCTs at the physician or practice level, so we graded these three studies as at unclear risk of bias.
Blinding
Rapid test results were used in decision‐making, thus clinicians and participants were considered to be non‐blinded, resulting in a judgement of high risk of bias for all included RCTs.
Incomplete outcome data
The numbers of participants with missing data on prescribed antibiotics were as follows: one in Little 2013a (intervention group); one in Little 2013b (control group); 14 in Llor 2011a (10 in the control group and four in the intervention group); nine in Maltezou 2008 (control group); and none in Worrall 2007a.
The numbers of participants with missing data on dispensed antibiotics were as follows: 99 in Little 2013a; 178 in Little 2013b; 14 in Llor 2011a; nine in Maltezou 2008; and none in Worrall 2007a.
Selective reporting
We assessed the risk of selective reporting as low in four studies (Little 2013a; Little 2013b; Maltezou 2008; Worrall 2007a). We judged the risk of selective reporting as high in one study because of inconsistencies in outcome reporting (Llor 2011a).
Other potential sources of bias
None.
Effects of interventions
See: Table 1
COMPARISON 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined)
Primary outcomes
1. Number of participants provided with an antibiotic prescription
Five trials (2545 participants) reported on this outcome. The proportion of participants who received a prescription of antibiotics ranged from 32% to 46% in the intervention group and from 57% to 70% in the control group. The summary result for all included trials showed a statistically significant effect of using a rapid test on the number of participants provided with an antibiotic prescription (risk difference (RD) −0.25, 95% confidence interval (CI) −0.31 to −0.18; P < 0.001; I2 = 62%; moderate‐certainty evidence; Analysis 1.1; Figure 4). We did not use a funnel plot to assess reporting bias because of the low number of included trials. See Table 1.
1.1. Analysis.

Comparison 1: Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), Outcome 1: Number of participants provided with an antibiotic prescription
4.

Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), outcome: 1.1 Number of participants provided with an antibiotic prescription.
2. Number of participants with an antibiotic dispensed
Two trials (900 participants) reported on this outcome (Little 2013a; Little 2013b). In the intervention groups, the antibiotic dispensing rate was 35% in both trials. In the control groups, antibiotic dispensing rates ranged from 37% to 47%. The summary result for the two included trials showed a non‐significant effect of using a rapid test on the number of antibiotic prescriptions dispensed (RD −0.07, 95% CI −0.17 to 0.02; P = 0.13; I2 = 53%; low‐certainty evidence; Analysis 1.2; Figure 5). See Table 1.
1.2. Analysis.

Comparison 1: Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), Outcome 2: Number of participants with an antibiotic dispensed
5.

Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), outcome: 1.2 Number of participants with an antibiotic dispensed.
Secondary outcomes
1. Duration of sore throat symptoms
No trials reported on this outcome.
2. Duration of other symptoms (e.g. fever)
No trials reported on this outcome.
3. Quality of life measures
No trials reported on this outcome.
4. Number of participants with a complication attributed to the index infection (e.g. quinsy, acute rheumatic fever)
Four trials (2075 participants) reported on this outcome. Two trials reported that no such complications were observed (Llor 2011a; Maltezou 2008). Two trials reported suppurative complications (Little 2013a; Little 2013b). The summary odds ratio (OR) was 0.85, 95% CI 0.03 to 26.65; P = 0.93; I2 = 62%; very low‐certainty evidence; Analysis 1.3; Figure 6, which means that participants in the rapid testing group were less likely to develop complications of the index infection, but this association was not statistically significant. See Table 1.
1.3. Analysis.

Comparison 1: Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), Outcome 3: Number of participants with a complication attributed to the index infection
6.

Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), outcome: 1.6 Number of participants with a complication attributed to the index infection.
5. Number of participants in need of re‐consultation by the end of follow‐up
Two trials (1161 participants) reported on this outcome (Little 2013a; Little 2013b). The summary OR was 1.12, 95% CI 0.57 to 2.21; P = 0.74; I2 = 59%; low‐certainty evidence; Analysis 1.4, which means that participants in the rapid testing group were more likely to be in need of re‐consultation by the end of study follow‐up, but this association was not statistically significant. See Table 1.
1.4. Analysis.

Comparison 1: Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined), Outcome 4: Number of participants in need of re‐consultation by the end of follow‐up
6. Number of participants in need of hospital admission by the end of follow‐up
No trials reported on this outcome.
7. Number of satisfied participants
No trials reported on this outcome.
8. Number of participants with an adverse event attributed to the rapid test (i.e. discomfort, dissatisfaction, vomiting).
No trials reported on this outcome.
COMPARISON 2: Rapid test alone versus clinical grounds (with scoring system)
Primary outcomes
1. Number of participants provided with an antibiotic prescription
One trial (256 participants) reported on this outcome (Worrall 2007a). This study showed a statistically significant effect of using an RADT on the number of participants provided with an antibiotic prescription (RD −0.29, 95% CI −0.40 to −0.17; P < 0.001; Analysis 2.1).
2.1. Analysis.

Comparison 2: Comparison 2: Rapid test alone versus clinical grounds (with scoring system), Outcome 1: Number of participants provided with an antibiotic prescription
2. Number of participants with an antibiotic dispensed
No trials reported data on this outcome.
Secondary outcomes
No trials reported on any of the secondary outcomes.
COMPARISON 3: Rapid test alone versus clinical grounds (without scoring system)
Primary outcomes
1. Number of participants provided with an antibiotic prescription
Three trials (1129 participants) reported on this outcome (Llor 2011a; Maltezou 2008; Worrall 2007a). These studies showed a statistically significant effect of using an RADT on the number of participants provided with an antibiotic prescription (RD −0.29, 95% CI −0.40 to −0.19; P < 0.001; I2 = 71%; Analysis 3.1).
3.1. Analysis.

Comparison 3: Comparison 3: Rapid test alone versus clinical grounds (without scoring system), Outcome 1: Number of participants provided with an antibiotic prescription
2. Number of participants with an antibiotic dispensed
No trials reported data on this outcome.
Secondary outcomes
No trials reported on any of the secondary outcomes.
COMPARISON 4: Rapid test used with a scoring system versus clinical grounds (with scoring system)
Primary outcomes
1. Number of participants provided with an antibiotic prescription
Three trials (1416 participants) reported on this outcome (Little 2013a; Little 2013b; Worrall 2007a). These studies showed a statistically significant effect of using an RADT on the number of participants provided with an antibiotic prescription (RD −0.21, 95% CI −0.26 to −0.16; P < 0.001; I2 = 0%; Analysis 4.1).
4.1. Analysis.

Comparison 4: Comparison 4: Rapid test used with a scoring system versus clinical grounds (with scoring system), Outcome 1: Number of participants provided with an antibiotic prescription
2. Number of participants with an antibiotic dispensed
Two trials (900 participants) reported on this outcome (Little 2013a; Little 2013b). The pooled result showed a non‐significant effect of using an RADT on the number of antibiotic prescriptions dispensed (RD −0.07, 95% CI −0.17 to 0.02; P = 0.13; I2 = 53%; Analysis 4.2).
4.2. Analysis.

Comparison 4: Comparison 4: Rapid test used with a scoring system versus clinical grounds (with scoring system), Outcome 2: Number of participants with an antibiotic dispensed
Secondary outcomes
No trials reported on any of the secondary outcomes.
COMPARISON 5: Rapid test used with a scoring system versus clinical grounds (without scoring system)
Primary outcomes
1. Number of participants provided with an antibiotic prescription
One trial (214 participants) reported on this outcome (Worrall 2007a). This study showed a statistically significant effect of using an RADT on the number of participants provided with an antibiotic prescription (RD −0.20, 95% CI −0.34 to −0.07; P = 0.003; Analysis 5.1).
5.1. Analysis.

Comparison 5: Comparison 5: Rapid test used with a scoring system versus clinical grounds (without scoring system), Outcome 1: Number of participants provided with an antibiotic prescription
2. Number of participants with an antibiotic dispensed
No trials reported data on this outcome.
Secondary outcomes
No trials reported on any of the secondary outcomes.
Subgroup analysis and investigation of heterogeneity
1. Children (≤ 18 years) versus adults (> 18 years)
Three RCTs included both adults and children (Little 2013a; Little 2013b; Llor 2011a); one RCT included only children (Maltezou 2008); and one RCT included only adults (Worrall 2007a). We judged the data as too scarce to permit subgroup analysis.
2. Type of rapid test (latex agglutination, enzyme immunoassay, or optical immunoassay)
All included studies used an enzyme immune‐assay in the intervention group, therefore we could not perform this pre‐planned subgroup analysis.
3. Setting: office‐based versus hospital‐based
Two trials were conducted in general practitioner practices (Little 2013a; Little 2013b), one in family doctors’ offices (Worrall 2007a), one in paediatricians’ offices (Maltezou 2008); and one in primary healthcare centres (Llor 2011a). No trial was hospital‐based. We could therefore not perform this pre‐planned subgroup analysis.
Sensitivity analysis
The following sensitivity analyses apply to Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined).
1. RCTs with low risk of bias according to the 'Risk of bias' tool
We judged three RCTs as at low overall risk of bias (Little 2013a; Little 2013b; Worrall 2007a). The summary estimate of the number of participants provided with an antibiotic prescription was comparable when restricting to RCTs judged as at low overall risk of bias (1646 participants): RD −0.22, 95% CI −0.27 to −0.17; P < 0.001; I2 = 0%; Analysis 6.1.
6.1. Analysis.

Comparison 6: Sensitivity analysis: studies at low risk of bias, Outcome 1: Sensitivity analysis: studies at low risk of bias
2. RCTs where individual participants were randomised (versus cluster‐RCTs)
The unit of randomisation was the participant in two trials (Little 2013a; Little 2013b), the physician in two trials (Maltezou 2008; Worrall 2007a), and the healthcare centre in one trial (Llor 2011a). The summary estimate of the number of participants provided with an antibiotic prescription was stable when restricting to RCTs in which individual participants were randomised (1176 participants): RD −0.21, 95% CI −0.27 to −0.16; P < 0.001; I2 = 0%; Analysis 7.1.
7.1. Analysis.

Comparison 7: Sensitivity analysis: Studies with individual randomisation, Outcome 1: Sensitivity analysis: Studies with individual randomisation
3. Imputation of missing outcome data as intervention failures (i.e. receiving antibiotics)
The numbers of participants with missing data on prescribed antibiotics were as follows: one in Little 2013a (intervention group); one in Little 2013b (control group); 14 in Llor 2011a (10 in the control group and four in the intervention group); nine in Maltezou 2008 (control group); none in Worrall 2007a. The summary estimate of the number of participants provided with an antibiotic prescription was stable when imputing missing values as intervention failures (2557 participants): RD −0.25, 95% CI −0.32 to −0.18; P < 0.001; I2 = 61%; Analysis 8.1.
8.1. Analysis.

Comparison 8: Sensitivity analysis: Missing data as failures, Outcome 1: Number of participants provided with an antibiotic
Discussion
Summary of main results
We identified five RCTs evaluating the effect of using an RADT on antibiotic prescription in people with a sore throat in primary care settings, encompassing a total of 2891 participants (2545 after adjusting for clustering). We found that using RADTs reduced antibiotic prescriptions by 25% absolute risk reduction (RD) (95% CI ‐31% to ‐18%); antibiotic dispensing was not significantly affected (RD ‐7%, 95% CI ‐17% to 2%; data from only two RCTs).
Overall completeness and applicability of evidence
No data were reported concerning most of our prespecified secondary outcomes. Two included studies reported that no pharyngitis‐related complications occurred during follow‐up, whilst reporting observed side effects from antibiotic use, mostly skin rash and diarrhoea (Llor 2011a; Maltezou 2008). Two trials reported both suppurative complications and side effects (Little 2013a; Little 2013b), and one trial did not report information about suppurative complications and antibiotic side effects (Worrall 2007a). Hence, the risk‐to‐benefit balance between side effects from antibiotic use and complications resulting from withholding antimicrobial treatment remains unclear. However, intervention trials are commonly underpowered for detecting adverse events, notably rare but severe side effects (Vandenbroucke 2004).
Rapid tests can be used alone or in combination with clinical scoring systems, such as the Centor score. Similarly, the control can be clinical grounds alone, with or without a scoring system. Unsurprisingly, the most extreme contrast led to the most extreme impact: when combining data from the three trials (1129 participants) that compared RADT (without a scoring system) against clinical grounds (without a scoring system), the reduction in the number of people provided with an antibiotic prescription rose to RD −29%, 95% CI −40% to −19% (Table 3). Of note, clinical scoring systems may perform poorly in children (Cohen 2015).
2. Summary of the various comparisons assessed in the review.
| Comparison | Intervention: management based on the results of rapid testing | Control: management based on clinical grounds | Number of trials | Number of participants | Number of participants provided with an antibiotic prescription, risk difference (95% confidence interval) |
| 1 | Rapid test with and without using a scoring system (arms combined) | Clinical grounds with and without using a scoring system (arms combined) | 5 | 2545 | −25% (−31% to −18%) |
| 2 | Rapid test for all | With a scoring system | 1 | 256 | −29% (−40% to −17%) |
| 3 | Rapid test for all | Without a scoring system | 3 | 1129 | −29% (−40% to −19%) |
| 4 | Rapid testing only if above a certain clinical score | With a scoring system | 3 | 1416 | −21% (−26% to −16%) |
| 5 | Rapid testing only if above a certain clinical score | Without a scoring system | 1 | 214 | −20% (−34% to −7%) |
Quality of the evidence
We assessed only three RCTs as at low overall risk of bias (Little 2013a; Little 2013b; Worrall 2007a), mainly due to poor reporting impeding methodological quality assessment and because blinding was impossible. The support for the impact of RADTs on antibiotics dispensing is weaker than for the impact on antibiotic prescribing, mainly due to sparse data (two RCTs). Moreover, the two RCTs reporting on antibiotic dispensing were performed by the same research team, thereby decreasing their external validity. The discrepancy between prescription rates and dispensing rates could be explained by the fact that, in order to obtain data on antibiotic dispenses, follow‐up was required. One could hypothesise that people more adherent to the study protocol were both more likely to take the antibiotics from the pharmacy and to participate in the study follow‐up. However, if follow‐up had instead included all participants, one could have expected a greater RD in antibiotic dispensing between intervention and control groups. Moreover, in both studies, antibiotic dispensing rates in the control groups were rather low (< 50%) (Little 2013a; Little 2013b). Participant behaviour with regard to filling their prescriptions may have been influenced by the knowledge that the trial was aimed at reducing antibiotic use.
Potential biases in the review process
The search strategy was developed by a medical librarian and included grey literature (e.g. conference abstracts and poster presentations). Also, as per Cochrane standards, the literature search was performed independently by two review authors.
Our meta‐analysis is based on aggregated data. An analysis of individual patient data would have been preferable and would have offered the possibility of performing multiple imputations of missing data and multivariate adjustments.
Agreements and disagreements with other studies or reviews
This review included RCTs originating from high‐income countries, involving both adults and children, and using recent RADT devices. Antibiotic prescription rates in the control groups ranged between 57% and 70%, which is consistent with reported antibiotic use for sore throat in high‐income countries (range 53% to 93% in Chahwakilian 2011, Fleming‐Dutra 2016, Hong 2011, Linder 2005, and Pouwels 2018).
In Worrall 2007a, the sample calculation was based on the trialists’ aim of detecting a 25% reduction in the rate of antibiotic prescribing, which is in line with our findings.
Our estimates are also coherent with results from non‐randomised evidence (i.e. observational studies) assessing the association between RADT and antibiotic use (range −16% to −23% in Ayanruoh 2009, Cardoso 2013, Humair 2006, and McIsaac 2004). For example, in Ayanruoh’s before‐after study, which included more than 8000 participants, implementing rapid testing led to a decrease in antibiotic prescription rates for children with pharyngitis (41.4% for those treated before implementing rapid testing versus 22.5% for those treated after implementing rapid testing; absolute RD −18.9%) (Ayanruoh 2009).
This is the first systematic review assessing the clinical impact of rapid testing in sore throat, but several reviews have already assessed the diagnostic accuracy of such rapid tests for sore throat. These reviews have shown that rapid tests are highly accurate, with a sensitivity of about 85% and a specificity of about 95% to detect streptococcal cases, with throat culture as the reference standard (Cohen 2016; Lean 2014). In contrast, a large‐scale validation study including 142,081 patients ≥ 15 years old showed that the Centor score poorly correlates with the risk of strep throat (proportion of group A streptococcus‐positives: score 0, 7%; score 1, 12%; score 2, 21%; score 3, 38%; score 4, 57%) (Fine 2012). The same applies to children (Cohen 2015). Because RADTs are highly specific, they may have a greater impact on antibiotic prescribing than clinical scoring systems.
Other point‐of‐care interventions are available to guide antibiotic prescribing in primary care. In a recent Cochrane Review based on six trials, C‐reactive protein testing had a significant impact on antibiotic prescribing in participants with acute respiratory infections in primary care settings (pooled risk ratio (RR) 0.78, 95% CI 0.66 to 0.92) (Aabenhus 2014). In comparison, our effect size was −25% in absolute RD, which is equivalent to an RR of 0.62 (95% CI 0.54 to 0.70), showing that RADTs might have a greater impact than C‐reactive protein testing.
Authors' conclusions
Implications for practice.
Sore throat is most often a mild and self‐limiting disease, even when due to group A streptococcus (GAS). Antibiotic treatment may be indicated to prevent suppurative and non‐suppurative complications (e.g. acute rheumatic fever) and reduce symptom duration and infection spread, but absolute benefits are modest (Spinks 2013), notably in high‐income countries.
We found a −25% risk difference in antibiotic prescribing when using rapid testing compared to management based on clinical grounds alone in people with sore throat. With increasing antimicrobial resistance, a rapid antigen detection test (RADT) may be useful in the clinical management of people with a sore throat. The cost of a rapid test is equivalent to that of a course of a first‐line antibiotic such as ampicillin. Rapid tests do not require specific equipment and can be performed at the point‐of‐care within 5 to 10 minutes by personnel with limited training. Hence, rapid tests seem feasible in various settings, including low‐resource countries. In settings where it is recommended to treat GAS pharyngitis with antibiotics, patients, physicians, and policymakers should prioritise RADTs in their attempt to reduce antimicrobial resistance.
Implications for research.
Additional randomised controlled trials (RCTs) should be conducted to explore the robustness of our estimates. Whilst we observed a significant reduction in the use of antibiotics, we were not able to assess possible sources of heterogeneity, such as differences across age groups (children versus adults) and settings (office‐based versus hospital‐based physicians). We also encourage future trials to investigate not only the effect of RADTs on antibiotic use, but also patient‐relevant outcomes such as the duration of other symptoms of sore throat and fever, quality of life measures, and patient satisfaction. The impact of implementing rapid tests on the acquisition of antibiotic‐resistant bacteria may also be of interest. Also, RADTs should be compared to other point‐of‐care tests available in primary care, such as C‐reactive protein. A new generation of molecular point‐of‐care tests for detecting GAS is now on the market; first evaluations show that they may be oversensitive compared to throat culture, which might negatively impact antimicrobial stewardship efforts (Tanz 2009). We included RCTs conducted in high‐income countries (Canada, Greece, Spain, and the UK), but the impact of rapid testing on antibiotic prescribing might be even more favourable in low‐ and middle‐income settings with high antibiotic use and currently low access to point‐of‐care tests (Joachim 2010).
History
Protocol first published: Issue 11, 2016 Review first published: Issue 6, 2020
Acknowledgements
We thank Mr René Spijker (Dutch Cochrane Centre, University Medical Center, Utrecht, the Netherlands) and Mr David Honeyman (Faculty Library, Bond University, Australia) for their assistance in the literature search. We thank Dr Maltezou and Dr Llor for clarifying aspects of their trial. We are grateful to the members of the Cochrane Acute Respiratory Infections Group for their comments and support. We also thank the following people for commenting on the draft protocol and full‐text review (in alphabetical order): Bruce Arroll, Itzhak Brook, Ann Fonfa, Gail Haywood, Teresa Neeman, Amanda Roberts, Knut Schroeder, Anneliese Spinks, Conor Teljeur.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
| # | Searches |
| 1 | exp Pharyngitis/ |
| 2 | pharyngitis.tw. |
| 3 | Tonsillitis/ |
| 4 | tonsillitis.tw. |
| 5 | (tonsillopharyngitis or pharyngotonsillitis).tw. |
| 6 | (sore* adj2 throat*).tw. |
| 7 | ((throat* or pharyn* or tonsil*) adj3 (infect* or inflam* or strep*)).tw. |
| 8 | Pharynx/mi [Microbiology] |
| 9 | Streptococcal Infections/ |
| 10 | (strep* adj5 (throat* or pharyn* or tonsil*)).tw. |
| 11 | ("group a" adj5 streptococc*).tw. |
| 12 | gabhs.tw. |
| 13 | (beta‐hemoly* or beta‐haemoly*).tw. |
| 14 | lancefield group a.tw. |
| 15 | Streptococcus pyogenes/ |
| 16 | (streptococcus pyogenes or "s. pyogenes" or "s.pyogenes").tw. |
| 17 | or/1‐16 |
| 18 | Immunoassay/ |
| 19 | exp Immunoenzyme Techniques/ |
| 20 | (enzyme adj2 (immunoassay* or immuno‐assay* or immunosorbent)).tw. |
| 21 | Immunochromatography/ |
| 22 | immunochromatograph*.tw. |
| 23 | Immunosorbent Techniques/ |
| 24 | exp Enzyme‐Linked Immunosorbent Assay/ |
| 25 | (elisa or elisas or eia or eias).tw. |
| 26 | (sandwich* adj2 assay*).tw. |
| 27 | (lateral flow adj2 assay).tw. |
| 28 | (optical adj2 (immunoassay* or immuno‐assay*)).tw. |
| 29 | (oia or oias).tw. |
| 30 | Antigens, Bacterial/ |
| 31 | Reagent Kits, Diagnostic/ |
| 32 | Point‐of‐Care Systems/ |
| 33 | ((rapid or "point of care" or "near patient" or poc or poct or bedside) adj5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*)).tw. |
| 34 | (radt or radts or rdt or rdts).tw. |
| 35 | (antigen* adj3 detect*).tw. |
| 36 | or/18‐35 |
| 37 | 17 and 36 |
| 38 | ((randomized controlled trial or controlled clinical trial).pt. or drug therapy.fs. or (randomized or randomised or placebo or randomly or trial or groups).ab.) not (animals/ not (humans/ and animals/)) [Cochrane highly sensitive filter 2008] |
| 39 | 37 and 38 |
Appendix 2. Embase (Elsevier) search strategy
| # | Searches |
| 1 | 'pharyngitis'/exp |
| 2 | pharyngitis:ti,ab |
| 3 | tonsillitis:ti,ab |
| 4 | tonsillopharyngitis:ti,ab OR pharyngotonsillitis:ti,ab |
| 5 | (sore* NEAR/2 throat*):ti,ab |
| 6 | ((throat* OR pharyn* OR tonsil*) NEAR/3 (infect* OR inflam* OR strep*)):ti,ab |
| 7 | 'pharynx'/de |
| 8 | 'streptococcus infection'/de OR 'group a streptococcal infection'/de OR 'streptococcal pharyngitis'/de |
| 9 | (strep* NEAR/5 (throat* OR pharyn* OR tonsil*)):ti,ab |
| 10 | ('group a' NEAR/5 streptococc*):ti,ab |
| 11 | gabhs:ti,ab |
| 12 | 'beta hemoly*':ti,ab OR 'beta haemoly*':ti,ab |
| 13 | 'lancefield group a':ti,ab |
| 14 | 'streptococcus pyogenes'/de |
| 15 | 'streptococcus pyogenes':ti,ab OR 's pyogenes':ti,ab OR s pyogenes:ti,ab |
| 16 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 |
| 17 | 'immunoassay'/exp |
| 18 | (enzyme NEAR/2 (immunoassay* OR 'immuno assay*' OR immunosorbent)):ti,ab |
| 19 | 'immunoaffinity chromatography'/de |
| 20 | immunochromatograph*:ti,ab |
| 21 | 'immunoadsorption'/de |
| 22 | elisa:ti,ab OR elisas:ti,ab OR eia:ti,ab OR eias:ti,ab |
| 23 | (sandwich* NEAR/2 assay*):ti,ab |
| 24 | ('lateral flow' NEAR/2 assay):ti,ab |
| 25 | (optical NEAR/2 (immunoassay* OR 'immuno assay*')):ti,ab |
| 26 | oia:ti,ab OR oias:ti,ab |
| 27 | 'bacterial antigen'/exp |
| 28 | 'diagnostic kit'/exp |
| 29 | 'hospital information system'/de OR 'point of care system'/de OR 'point of care testing'/de |
| 30 | ((rapid OR 'point of care' OR 'near patient' OR poc OR poct OR bedside) NEAR/5 (test OR tests OR testing OR detect* OR diagnos* OR screen* OR kit OR kits OR assay*)):ti,ab |
| 31 | radt:ti,ab OR radts:ti,ab OR rdt:ti,ab OR rdts:ti,ab |
| 32 | (antigen* NEAR/3 detect*):ti,ab |
| 33 | #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 |
| 34 | #16 AND #33 |
| 35 | random* OR factorial OR crossover OR placebo OR blind OR blinded OR assign OR assigned OR allocate OR allocated OR 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp NOT ('animal'/exp NOT 'animal'/exp AND 'human'/exp)) |
| 36 | #34 AND #35 |
Appendix 3. Web of Science (Clarivate Analytics) search strategy
Databases: Web of Science (Science Citation Index Expanded (SCI‐EXPANDED) 1900‐present and Conference Proceedings Citation Index‐ Science (CPCI‐S)) 1990‐present
Topic=(pharyngitis or tonsillitis or tonsillopharyngitis or pharyngotonsillitis or (sore* NEAR/2 throat*) or ((throat* or pharyn* or tonsil*) NEAR/3 (infect* or inflam* or strep*)) or (strep* NEAR/5 (throat* or pharyn* or tonsil*)) or ("group a" NEAR/5 streptococc*) or gabhs or beta‐hemoly* or beta‐haemoly* or "lancefield group a" or "streptococcus pyogenes" or "s. pyogenes" or "s.pyogenes") AND ((enzyme NEAR/2 (immunoassay* or immuno‐assay* or immunosorbent)) or immunochromatograph* or elisa or elisas or eia or eias or (sandwich* NEAR/2 assay*) or (lateral flow NEAR/2 assay) or (optical NEAR/2 (immunoassay* or immuno‐assay*)) or oia or oias or ((rapid or "point of care" or "near patient" or poc or poct or bedside) NEAR/5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*)) or radt or radts or rdt or rdts or (antigen* NEAR/3 detect*))
AND
Topic=(random* or placebo* or allocat* or crossover* or "cross over" or ((singl* or doubl*) NEAR/1 blind*)) OR Title=(trial)
Appendix 4. LILACS (BIREME) search strategy
(mh:C07.550.781$ OR pharyngitis OR faringit* OR tonsillitis OR tonsilit* OR (tonsillopharyngitis OR pharyngotonsillitis) OR "sore throat" OR "dolor de garganta" OR "dor de garganta" OR "sore throats" OR "dolores de garganta" OR "dores de garganta" OR ((throat* OR garganta OR pharyn* OR faringe OR tonsil* OR amígdalas) AND (infect* OR infección* OR infecção OR infecções OR inflam* OR enconado OR inflama* OR strep* OR estreptoc*)) OR mh:pharynx OR mh:"Streptococcal Infections" OR ("group a" AND streptococc*) OR gabhs OR (beta‐hemoly* OR beta‐haemoly*) OR "lancefield group a" OR mh:"Streptococcus pyogenes" OR ("streptococcus pyogenes" OR "s. pyogenes" OR "s.pyogenes")) AND (mh:immunoassay OR mh:E05.478.566.350$ OR ((enzyme OR enzima) AND (immunoassay* OR inmunoensayo OR imunoensaio OR immuno‐assay* OR immunosorbent)) OR mh:immunochromatography OR immunochromatograph* OR inmunocromatografía OR imunocromatografia OR mh:"Immunosorbent Techniques" OR "técnicas de inmunoadsorción" OR "técnicas de Imunoadsorção" OR mh:"Enzyme‐Linked Immunosorbent Assay" OR "ensayo de inmunoadsorción enzimática" OR "ensaio de imunoadsorção enzimática" OR (elisa OR elisas OR eia OR eias) OR (sandwich* AND assay*) OR ("lateral flow" AND assay) OR (optical AND (immunoassay* OR immuno‐assay*)) OR (oia OR oias) OR mh:"Antigens, Bacterial" OR mh:"Reagent Kits, Diagnostic" OR mh:point‐of‐care systems OR "sistemas de atención de punto" OR "sistemas automatizados de assistência junto ao leito" OR ((rapid OR "point of care" OR "near patient" OR poc OR poct OR bedside) AND (test OR tests OR testing OR detect* OR diagnos* OR screen* OR kit OR kits OR assay*)) OR (radt OR radts OR rdt OR rdts) OR (antigen* AND detect*)) AND (instance:"regional") AND ( db:("LILACS"))
Appendix 5. CENTRAL (Cochrane Library) search strategy
| # | Searches |
| 1 | [mh Pharyngitis] |
| 2 | pharyngitis:ti,ab |
| 3 | tonsillitis:ti,ab |
| 4 | (tonsillopharyngitis or pharyngotonsillitis):ti,ab |
| 5 | (sore* near/2 throat*):ti,ab |
| 6 | ((throat* or pharyn* or tonsil*) near/3 (infect* or inflam* or strep*)):ti,ab |
| 7 | [mh ^Pharynx] |
| 8 | [mh ^"Streptococcal Infections"] |
| 9 | (strep* near/5 (throat* or pharyn* or tonsil*)):ti,ab |
| 10 | ("group a" near/5 streptococc*):ti,ab |
| 11 | gabhs:ti,ab |
| 12 | (beta‐hemoly* or beta‐haemoly*):ti,ab |
| 13 | "lancefield group a":ti,ab |
| 14 | [mh ^"Streptococcus pyogenes"] |
| 15 | ("streptococcus pyogenes" or "s. pyogenes" or s.pyogenes):ti,ab |
| 16 | {or #1‐#15} |
| 17 | [mh ^Immunoassay] |
| 18 | [mh "Immunoenzyme Techniques"] |
| 19 | (enzyme near/2 (immunoassay* or immuno‐assay* or immunosorbent)):ti,ab |
| 20 | [mh ^Immunochromatography] |
| 21 | immunochromatograph*:ti,ab |
| 22 | [mh ^"Immunosorbent Techniques"] |
| 23 | [mh "Enzyme‐Linked Immunosorbent Assay"] |
| 24 | (elisa or elisas or eia or eias):ti,ab |
| 25 | (sandwich* near/2 assay*):ti,ab |
| 26 | ("lateral flow" near/2 assay):ti,ab |
| 27 | (optical near/2 (immunoassay* or immuno‐assay*)):ti,ab |
| 28 | (oia or oias):ti,ab |
| 29 | [mh "Antigens, Bacterial"] |
| 30 | [mh "Reagent Kits, Diagnostic"] |
| 31 | [mh "Point‐of‐Care Systems"] |
| 32 | ((rapid or "point of care" or "near patient" or poc or poct or bedside) near/5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*)):ti,ab |
| 33 | (radt or radts or rdt or rdts):ti,ab |
| 34 | (antigen* near/3 detect*):ti,ab |
| 35 | {or #17‐#34} |
| 36 | #16 and #35 |
Appendix 6. CINAHL (EBSCO) search strategy
| # | Searches |
| 1 | MH "Pharyngitis" |
| 2 | TI pharyngitis OR AB pharyngitis |
| 3 | MH "Tonsillitis" |
| 4 | TI tonsillitis OR AB tonsillitis |
| 5 | TI pharyngotonsillitis OR AB pharyngotonsillitis OR TI tonsillopharyngitis OR AB tonsillopharyngitis |
| 6 | TI (sore* N2 throat*) OR AB (sore* N2 throat*) |
| 7 | TI ((throat* or pharyn* or tonsil*) N3 (infect* or inflam* or strep*)) OR AB ((throat* or pharyn* or tonsil*) N3 (infect* or inflam* or strep*)) |
| 8 | MH "Pharynx/MI" |
| 9 | MH "Streptococcal Infections" |
| 10 | TI (strep* N5 (throat* or pharyn* or tonsil*)) OR AB (strep* N5 (throat* or pharyn* or tonsil*)) |
| 11 | TI ("group a" N5 streptococc*) OR AB ("group a" N5 streptococc*) |
| 12 | TI gabhs OR AB gabhs |
| 13 | TI (beta‐hemoly* or beta‐haemoly*) OR AB (beta‐hemoly* or beta‐haemoly*) |
| 14 | TI "lancefield group a" OR AB "lancefield group a" |
| 15 | MH "Streptococcus" |
| 16 | TI ("streptococcus pyogenes" or "s. pyogenes" or "s.pyogenes") OR AB ("streptococcus pyogenes" or "s. pyogenes" or "s.pyogenes") |
| 17 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 |
| 18 | MH "Immunoassay" |
| 19 | MH "Immunoenzyme Techniques" |
| 20 | TI (enzyme N2 (immunoassay* or immuno‐assay* or immunosorbent)) OR AB (enzyme N2 (immunoassay* or immuno‐assay* or immunosorbent)) |
| 21 | TI immunochromatograph* OR AB immunochromatograph* |
| 22 | MH "Immunosorbent Techniques" |
| 23 | MH "Enzyme‐Linked Immunosorbent Assay" |
| 24 | TI (elisa or elisas or eia or eias) OR AB (elisa or elisas or eia or eias) |
| 25 | TI (sandwich* N2 assay*) OR AB (sandwich* N2 assay*) |
| 26 | TI ("lateral flow" N2 assay) OR TI ("lateral flow" N2 assay) |
| 27 | TI (optical N2 (immunoassay* or immuno‐assay*)) OR AB (optical N2 (immunoassay* or immuno‐assay*)) |
| 28 | TI (oia or oias) OR AB (oia or oias) |
| 29 | MH "Antigens, Bacterial+" |
| 30 | MH "Reagent Kits, Diagnostic+" |
| 31 | MH "Clinical Information Systems+" OR MH "Point‐of‐Care Testing" |
| 32 | TI ((rapid or "point of care" or "near patient" or poc or poct or bedside) N5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*)) OR AB ((rapid or "point of care" or "near patient" or poc or poct or bedside) N5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*)) |
| 33 | TI (radt or radts or rdt or rdts) OR AB (radt or radts or rdt or rdts) |
| 34 | TI (antigen* N3 detect*) OR AB (antigen* N3 detect*) |
| 35 | S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 |
| 36 | S17 AND S35 |
Data and analyses
Comparison 1. Comparison 1: Rapid test (with and without scoring system combined) versus clinical grounds (with and without scoring system combined).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number of participants provided with an antibiotic prescription | 5 | 2545 | Risk Difference (M‐H, Random, 95% CI) | ‐0.25 [‐0.31, ‐0.18] |
| 1.2 Number of participants with an antibiotic dispensed | 2 | 900 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.17, 0.02] |
| 1.3 Number of participants with a complication attributed to the index infection | 4 | 2075 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.03, 26.65] |
| 1.4 Number of participants in need of re‐consultation by the end of follow‐up | 2 | 1161 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.57, 2.21] |
Comparison 2. Comparison 2: Rapid test alone versus clinical grounds (with scoring system).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number of participants provided with an antibiotic prescription | 1 | 256 | Risk Difference (M‐H, Random, 95% CI) | ‐0.29 [‐0.40, ‐0.17] |
Comparison 3. Comparison 3: Rapid test alone versus clinical grounds (without scoring system).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number of participants provided with an antibiotic prescription | 3 | 1129 | Risk Difference (M‐H, Random, 95% CI) | ‐0.29 [‐0.40, ‐0.19] |
Comparison 4. Comparison 4: Rapid test used with a scoring system versus clinical grounds (with scoring system).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Number of participants provided with an antibiotic prescription | 3 | 1416 | Risk Difference (M‐H, Random, 95% CI) | ‐0.21 [‐0.26, ‐0.16] |
| 4.2 Number of participants with an antibiotic dispensed | 2 | 900 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.17, 0.02] |
Comparison 5. Comparison 5: Rapid test used with a scoring system versus clinical grounds (without scoring system).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Number of participants provided with an antibiotic prescription | 1 | 214 | Risk Difference (M‐H, Random, 95% CI) | ‐0.20 [‐0.34, ‐0.07] |
Comparison 6. Sensitivity analysis: studies at low risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Sensitivity analysis: studies at low risk of bias | 3 | 1646 | Risk Difference (M‐H, Random, 95% CI) | ‐0.22 [‐0.27, ‐0.17] |
Comparison 7. Sensitivity analysis: Studies with individual randomisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Sensitivity analysis: Studies with individual randomisation | 2 | 1176 | Risk Difference (M‐H, Random, 95% CI) | ‐0.21 [‐0.27, ‐0.16] |
Comparison 8. Sensitivity analysis: Missing data as failures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Number of participants provided with an antibiotic | 5 | 2557 | Risk Difference (M‐H, Random, 95% CI) | ‐0.25 [‐0.32, ‐0.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Little 2013a.
| Study characteristics | ||
| Methods |
Clinical setting: general practices Single‐ or multicentre study: multicentre Country of study: United Kingdom Unit of allocation: individual participants Inclusion criteria: people aged ≥ 3 years presenting with acute sore throat (2 weeks or less of sore throat) and an abnormal looking throat (erythema and/or pus) Exclusion criteria: non‐infective causes of sore throat and inability of participant or parent/guardian to consent Follow‐up:
|
|
| Participants |
Number of clusters (n): not applicable Number of participants (n): 424 (as in Table 1 of the article) Participant characteristics:
|
|
| Interventions |
Management in intervention group(s): RADT in combination with clinical examination/scoring system (“Group 3”: clinical score 0 to 1, no antibiotics or rapid antigen test; score of 2, delayed antibiotic prescription without rapid testing; scores ≥ 3, rapid antigen test with antibiotics not offered if negative result). Type of RADT system used: enzyme immunoassay Commercial name and brand of the RADT: IMI TestPack Plus Strep A (Inverness Medical) Management in control group(s): clinical grounds with a scoring system (“Group 2”: clinical score 0 to 1, no antibiotics; score 2 to 3, delayed antibiotics; score ≥ 4, immediate antibiotics). |
|
| Outcomes |
Primary outcome(s): symptom severity (mean score of soreness and difficulty swallowing in days 2 to 4) Secondary outcome(s):
|
|
| Notes |
Type of report: journal article Source(s) of funding: funded by the National Institute for Health Research Heath Technology Assessment (HTA) Programme (project number 05/10/01) RCT registration number: ISRCTN32027234 The PRISM RCT had 2 parts, each relying on a different clinical scoring system. The results of the first part of the trial, obtained with Score 1, are presented in the online appendices (and here referred to as Little 2013b). The results of the second part of the trial, obtained with Score 2 (acronym FeverPAIN), are presented in the text as the main findings (and here referred to as Little 2013a). We assumed that the methods were similar for the 2 parts of the trial. The “Delayed antibiotics” group (“Group 1”) was excluded because by definition all participants were prescribed antibiotics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were individually randomised with a web based computer randomisation service to one of three groups. [...] Randomisation used permuted block sizes of 3, 6, 9, and 12, which were also randomly chosen." |
| Allocation concealment (selection bias) | Low risk | Web‐based computer randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are discrepancies between participants included in the initial assessments and in the follow‐up (20% to 25% reduction in participants assessed for the outcome “antibiotic use” compared to initial number of participants), without any explanation. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the Methods section are presented in the Results section. There is concordance between protocol and article. |
| Other bias | Low risk | None |
Little 2013b.
| Study characteristics | ||
| Methods |
Clinical setting: see Little 2013a Single‐ or multicentre study: see Little 2013a Country of study: see Little 2013a Unit of allocation: see Little 2013a Inclusion criteria: see Little 2013a Exclusion criteria: see Little 2013a Follow‐up: see Little 2013a |
|
| Participants |
Number of clusters (n): not applicable Number of participants (n): 752 (as in Table B of the article) Participant characteristics:
|
|
| Interventions |
Management in intervention group(s): RADT in combination with clinical examination/scoring system Type of RADT system used: see Little 2013a Commercial name and brand of the RADT: see Little 2013a Management in control group(s): clinical grounds with a scoring system ("Score 1") |
|
| Outcomes |
Primary outcome(s): see Little 2013a Secondary outcome(s): see Little 2013a |
|
| Notes | See Little 2013a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were individually randomised with a web based computer randomisation service to one of three groups (see below). Randomisation used permuted block sizes of 3, 6, 9, and 12, which were also randomly chosen." |
| Allocation concealment (selection bias) | Low risk | Web‐based computer randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are discrepancies between participants included in the initial assessments and in the follow‐up (20% to 25% reduction in participants assessed for the outcome “antibiotic use” compared to initial number of participants), without any explanation. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the Methods section are presented in the Results section. There is concordance between protocol and article. |
| Other bias | Low risk | None |
Llor 2011a.
| Study characteristics | ||
| Methods |
Clinical setting: primary healthcare centres Single‐ or multicentre study: multicentre Country of study: Spain Unit of allocation: clusters Inclusion criteria: patients aged 14 to 60 years diagnosed with acute pharyngitis with 1 or more Centor criteria (fever, sore throat, tonsillar exudate, tender cervical nodes, and/or absence of cough). Exclusion criteria: patients with more than 5 episodes of pharyngitis over the last year; those with immunosuppressed condition, such as active neoplasm, AIDS, or reception of chemotherapy, radiotherapy, steroids, and/or immunosuppressive therapy; those with heart valve disease; rheumatic fever; an episode of pharyngitis treated with antibiotics in the previous 15 days; and those who had tonsillectomy. Follow‐up: “evolution within the first month” (no more information reported). |
|
| Participants |
Number of clusters (n): 20 centres Number of participants (n): 557 enrolled, 543 included in the analysis Participant characteristics:
|
|
| Interventions |
Management in intervention group(s): RADT alone (“Physicians allocated to the intervention group were provided with RADT”) Type of RADT system used: enzyme immunoassay Commercial name and brand of the RADT: OSOM Strep A test (Genzyme) Management in control group(s): clinical grounds without a scoring system (“Those assigned to the control group managed streptococcal pharyngitis with only clinical criteria”) |
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
|
| Notes |
Type of report: journal article Source(s) of funding: funded by the Fondo de Investigaciones Sanitarias, the University and Innovation Department of Spain, and by the Catalan Society of Family Medicine. The Rapid Test Device OSOM StrepA of Genzyme was provided by Leti. All the trial authors declare they have received no honoraria from the Leti Laboratory for undertaking this study. Carl Llor declares having received tests free of charge from Leti for investigational studies. In the last 3 years Leti has covered the travel and accommodation costs for seeking the inclusion of physicians in a control group in the Happy Audit study in Murcia. Leti also covered the accommodation costs during an international congress on respiratory disease in primary care within the last year. RCT registration number: ISRCTN23587778 We contacted the authors to confirm that physicians in the control group were not invited to prescribe antibiotics based on clinical criteria (although some of them might be aware of the Centor criteria). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participating primary healthcare centres were randomised to the intervention or to the control arm of the study, with an allocation ratio of 1:1, by a random sequence generated by a computer program." |
| Allocation concealment (selection bias) | Unclear risk | No description exists of any concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were incomplete for 14 out of 557 participants without any explanation. Elsewhere, there is existence of incomplete outcome data, such as with regards to evolution of pharyngitis, and without explanation. |
| Selective reporting (reporting bias) | High risk | Missing data in outcome in the intervention group and in the control group (complete data for 274 out of 281 and 237 out of 262 participants, respectively). |
| Other bias | Low risk | None |
Maltezou 2008.
| Study characteristics | ||
| Methods |
Clinical setting: offices of private‐practice paediatricians Single‐ or multicentre study: multicentre Country of study: Greece Unit of allocation: clusters Inclusion criteria: children aged 2 to 14 years with clinical evidence of pharyngitis including at least 1 of the following 4 criteria: fever (> 38.0 °C), tonsillar exudate, tender enlarged anterior cervical lymph nodes, and absence of cough. Exclusion criteria: having received antibiotics within the previous week or being immunocompromised. Follow‐up: 3 weeks after enrolment, paediatricians called their participants for follow‐up information (clinical course and complications, if any). |
|
| Participants |
Number of clusters (n): 17 Number of participants (n): 639 Participant characteristics:
|
|
| Interventions |
Management in intervention group(s): RADT alone (“Application of the RADT in children with at least one clinical criterion and prescription of antibiotics only if positive; throat culture was also taken and if positive, but RADT‐negative, prescription of antibiotics was done 48 h later” (Group B)). Type of RADT system used: enzyme immunoassay Commercial name and brand of the RADT: Link 2 Strep A Rapid Test (Becton‐Dickinson) Management in control group(s): clinical grounds without a scoring system ("Decision to prescribe antibiotics by clinical criteria only" ("Group A")). |
|
| Outcomes |
|
|
| Notes |
Type of report: journal article Source(s) of funding: funded by The Hellenic Center for Disease Control and Prevention (Athens, Greece) RCT registration number: not reported “Group C” was excluded because paediatricians in this group managed children using the RADT/culture strategy without being randomised. We contacted the trial authors to confirm that this was a cluster‐RCT and that paediatricians in Group A were not invited to prescribe antibiotics based on clinical criteria (although they had to collect Centor criteria for the study). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From correspondence with the trial authors, the following information was provided: "it was a cluster randomized trial, with private‐practice pediatricians being randomized to strategy A or B (whereas hospital‐affiliated pediatricians were all assigned to strategy C)". Thus, not all were randomised. |
| Allocation concealment (selection bias) | Unclear risk | No description exists of any concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are unexplained inconsistencies, e.g. between the percentages mentioned in Table 3 and the actual percentage when dividing the number of prescriptions with the participants in each group. There is no mentioning of handling, or existence, of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the Methods section are presented in the Results section. There is no protocol to be used for comparison regarding outcomes mentioned. |
| Other bias | Low risk | None |
Worrall 2007a.
| Study characteristics | ||
| Methods |
Clinical setting: family doctors’ offices Single‐ or multicentre study: multicentre Country of study: Canada Unit of allocation: clusters Inclusion criteria: patients aged 19 years or older who presented with acute sore throat as their primary symptom Exclusion criteria: not reported Follow‐up: not reported |
|
| Participants |
Number of clusters (n): 37 Number of participants (n): 533 Participant characteristics:
|
|
| Interventions |
Management in intervention group(s):
Type of RADT system used: enzyme immunoassay Commercial name and brand of the RADT: Clearview Exact Strep A dipstick (Wampole Laboratories) Management in control group(s):
|
|
| Outcomes |
Primary outcome(s): rate of antibiotic prescribing Secondary outcome(s): types of antibiotics prescribed |
|
| Notes |
Type of report: journal article Source(s) of funding: not reported RCT registration number: not reported The trial had 4 arms: 1. Usual practice (“Control arm”), 2. Decision rule only (“STDR arm”), 3. Rapid antigen test only (“RADT arm”), 4. Decision rule and antigen test combined (“STDR and RADT arm“). The clinical scoring system used was a slightly modified Centor score. There is no report of participant characteristics. The authors explain that they ”did not ask doctors to record clinical or demographic characteristics of the patients” because they “wanted the doctors to do as little extra work as possible”. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 40 physicians who agreed to take part in the study were randomly allocated to 1 of 4 trial arms [...]." No description exists on the generation of the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No description exists of any concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding is impossible in this context, and this lack of blinding is likely to influence the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants entered into the study appear to have been assessed for antibiotic prescription. There is no mentioning of handling, or existence, of incomplete outcome data (exclusion, attrition, etc.), but the risk of bias is considered low. |
| Selective reporting (reporting bias) | Low risk | Unclear description of what outcomes are included in study; despite this, relevant outcomes are reported in the Results. There is no protocol to be used for comparison regarding outcomes mentioned. |
| Other bias | Low risk | None |
GABHS: group A beta‐haemolytic streptococcus RADT: rapid antigen detection test RCT: randomised controlled trial SD: standard deviation STDR: sore throat decision rule
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Najjar 2008 | Diagnostic accuracy study |
| Alper 2013 | Diagnostic accuracy study |
| Bird 2018 | Observational study |
| Bottaro 2007 | Observational study |
| Buchbinder 2007 | Diagnostic accuracy study |
| Cardoso 2013 | Diagnostic accuracy study |
| Cohen 2017 | Guidelines |
| Contessotto 2000 | Diagnostic accuracy study |
| Dodd 2018 | Observational study |
| Frost 2019 | Management study |
| Harris 1995 | Diagnostic accuracy study |
| Hedges 1991 | Management study |
| Humair 2006 | Diagnostic accuracy study |
| Lieu 1986 | Diagnostic accuracy study |
| Little 2014a | Duplicate |
| Little 2014b | Duplicate |
| Llor 2011b | Observational study |
| Llor 2014a | Observational study |
| Llor 2014b | Observational study |
| Llor 2018 | Observational study |
| Llor 2019 | Observational study |
| Luo 2019 | Management study |
| Madurell 2010 | Study protocol |
| Maizia 2012 | Cost‐effectiveness study |
| Majeed 1993 | Cost‐effectiveness study |
| Makela 1989 | Diagnostic accuracy study |
| McGinn 2013 | Different intervention |
| McIsaac 2004 | Diagnostic accuracy study |
| NCT03744832 | Study protocol |
| Orda 2016 | Diagnostic accuracy study |
| Papastergiou 2018 | Observational study |
| Ralph 2019 | Diagnostic accuracy study |
| Rao 2019 | Diagnostic accuracy study |
| Regueras 2012 | Diagnostic accuracy study |
| Reichardt 2009 | Observational study |
| Tanz 2018 | Observational study |
| Thornton 2017 | Observational study |
| True 1986 | Diagnostic accuracy study |
| Worrall 2007b | Duplicate |
| Worrall 2007c | Duplicate |
Characteristics of studies awaiting classification [ordered by study ID]
Wächtler 2018.
| Methods | Cluster‐randomised trial |
| Participants | 520 participants with sore throat |
| Interventions | The study had 3 arms:
|
| Outcomes | Antibiotic prescription rate |
| Notes | Preliminary results presented at a conference in 2018. We have contacted the trial authors for more information. |
DEGAM: German College of General Practitioners and Family Physicians RADT: rapid antigen detection test
Differences between protocol and review
We could not report on several outcomes due to lack of data.
We did not contact trial authors to obtain intraclass correlation coefficients.
We did not use GRADEpro GDT software to produce the Table 1.
We rephrased the Objectives of the review from 'To assess the efficacy and safety of using rapid tests at the point‐of‐care to guide antibiotic prescriptions as compared to management based on clinical grounds' (protocol) to 'To assess the efficacy and safety of strategies based on rapid tests to guide antibiotic prescriptions for sore throat in primary care settings' (review). The rationale for the change was that some studies do not only evaluate rapid tests, but strategies based on rapid tests (e.g. combinations of clinical scores and rapid tests).
We modified the wording of our second primary outcome from ‘Number of antibiotic prescriptions dispensed’ (protocol) to ‘Number of participants with an antibiotic dispensed’ (review), for the sake of consistency in wording across outcomes.
For Comparison 1, we had planned to create a 'Summary of findings' table with the following outcomes: number of participants provided with an antibiotic prescription; number of antibiotic prescriptions dispensed; duration of sore throat symptoms; duration of other symptoms (e.g. fever); number of participants with a complication attributed to the index infection (e.g. quinsy, acute rheumatic fever); number of participants in need of re‐consultation by the end of follow‐up; and number of participants in need of hospital admission by the end of follow‐up (protocol). In the review, our final 'Summary of findings' table only included outcomes for which we could extract data in the included studies (i.e. number of participants provided with an antibiotic prescription; number of participants with an antibiotic dispensed; number of participants with a complication attributed to the index infection; and number of participants in need of re‐consultation by the end of follow‐up).
Contributions of authors
Original idea for the review: JFC and MC.
First draft of the protocol: JFC and MC.
Input and editing on the protocol: RC, NH, JYP.
Study selection and data extraction: JFC, NH, JYP.
Data analysis: JFC and NH.
First draft of the manuscript: JFC and NH.
Critical revisions to the manuscript: All authors.
Study supervision: MC.
Sources of support
Internal sources
-
Cochrane Review Support Programme (CRSP), UK
Cochrane Review Support Programme (CRSP) grants aim to financially support the publication of high‐priority Cochrane Reviews.
External sources
-
French Ministry of Health, France
Research grant PHRC régional AOR 12089 (2012)
Declarations of interest
Jérémie F Cohen: None known.
Jean‐Yves Pauchard: None known.
Nils Hjelm: I have received three scholarships (one scholarship for conducting research abroad as part of my medical studies at the Lund University; one scholarship for conducting research in France; and one scholarship from the Erasmus Funding Programme for conducting research in another European Union country). None of the received scholarships are regarded as potential conflicts of interest.
Robert Cohen: RC’s institution, ACTIV, has received research grant support from Pfizer, GlaxoSmithKline, Merck, and Sanofi Pasteur MSD. RC reports personal fees from Pfizer, GlaxoSmithKline, Merck, Sanofi, and AstraZeneca, outside the submitted work. These potential interests are only in the field of vaccines.
Martin Chalumeau: No financial competing interest in relation to the present systematic review.
JFC, RC, and MC have conducted diagnostic accuracy studies about rapid tests for strep throat in which rapid tests kits were provided by the manufacturer (Dectrapharm/Biosynex). These studies were not in the scope of the present review and thus were not included in the review.
New
References
References to studies included in this review
Little 2013a {published data only}
- Little P, Hobbs FD, Moore M, Mant D, Williamson I, McNulty C, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013;347:f5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2013b {published data only}
- Little P, Hobbs FD, Moore M, Mant D, Williamson I, McNulty C, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013;347:f5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llor 2011a {published data only}
- Llor C, Madurell J, Balagué-Corbella M, Gómez M, Cots JM. Impact on antibiotic prescription of rapid antigen detection testing in acute pharyngitis in adults: a randomised clinical trial. British Journal of General Practice 2011;61:e244-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltezou 2008 {published data only}
- Maltezou HC, Tsagris V, Antoniadou A, Galani L, Douros C, Katsarolis I, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. Journal of Antimicrobial Chemotherapy 2008;62:1407–12. [DOI] [PubMed] [Google Scholar]
Worrall 2007a {published data only}
- Worrall G, Hutchinson J, Sherman G, Griffiths J. Diagnosing streptococcal sore throat in adults: randomized controlled trial of in-office aids. Canadian Family Physician 2007;53:666-71. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Najjar 2008 {published data only}
- Al-Najjar FY, Uduman SA. Clinical utility of a new rapid test for the detection of group A Streptococcus and discriminate use of antibiotics for bacterial pharyngitis in an outpatient setting. International Journal of Infectious Diseases 2008;12:308-11. [DOI] [PubMed] [Google Scholar]
Alper 2013 {published data only}
- Alper Z, Uncu Y, Akalin H, Ercan I, Sinirtas M, Bilgel NG. Diagnosis of acute tonsillopharyngitis in primary care: a new approach for low-resource settings. Journal of Chemotherapy 2013;25:148-55. [DOI] [PubMed] [Google Scholar]
Bird 2018 {published data only}
- Bird C, Winzor G, Lemon K, Moffat A, Newton T, Gray J. A pragmatic study to evaluate the use of a rapid diagnostic test to detect group A streptococcal pharyngitis in children with the aim of reducing antibiotic use in a UK emergency department. Pediatric Emergency Care 2018 Jul 24 [Epub ahead of print]. [DOI: 10.1097/PEC.0000000000001560] [DOI] [PubMed]
Bottaro 2007 {published data only}
- Bottaro G, Morselli I. Epidemiology and clinical pictures of pharyngitis: report on the activity of a family paediatrician. Pediatria Medica e Chirurgica 2007;29:326-30. [PubMed] [Google Scholar]
Buchbinder 2007 {published data only}
- Buchbinder N, Benzdira A, Belgaid A, Dufour D, Paon JC, Morel A, et al. Streptococcal pharyngitis in the pediatric emergency department: value and impact of rapid antigen detection test. Archives de Pédiatrie 2007;14:1057-61. [DOI] [PubMed] [Google Scholar]
Cardoso 2013 {published data only}
- Cardoso DM, Gilio AE, Hsin SH, Machado BM, Paulis M, Lotufo JP, et al. Impact of the rapid antigen detection test in diagnosis and treatment of acute pharyngotonsillitis in a pediatric emergency room. Revista Paulista de Pediatria 2013;31:4-9. [DOI] [PubMed] [Google Scholar]
Cohen 2017 {published data only}
- Cohen R, Haas H, Lorrot M, Biscardi S, Romain O, Vie Le Sage F, et al. Antimicrobial treatment of ENT infections. Archives de Pédiatrie 2017;24:S9-16. [DOI] [PubMed] [Google Scholar]
Contessotto 2000 {published data only}
- Contessotto Spadetto C, Camara Simon M, Aviles Ingles MJ, Ojeda Escuriet JM, Cascales Barcelo I, Rodriguez Sanchez F. Rational use of antibiotics in pediatrics: impact of a rapid test for detection of beta-haemolytic group A streptococci in acute pharyngotonsillitis. Anales Espanoles de Pediatria 2000;52:212-9. [PubMed] [Google Scholar]
Dodd 2018 {published data only}
- Dodd M, Adolphe A, Parada A, Brett M, Culbreath K, Mercier RC. Clinical impact of a rapid streptococcal antigen test on antibiotic use in adult patients. Diagnostic Microbiology and Infectious Disease 2018;91:339-44. [DOI] [PubMed] [Google Scholar]
Frost 2019 {published data only}
- Frost HM, Fritsche TR, Hall MC. Beta-hemolytic nongroup A streptococcal pharyngitis in children. Journal of Pediatrics 2019;206:268-73.e1. [DOI] [PubMed] [Google Scholar]
Harris 1995 {published data only}
- Harris R, Paine D, Wittler R, Bruhn F. Impact on empiric treatment of group A streptococcal pharyngitis using an optical immunoassay. Clinical Pediatrics 1995;34:122-7. [DOI] [PubMed] [Google Scholar]
Hedges 1991 {published data only}
- Hedges JR, Singal BM, Estep JL. The impact of a rapid screen for streptococcal pharyngitis on clinical decision making in the emergency department. Medical Decision Making 1991;11:119-24. [DOI] [PubMed] [Google Scholar]
Humair 2006 {published data only}
- Humair JP, Revaz SA, Bovier P, Stalder H. Management of acute pharyngitis in adults: reliability of rapid streptococcal tests and clinical findings. Archives of Internal Medicine 2006;166:640-4. [DOI] [PubMed] [Google Scholar]
Lieu 1986 {published data only}
- Lieu TA, Fleisher GR, Schwartz JS. Clinical performance and effect on treatment rates of latex agglutination testing for streptococcal pharyngitis in an emergency department. Pediatric Infectious Disease 1986;5:655-9. [DOI] [PubMed] [Google Scholar]
Little 2014a {published data only}
- Little P, Moore M, Hobbs FD, Mant D, McNulty C, Williamson I, et al. Randomised controlled trial of a clinical score and rapid antigen detection test for sore throats. Health Technology Assessment 2014;18:29-39. [Google Scholar]
Little 2014b {published data only}
- Little P, Hobbs FD, Moore M, Mant D, Williamson I, McNulty C, et al. PRImary care Streptococcal Management (PRISM) study: in vitro study, diagnostic cohorts and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technology Assessment 2014;18:vii-xxv, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llor 2011b {published data only}
- Llor C, Cots JM, Gonzalez Lopez-Valcarcel B, Dios Alcantara J, Garcia G, Arranz J, et al. Effect of two interventions on reducing antibiotic prescription in pharyngitis in primary care. Journal of Antimicrobial Chemotherapy 2011;66:210-5. [DOI] [PubMed] [Google Scholar]
Llor 2014a {published data only}
- Llor C, Cots JM, Hernández S, Ortega J, Arranz J, Monedero MJ, et al. Effectiveness of two types of intervention on antibiotic prescribing in respiratory tract infections in primary care in Spain: Happy Audit Study. Atencion Primaria 2014;46:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llor 2014b {published data only}
- Llor C, Bjerrum L, Munck A, Cots JM, Hernández S, Moragas A. Access to point-of-care tests reduces the prescription of antibiotics among antibiotic-requesting subjects with respiratory tract infections. Respiratory Care 2014;59:1918-23. [DOI] [PubMed] [Google Scholar]
Llor 2018 {published data only}
- Llor C, Bjerrum L, Molero JM, Moragas A, González López-Valcárcel B, Monedero MJ, et al. Long-term effect of a practice-based intervention (HAPPY AUDIT) aimed at reducing antibiotic prescribing in patients with respiratory tract infections. Journal of Antimicrobial Chemotherapy 2018;73:2215-22. [DOI] [PubMed] [Google Scholar]
Llor 2019 {published data only}
- Llor C, Molero JM, Moragas A, Cordoba G, Bjerrum L. Use of point-of-care tests and antibiotic prescribing in sore throat and lower respiratory infections by general practitioners. Enfermedades Infecciosas y Microbiología Clínica 2019;38:21-4. [DOI: 10.1016/j.eimc.2019.02.005] [DOI] [PubMed] [Google Scholar]
Luo 2019 {published data only}
- Luo R, Sickler J, Vahidnia F, Lee YC, Frogner B, Thompson M. Diagnosis and management of group A streptococcal pharyngitis in the United States, 2011-2015. BMC Infectious Diseases 2019;19:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madurell 2010 {published data only}
- Madurell J, Balague M, Gomez M, Cots JM, Llor C. Impact of rapid antigen detection testing on antibiotic prescription in acute pharyngitis in adults. FARINGOCAT STUDY: a multicentric randomized controlled trial. BMC Family Practice 2010;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maizia 2012 {published data only}
- Maizia A, Letrilliart L, Colin C. Diagnostic strategies for acute tonsillitis in France: a cost-effectiveness study. Presse Medicale 2012;41:e195-203. [DOI] [PubMed] [Google Scholar]
Majeed 1993 {published data only}
- Majeed HA, al-Doussary L, Moussa MM, Yusuf AR, Suliman AH. Office diagnosis and management of group A streptococcal pharyngitis employing the rapid antigen detecting test. A 1-year prospective study of reliability and cost in primary care centres. Annals of Tropical Paediatrics 1993;13:65-72. [DOI] [PubMed] [Google Scholar]
Makela 1989 {published data only}
- Makela M. Effect of latex agglutination test on prescribing for group A streptococcal throat disease in primary care. Scandinavian Journal of Infectious Diseases 1989;21:161-7. [DOI] [PubMed] [Google Scholar]
McGinn 2013 {published data only}
- McGinn TG, McCullagh L, Kannry J, Knaus M, Sofianou A, Wisnivesky JP, et al. Efficacy of an evidence-based clinical decision support in primary care practices: a randomized clinical trial. JAMA Internal Medicine 2013;173:1584-91. [DOI] [PubMed] [Google Scholar]
McIsaac 2004 {published data only}
- McIsaac WJ, Kellner JD, Aufricht P, Vanjaka A, Low DE. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA 2004;291:1587-95. [DOI] [PubMed] [Google Scholar]
NCT03744832 {published data only}
- NCT03744832. Point of care streptococcal pharyngitis testing. clinicaltrials.gov/ct2/show/NCT03744832 (first posted 16 November 16 2018).
Orda 2016 {published data only}
- Orda U, Mitra B, Orda S, Fitzgerald M, Gunnarsson R, Rofe G, et al. Point of care testing for group A streptococci in patients presenting with pharyngitis will improve appropriate antibiotic prescription. Emergency Medicine Australasia 2016;28:199-204. [DOI] [PubMed] [Google Scholar]
Papastergiou 2018 {published data only}
- Papastergiou J, Trieu CR, Saltmarche D, Diamantouros A. Community pharmacist-directed point-of-care group A Streptococcus testing: evaluation of a Canadian program. Journal of the American Pharmacists Association 2018;58:450-6. [DOI] [PubMed] [Google Scholar]
Ralph 2019 {published data only}
- Ralph AP, Holt DC, Islam S, Osowicki J, Carroll DE, Tong SYC, et al. Potential for molecular testing for group A Streptococcus to improve diagnosis and management in a high-risk population: a prospective study. Open Forum Infectious Diseases 2019;6:ofz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2019 {published data only}
- Rao A, Berg B, Quezada T, Fader R, Walker K, Tang S, et al. Diagnosis and antibiotic treatment of group A streptococcal pharyngitis in children in a primary care setting: impact of point-of-care polymerase chain reaction. BMC Pediatrics 2019;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Regueras 2012 {published data only}
- Regueras De Lorenzo G, Santos Rodríguez PM, Villa Bajo L, Pérez Guirado A, Arbesú Fernández E, Barreiro Hurlé L, et al. Use of the rapid antigen technique in the diagnosis of Streptococcus pyogenes pharyngotonsillitis. Anales de Pediatria 2012;77:193-9. [DOI] [PubMed] [Google Scholar]
Reichardt 2009 {published data only}
- Reichardt B, Pichlhofer O, Zehetmayer S, Maier M. Current diagnosis of acute pharyngitis. Wiener Medizinische Wochenschrift 2009;159:202-6. [DOI] [PubMed] [Google Scholar]
Tanz 2018 {published data only}
- Tanz RR, Zheng XT, Carter DM, Steele MC, Shulman ST. Caution needed: molecular diagnosis of pediatric group A streptococcal pharyngitis. Journal of the Pediatric Infectious Disease Society 2018;7:e145-7. [DOI] [PubMed] [Google Scholar]
Thornton 2017 {published data only}
- Thornton HV, Hay AD, Redmond NM, Turnbull SL, Christensen H, Peters TJ, et al. Throat swabs in children with respiratory tract infection: associations with clinical presentation and potential targets for point-of-care testing. Family Practice 2017;34:407-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
True 1986 {published data only}
- True BL, Carter BL, Driscoll CE, House JD. Effect of a rapid diagnostic method on prescribing patterns and ordering of throat cultures for streptococcal pharyngitis. Journal of Family Practice 1986;23:215-9. [PubMed] [Google Scholar]
Worrall 2007b {published data only}
- Worrall G, Hutchinson J, Sherman G, Griffiths J. Diagnosing streptococcal sore throat in adults - randomized controlled trial of in-office aids. Canadian Family Physician 2007;53:667-71. [PMC free article] [PubMed] [Google Scholar]
Worrall 2007c {published data only}
- Worrall G, Hutchinson J, Sherman G, Griffiths J. Diagnosing streptococcal sore throat in adults: randomized controlled trial of in-office aids. [Erratum appears in Can Fam Physician. 2007 Jun;53(6):1006]. Canadian Family Physician 2007;53:666-71. [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Wächtler 2018 {unpublished data only}
- Wächtler H, Kaduszkiewicz H, Hansmann-Wiest J, Hedderich J, Wiese B, Maas S, et al. Antibiotics for sore throat in Germany: influence of a guideline or an additional Strep-test on antibiotic prescriptions. In: General Practice Research on Infections Network. 2018. [TRIAL IDENTIFIER: DRKS00013018]
Additional references
Aabenhus 2014
- Aabenhus R, Jensen JU, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010130.pub2] [DOI] [PubMed] [Google Scholar]
AAP 2015
- Kimberlin DW (editor). Red Book: 2015 Report of the Committee on Infectious Diseases. 30th edition. Elk Grove Village, IL: American Academy of Pediatrics, 2015. [Google Scholar]
Adams 2004
- Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. Journal of Clinical Epidemiology 2004;57(8):785-94. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayanruoh 2009
- Ayanruoh S, Waseem M, Quee F, Humphrey A, Reynolds T. Impact of rapid streptococcal test on antibiotic use in a pediatric emergency department. Pediatric Emergency Care 2009;25(11):748-50. [DOI] [PubMed] [Google Scholar]
Carlet 2012
- Carlet J. World Alliance Against Antibiotic Resistance (WAAR): safeguarding antibiotics. Intensive Care Medicine 2012;38(10):1723-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
CDC 2012
- Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2012 state and national summary tables. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf.
Chahwakilian 2011
- Chahwakilian P, Huttner B, Schlemmer B, Harbarth S. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. Journal of Antimicrobial Chemotherapy 2011;66(12):2872-9. [DOI] [PubMed] [Google Scholar]
Cohen 2015
- Cohen JF, Cohen R, Levy C, Thollot F, Benani M, Bidet P, et al. Selective testing strategies for diagnosing group A streptococcal infection in children with pharyngitis: a systematic review and prospective multicentre external validation study. Canadian Medical Association Journal 2015;187(1):23-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2016
- Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD010502.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebell 2000
- Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA 2000;284(22):2912-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fine 2012
- Fine AM, Nizet V, Mandl KD. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Archives of Internal Medicine 2012;172(11):847-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming‐Dutra 2016
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73. [DOI] [PubMed] [Google Scholar]
Gerber 2004
- Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clinical Microbiology Reviews 2004;17(3):571-80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goossens 2005
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365(9459):579-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hong 2011
- Hong SY, Taur Y, Jordan MR, Wanke C. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines. Journal of Evaluation in Clinical Practice 2011;17(6):1176-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joachim 2010
- Joachim L, Campos D Jr, Smeesters PR. Pragmatic scoring system for pharyngitis in low-resource settings. Pediatrics 2010;126(3):e608-14. [DOI] [PubMed] [Google Scholar]
Laxminarayan 2013
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance - the need for global solutions. Lancet Infectious Diseases 2013;13(12):1057-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lean 2014
- Lean WL, Arnup S, Danchin M, Steer AC. Rapid diagnostic tests for group A streptococcal pharyngitis: a meta-analysis. Pediatrics 2014;134(4):771-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Linder 2005
- Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA 2005;294(18):2315-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Little 2014
- Little P, Hobbs FR, Moore M, Mant D, Williamson I, McNulty C, et al. PrImary care Streptococcal Management (PRISM) study: in vitro study, diagnostic cohorts and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technology Assessment 2014;18(6):vii-xxv. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Pouwels 2018
- Pouwels KB, Dolk FCK, Smith DRM, Robotham JV, Smieszek T. Actual versus 'ideal' antibiotic prescribing for common conditions in English primary care. Journal of Antimicrobial Chemotherapy 2018;73(Suppl 2):19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shaikh 2010
- Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 2010;126(3):e557-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shaikh 2012
- Shaikh N, Swaminathan N, Hooper EG. Accuracy and precision of the signs and symptoms of streptococcal pharyngitis in children: a systematic review. Journal of Pediatrics 2012;160(3):487-93.e3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shulman 2012
- Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2012;55(10):e86-102. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinks 2013
- Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD000023.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanz 2009
- Tanz RR, Gerber MA, Kabat W, Rippe J, Seshadri R, Shulman ST. Performance of a rapid antigen-detection test and throat culture in community pediatric offices: implications for management of pharyngitis. Pediatrics 2009;123(2):437-44. [DOI] [PubMed] [Google Scholar]
Vandenbroucke 2004
- Vandenbroucke JP. Benefits and harms of drug treatments. BMJ 2004;329(7456):2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wessels 2011
- Wessels MR. Clinical practice. Streptococcal pharyngitis. New England Journal of Medicine 2011;364(7):648-55. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cohen 2016b
- Cohen JF, Pauchard JY, Hjelm N, Cohen R, Chalumeau M. Efficacy and safety of rapid tests to guide antibiotic prescriptions for sore throat. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012431] [DOI] [PMC free article] [PubMed] [Google Scholar]


